Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8453
Peer-review started: March 19, 2021
First decision: April 29, 2021
Revised: May 14, 2021
Accepted: July 7, 2021
Article in press: July 7, 2021
Published online: October 6, 2021
Processing time: 192 Days and 20 Hours
Granular cell tumor (GCT) is a neurogenic tumor mainly occurring in the head and neck. GCT in the genitourinary system is extremely rare and only sporadic cases of urinary bladder GCT have been reported. Most urinary bladder GCT cases are benign and only two malignant cases have been reported. Due to its rarity, no consensus criteria for the treatment of urinary bladder GCT are available at present.
A 62-year-old Chinese woman was found to have a urinary bladder tumor without any clinical manifestations on physical examination. Cystoscopy revealed a semispherical shaped lesion measuring approximately 4.0 cm in diameter at the junction of the left wall and roof of the bladder, which was covered with normal bladder mucosa. Computed tomography scan demonstrated a high-density lesion on the left wall of the bladder, measuring approximately 2.9 cm × 2.4 cm with clear boundaries. Contrast-enhanced pelvic magnetic resonance imaging revealed a space-occupying lesion on the left wall of the bladder (non-mucosal origin/ external pressure), which was preliminarily suspected to be a desmoplastic fibroma or leiomyoma. In the context of the above findings, a pre-operative diagnosis of bladder leiomyoma was made. The patient consequently underwent a laparoscopic partial cystectomy. The resected bladder mass looked yellowish and well-demarcated, measuring 4.0 cm × 3.5 cm and infiltrated the muscular layer. The diagnosis of urinary bladder GCT was finally made by postoperative pathology, with positive immunohistochemical S-100 staining and negative pancytokeratin. The patient has been followed for 6 mo so far, with no tumor recurrence detected.
This case highlights the biological feature and differential diagnosis of urinary bladder GCT at the pathological and molecular levels. Transurethral resection of the bladder tumor and partial cystectomy are recommended in most urinary bladder GCT cases, while radical cystectomy is recommended in malignant cases.
Core Tip: ATP6AP1, ATP6AP2, BRD7 and GFRA2 gene mutations can potentially induce the progression of urinary bladder granular cell tumor. Characteristic biomarkers such as S-100, SOX10, CD56, pancytokeratin and HBM45 are essential for the diagnosis and differential diagnosis. Partial cystectomy is the treatment of choice to minimize recurrence and improve disease-free survival in benign cases. A relatively conservative transurethral resection of the bladder tumor can also be an alternative option according to the location of the primary tumor or at the patient's request. For malignant bladder granular cell tumors, radical surgical intervention with pelvic lymph node dissection is necessary.
- Citation: Wei MZ, Yan ZJ, Jiang JH, Jia XL. Atypical granular cell tumor of the urinary bladder: A case report. World J Clin Cases 2021; 9(28): 8453-8460
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8453.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8453
Granular cell tumor (GCT) is a neurogenic tumor usually occurring in head and neck regions. GCT of the genitourinary system such as urinary bladder is extremely rare. As only sporadic cases of bladder GCT have been reported, there are no explicit criteria for the treatment of this disease. Most bladder GCT cases are benign, and only two malignant cases have been reported, and distant metastasis of GCT from the primary organ to the bladder should not been neglected[1,2]. It is usually difficult to distinguish GCT from other neurogenic tumors by simply depending on radiologic and pathological findings without immunohistochemical (IHC) staining. In this article, we report a case of GCT occurring in the urinary bladder, and hope that it can help better understand this rare disease entity in the bladder.
A 62-year-old Chinese woman was found to have a "hypoechoic bladder space" without any specific complaints during a pelvic ultrasound examination at the local community health service center.
The patient had no presenting symptoms.
She had diabetes mellitus and left kidney agenesis.
On initial physical examination, her temperature was 36.7°C, blood pressure was 133/85 mmHg, heart rate was 84 bpm, and respiratory rate was 16 breaths/min. The clinical urological examination revealed no characteristic signs.
Routine blood, urine and stool tests were within the normal range, and microscopy for bacteria and fungi showed negative results. Laboratory tests showed high-sensitivity C-reactive protein of 17.97 mg/L (RR: 0.00-5.00 mg/L) and rheumatoid factor (RF) of 34.70 IU/mL (RR: < 20 IU/mL). Other biochemical and coagulation indicators were within the normal ranges. Tumor markers such as CA199, CA125, CEA and AFP which were used for routine screening for metastatic tumor from other primary organs such as the gastrointestinal tract, ovary, liver were not remarkable.
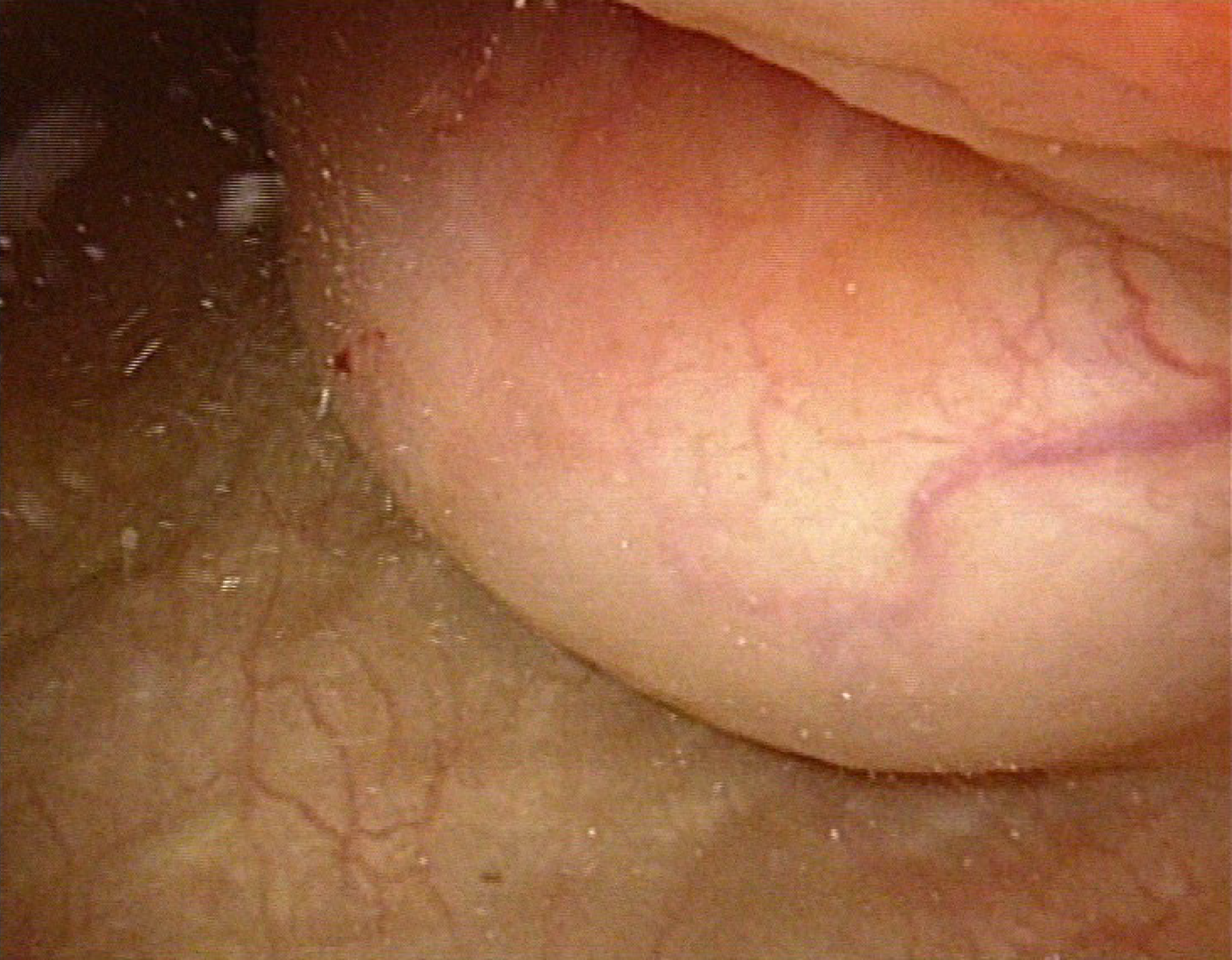
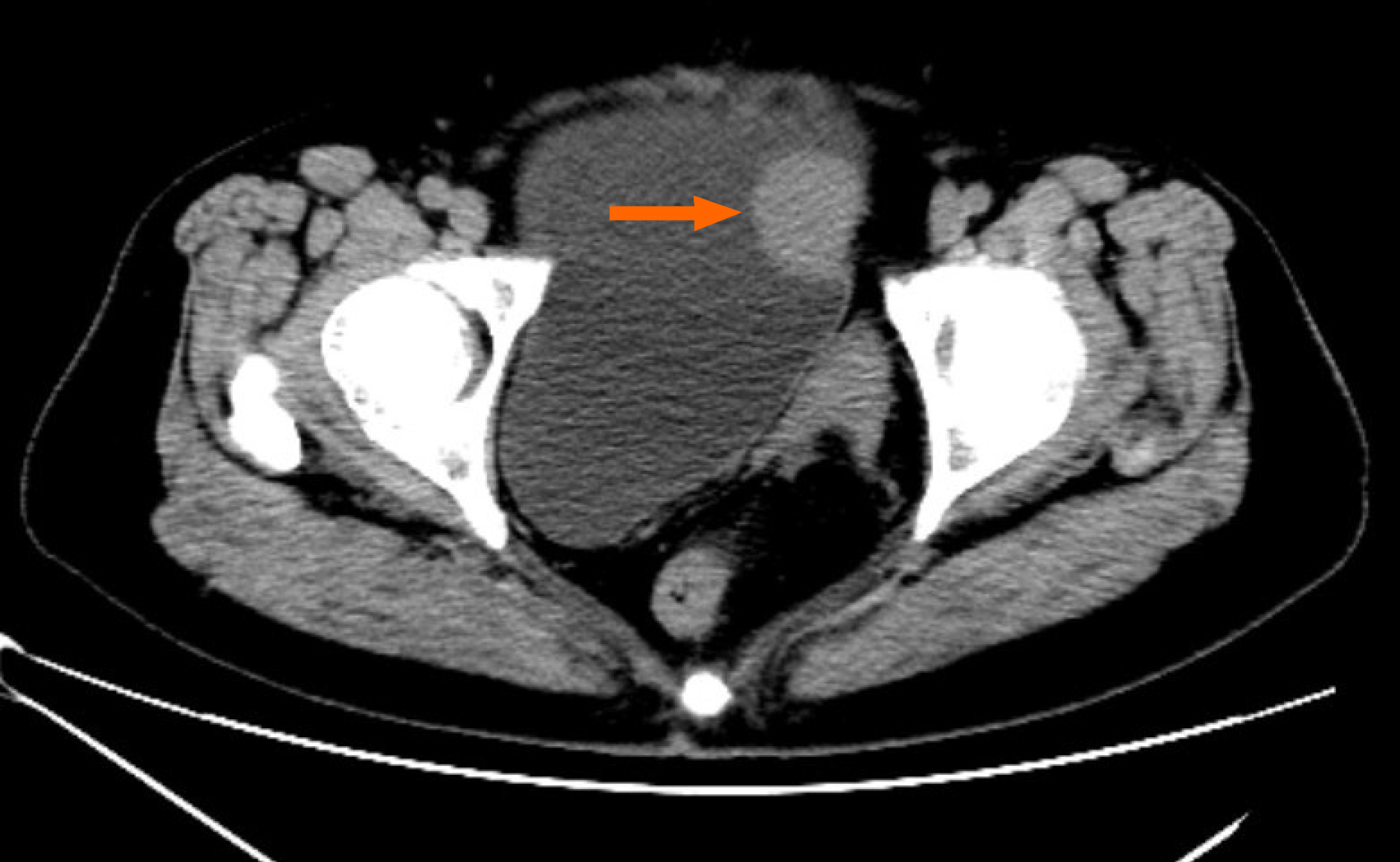
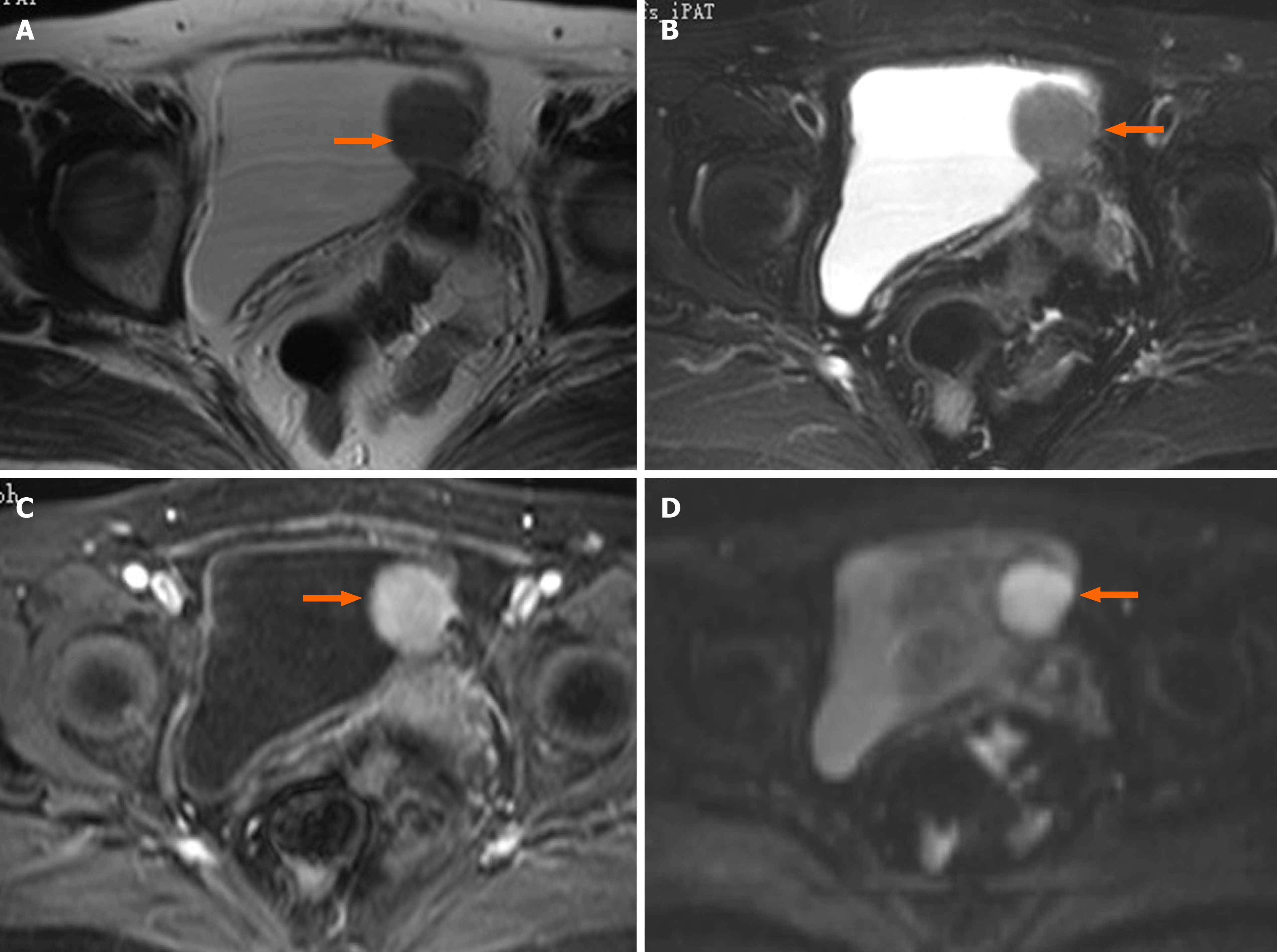
Cystoscopy revealed a semispherical shaped lesion measuring approximately 4.0 cm in diameter at the junction of the left wall and roof of the bladder, which was covered with normal bladder mucosa (Figure 1). An initial pelvic computed tomography (CT) scan revealed a high-density lesion on the left wall of the bladder, measuring was approximately 2.9 cm × 2.4 cm with clear boundaries, with a mean CT value of 44HU (Figure 2). Contrast-enhanced magnetic resonance imaging (MRI) revealed a space-occupying lesion (SOL) on the left wall of the bladder, the SOL did not originate from urothelium of the bladder wall but tended to generate externally into the bladder cavity (Figure 3), which was preliminarily suspected to be a desmoplastic fibroma or leiomyoma. MRI T1WI phase (Figure 3A) and T2WI (Figure 3B) sequences revealed round equalized signals; T1WI + fat suppression + enhanced sequences showed obvious enhancement (Figure 3C); the DWI phase revealed limited diffusion (Figure 3D).
Postoperative pathology of the resected specimen confirmed the diagnosis of bladder GCT, with positive IHC S-100 staining and negative pancytokeratin.
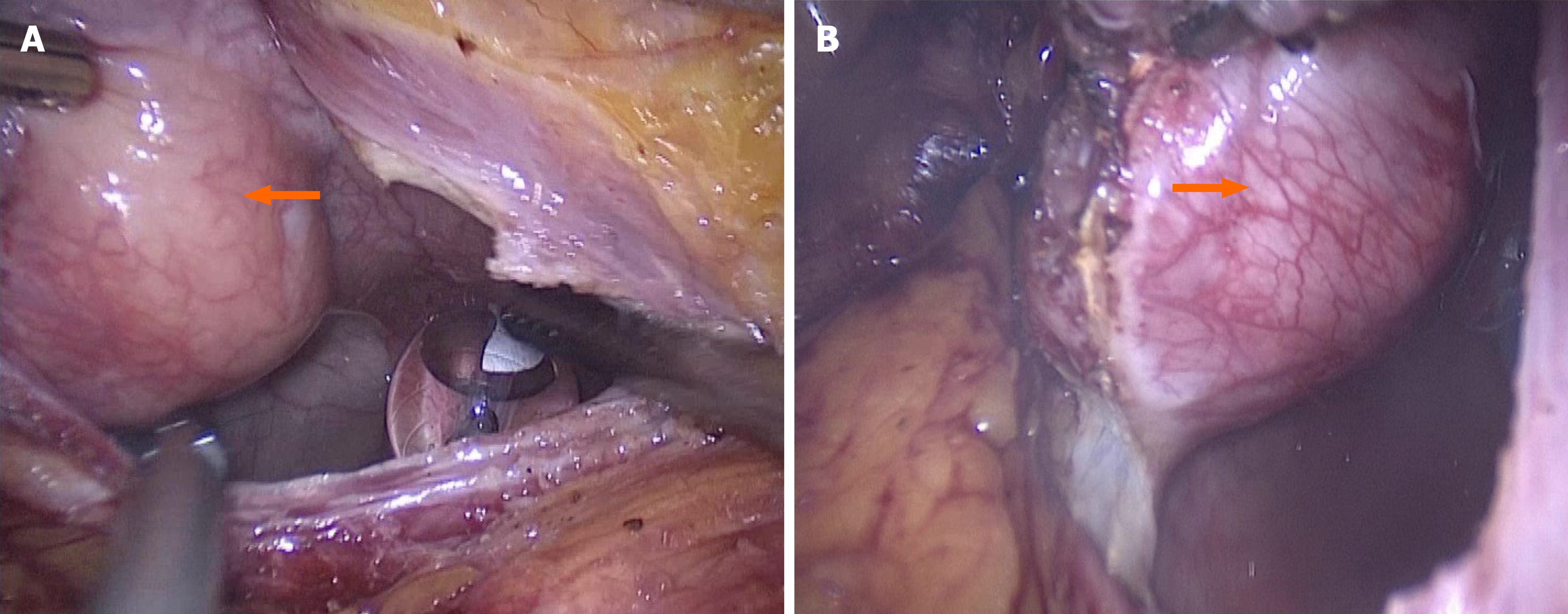
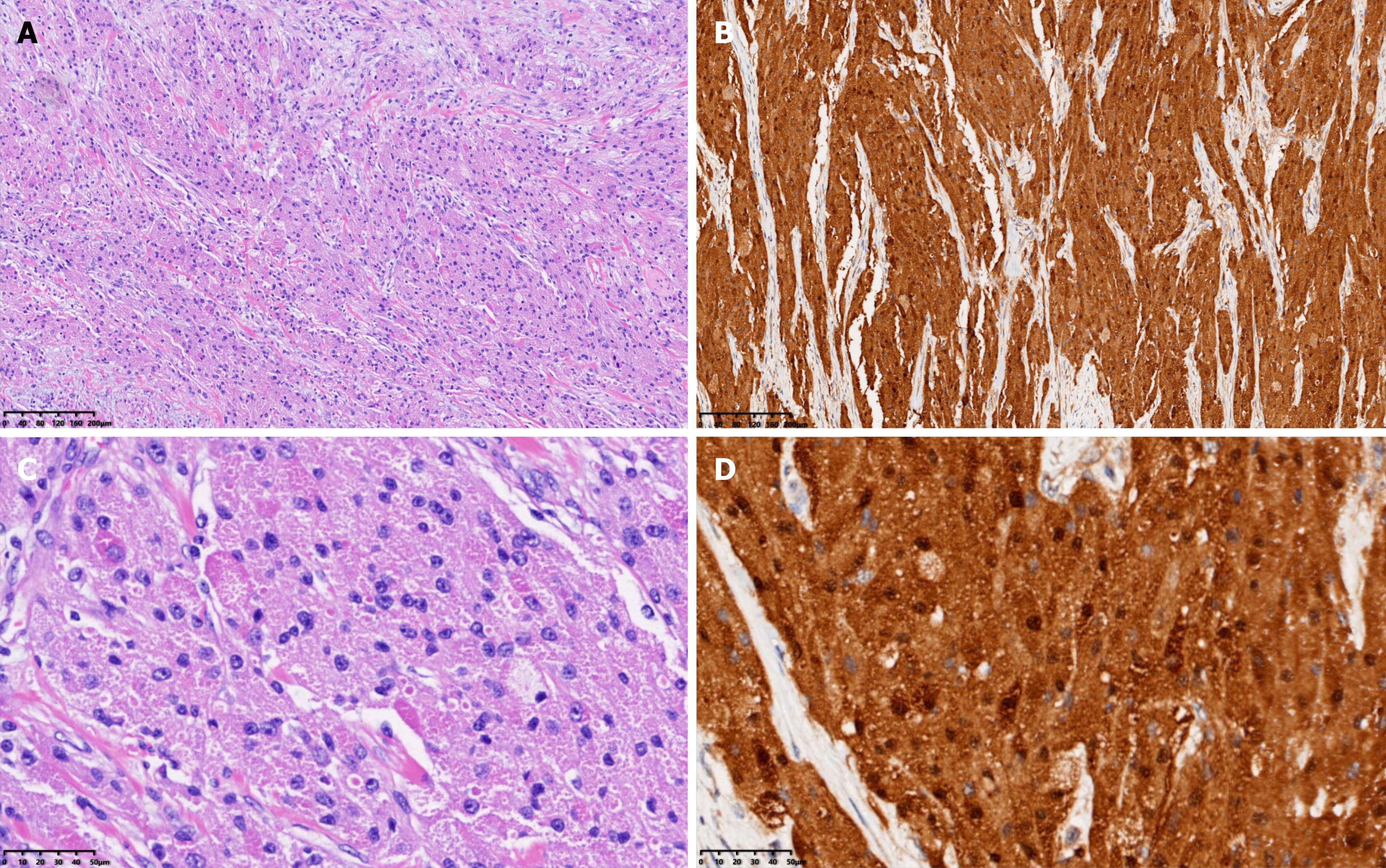
Based on the imaging findings and biochemical indicators, a pre-operative diagnosis of bladder leiomyoma was made, for which a laparoscopic partial cystectomy (Figure 4A and B) was successfully performed with an intraoperative blood loss of approximately 20 mL. The resected solid mass looked yellowish and well-demarcated, measuring approximately 4.0 cm × 3.5 cm, without obvious adhesion to the surrounding tissues under gross appearance. Frozen section examination of the specimen suggested the diagnosis of mesenchymal neurogenic or myogenic tumor with negative margins. Post-operative pathologic study demonstrated the diagnosis of atypical GCT, which infiltrated the muscular layer of the bladder with enlarged tumor cells and scattered pleomorphic cells. The nuclei were obvious and vacuolar, with an increased nuclei/cytoplasm ratio, nuclear mitosis 0-1/10HPF, and no necrotic lesions (Figure 5A). IHC showed positive expression of S-100 protein (Figure 5B), negative pancytokeratin, positive neuron-specific enolase (NSE), and weak positivity of both Ki-67 (5%) and CD-68.
The patient underwent positron emission tomography-CT (18F-fluorodeoxyglucose) three weeks after surgery, and showed multiple enlarged lymph nodes and increased fluorodeoxyglucose metabolism in the retroperitoneal and bilateral iliac vascular region, devoid of tumor metastasis. The patient did not receive adjuvant therapy, and was followed up with cystoscopy and CT scan of the urinary system every 3 mo. During a follow-up period of 6 mo, no evidence of recurrence or lymph node enlarge
GCT is a neurogenic tumor, first described as a myoblast mass of the tongue by Abrikosoff in 1926[3]. GCTs commonly occur in the oral cavity, digestive tract, skin, and subcutaneous tissue, and most cases occur in women aged 40-60 years. Genitourinary GCT is extremely rare, and only about 20 cases of urinary bladder GCT have been reported to date[2]. Distant metastasis of GCT from the primary organ should not be neglected.
Based on the currently available case reports, gross hematuria appears to be the most common clinical manifestation of urinary bladder GCT[4,5]. However, the patient in our case report did not present any apparent manifestations except the imaging findings, which were consistent with the pre-operative cystoscopic findings of undamaged mucosa of the solid mass.
GCT cells are larger than typical tumor cells, presenting as scattered polymorphic cells with polygonal and hyperchromatic nuclei and nuclear vacuoles[5]. GCT is mainly derived from Schwann cells and consists of large polymorphic cells containing a large granular cytoplasm. It is usually difficult to distinguish GCT from other neurogenic tumors and depends on cellular morphology and radiology. For instance, a schwannoma is completely composed of differentiated neoplastic Schwann cells, and a ganglioneuroma is mainly composed of mature Schwannian stroma and mature ganglion cells[6-8]. GCT often presents with atypical histological features, including spindling, increased mitotic features (> 2/10 HPF), nuclear pleomorphism, prominent nucleoli, a high nuclear/cytoplasm ratio, and necrosis[9].
The positive rate of Ki67 is usually less than 1% in benign GCT and ≥ 10% in malignant GCT. The GCT in our report was neurogenic, consistent with most reports[3,10-13], but the positive rate of Ki67 was 5%, which indirectly suggests that this may be the first reported case of atypical GCT of the bladder. The IHC profile of GCT is strongly positive for S-100 protein, SOX10, and CD68 but negative for pancytokeratin, which is partially consistent with the findings in the present case. Additionally, CD56, CD57, NSE, inhibin, calretinin, TFE3, PGP9.5, and vimentin are commonly positive in GCT[2]. It should be noted that malignant GCT tends to be misdiagnosed as melanoma due to positive IHC staining of S-100 and SOX10 in both cancers. However, HMB45 staining is positive in melanoma and negative in GCT[2,9].
The most recent studies have suggested that ATP6AP1 and ATP6AP2 gene mutations may lead to GCT cell proliferation by decreasing the lysosomal activities and that BRD7 and GFRA2 proteins of the RTK signaling pathway are highly expressed in malignant GCT[14-16].
Yoshida et al[17] reported that most urinary bladder GCT cases were pathologically diagnosed as benign tumors. However, although malignant cases are rare, local recurrence caused by incomplete resection of the primary tumor has been reported in sporadic cases, and accurate intra-operative biopsy is, therefore, necessary[17]. Given this context and based on the research of Abbas and other scholars in combination with the evaluation of the curative effect in our case, a preliminary consensus can still be reached that transurethral resection of bladder tumor (TURBt) or partial cystectomy may be the most common treatment for benign GCT of the bladder depending on the different positions of the tumor, and the prognosis is relatively optimistic[3]. One of the biological characteristics of GCT is its tendency to infiltrate tissues; if a GCT in other organs is not completely resected, it is likely to recur and continue to grow again. Sun et al[2] reported that recurrence occurred in more than 50% of cases of benign bladder GCT after the initial TURBt treatment and a full follow-up period.
Repeated TURBt of the lesion after diagnosis can reduce the possibility of recurrence. Compared with partial cystectomy, TURBt after the first recurrence may be associated with a higher recurrence rate[2]. Therefore, for both primary and recurrent benign bladder GCT cases, partial cystectomy can better ensure a negative incisal margin and reduce the possibility of recurrence. Extended radical bladder resection is often necessary for recurrent malignant GCT bladder tumors with a ureteral obstruction or more extensive tissue invasion. It is reported that radical cystectomy combined with bilateral pelvic lymphadenectomy can improve disease-free survival (DFS) and significantly improve the prognosis of malignant bladder GCT[2]. There is no clear evidence that patients with malignant bladder GCT should receive chemotherapy or radiotherapy. The current literature on malignant GCT derived from various body systems shows that the 5- and 10-year survival rates are 74% and 65%, respectively; the recurrence rate within 3-37 mo after pathological diagnosis is 32%-41%, and the metastasis rate is 11%-62%[18-20]. Therefore, the high risk of poor prognosis of malignant bladder GCT should not be ignored. After laparoscopic partial cystectomy in our case, the final pathological diagnosis was atypical GCT, suggesting the possibility of its malignant differentiation. Although this may indicate the malignant potency of the tumor, depending on the current consensus and promising follow-up result in our case, we finally performed a partial cystectomy instead of radical cystectomy to achieve better survival.
Furthermore, the enlargement of lymph nodes in the retroperitoneal and bilateral iliac vascular regions was detected by a positron emission tomography (PET) scan 3 wk after surgery; nevertheless, the routine CT scan during follow-up revealed no recurrence or apparent lymph node enlargement. In view of the above, we attribute the disparity of reports regarding the lymph nodes on PET and CT scans to the irritable inflammatory reaction. Although no recurrence has been detected within the 6-mo follow-up period, further close follow-up is necessary and radical resection may be required.
Significantly, the patient had left kidney agenesis and diabetes mellitus. Unilateral renal agenesis is usually accompanied by another ectopic kidney, nonrenal anomalies, and evidence of renal injury[21,22], such as contralateral renal hypertrophy, branchio-renal syndrome (commonly associated with hearing abnormalities), renal malfunction, and Müllerian defects[23] (e.g., uterine didelphys or vaginal duplication), which are common in girls. Unfortunately, depending on the illustrations of current reports, there is no relation between GCT and unilateral renal agenesis. In our case, the patient had a normal contralateral kidney size, which was not accompanied by hearing abnormality, renal injury (e.g., hypertension or proteinuria), abnormal serum creatinine or glomerular filtration rate; however, uterine didelphys was observed. We will continue to perform urinalysis in this patient during every follow-up to monitor the contralateral kidney function.
Bladder GCT is uncommon, and malignant GCT is extremely rare. IHC characteristics such as S-100, SOX10, CD56, HBM45, and pancytokeratin are essential for diagnosis. In addition, ATP6AP1, ATP6AP2, BRD7, and GFRA2 gene mutations cannot be neglected in terms of further diagnosis and targeting therapy. For benign bladder GCT, partial cystectomy is the treatment of choice because it can minimize recurrence and improve DFS. A relatively conservative TURBt can also be selected depending on the primary tumor location or upon the patient’s request. For malignant bladder GCT, radical surgical resection with pelvic lymph node dissection is necessary. The primary purposes of this case report were to attract more attention to this rare disease, help better understand the disease, and promote more research on specific biomarkers for the diagnosis and treatment of bladder GCT.
| 1. | Bedir R, Yılmaz R, Özdemir O, Uzun H. Granular cell tumor of the urinary bladder. Turk J Urol. 2017;43:383-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Sun Y, Reuter VE, Magi-Galluzzi C, Sankin A, Epstein JI. Granular Cell Tumor of the Bladder: A Report of Six Cases. Urology. 2018;121:203.e1-203.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Abbas F, Memon A, Siddiqui T, Kayani N, Ahmad NA. Granular cell tumors of the urinary bladder. World J Surg Oncol. 2007;5:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Lee C, Kim B, Song B, Park JH, Moon KC. Clinicopathologic Features of Benign Neurogenic Tumor of Urinary Bladder. Int J Surg Pathol. 2018;26:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Kang HW, Kim YW, Ha YS, Min YK, Kim WT, Kim YJ, Yun SJ, Lee SC, Kim WJ. Granular cell tumor of the urinary bladder. Korean J Urol. 2010;51:291-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 2162] [Article Influence: 216.2] [Reference Citation Analysis (2)] |
| 7. | Fletcher CD. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology. 2014;64:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 8. | Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:349-363. [PubMed] |
| 9. | Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22:779-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 463] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Maiorano E, Favia G, Napoli A, Resta L, Ricco R, Viale G, Altini M. Cellular heterogeneity of granular cell tumours: a clue to their nature? J Oral Pathol Med. 2000;29:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Rekhi B, Jambhekar NA. Morphologic spectrum, immunohistochemical analysis, and clinical features of a series of granular cell tumors of soft tissues: a study from a tertiary referral cancer center. Ann Diagn Pathol. 2010;14:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | An S, Jang J, Min K, Kim MS, Park H, Park YS, Kim J, Lee JH, Song HJ, Kim KJ, Yu E, Hong SM. Granular cell tumor of the gastrointestinal tract: histologic and immunohistochemical analysis of 98 cases. Hum Pathol. 2015;46:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Schoolmeester JK, Lastra RR. Granular cell tumors overexpress TFE3 without corollary gene rearrangement. Hum Pathol. 2015;46:1242-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Wei L, Liu S, Conroy J, Wang J, Papanicolau-Sengos A, Glenn ST, Murakami M, Liu L, Hu Q, Miles KM, Nowak DE, Liu B, Qin M, Bshara W, Omilian AR, Head K, Bianchi M, Burgher B, Darlak C, Kane J, Merzianu M, Cheney R, Fabiano A, Salerno K, Talati C, Khushalani NI, Trump DL, Johnson CS, Morrison CD. Whole-genome sequencing of a malignant granular cell tumor with metabolic response to pazopanib. Cold Spring Harb Mol Case Stud. 2015;1:a000380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Pareja F, Brandes AH, Basili T, Selenica P, Geyer FC, Fan D, Da Cruz Paula A, Kumar R, Brown DN, Gularte-Mérida R, Alemar B, Bi R, Lim RS, de Bruijn I, Fujisawa S, Gardner R, Feng E, Li A, da Silva EM, Lozada JR, Blecua P, Cohen-Gould L, Jungbluth AA, Rakha EA, Ellis IO, Edelweiss MIA, Palazzo J, Norton L, Hollmann T, Edelweiss M, Rubin BP, Weigelt B, Reis-Filho JS. Loss-of-function mutations in ATP6AP1 and ATP6AP2 in granular cell tumors. Nat Commun. 2018;9:3533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | Sekimizu M, Yoshida A, Mitani S, Asano N, Hirata M, Kubo T, Yamazaki F, Sakamoto H, Kato M, Makise N, Mori T, Yamazaki N, Sekine S, Oda I, Watanabe SI, Hiraga H, Yonemoto T, Kawamoto T, Naka N, Funauchi Y, Nishida Y, Honoki K, Kawano H, Tsuchiya H, Kunisada T, Matsuda K, Inagaki K, Kawai A, Ichikawa H. Frequent mutations of genes encoding vacuolar H+ -ATPase components in granular cell tumors. Genes Chromosomes Cancer. 2019;58:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Yoshida T, Hirai S, Horii Y, Yamauchi T. Granular cell tumor of the urinary bladder. Int J Urol. 2001;8:29-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Moten AS, Zhao H, Wu H, Farma JM. Malignant granular cell tumor: Clinical features and long-term survival. J Surg Oncol. 2018;118:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Thacker MM, Humble SD, Mounasamy V, Temple HT, Scully SP. Case report. Granular cell tumors of extremities: comparison of benign and malignant variants. Clin Orthop Relat Res. 2007;455:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Rose B, Tamvakopoulos GS, Yeung E, Pollock R, Skinner J, Briggs T, Cannon S. Granular cell tumours: a rare entity in the musculoskeletal system. Sarcoma. 2009;2009:765927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Westland R, Schreuder MF, Ket JC, van Wijk JA. Unilateral renal agenesis: a systematic review on associated anomalies and renal injury. Nephrol Dial Transplant. 2013;28:1844-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Westland R, Kurvers RA, van Wijk JA, Schreuder MF. Risk factors for renal injury in children with a solitary functioning kidney. Pediatrics. 2013;131:e478-e485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | O'Flynn O'Brien KL, Bhatia V, Homafar M, Gong YY, Winsten MT, Gerber J, Dietrich JE. The Prevalence of Müllerian Anomalies in Women with a Diagnosed Renal Anomaly. J Pediatr Adolesc Gynecol. 2021;34:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, Machado I, Shah OJ, Taskovska M, Yelamanchi R S-Editor: Gao CC L-Editor: Webster JR P-Editor: Xing YX