Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8461
Peer-review started: February 2, 2021
First decision: June 15, 2021
Revised: June 18, 2021
Accepted: August 16, 2021
Article in press: August 16, 2021
Published online: October 6, 2021
Processing time: 238 Days and 7 Hours
Renal cysts and diabetes (RCAD) syndrome is an autosomal dominant diabetic renal disease. Precise molecular diagnosis of RCAD syndrome has proven valuable for understanding its mechanism and personalized therapy.
A RCAD patient and her family were studied to investigate potential responsible genes by the whole exome sequencing (WES). Candidate pathogenic variants were validated by Sanger sequencing. The clinical characteristics of RCAD patient were collected from medical records. Unlike those typical RCAD patients, we observed renal manifestation and prediabetes phenotype, but not reproductive organ phenotype and hypomagnesaemia. A novel 7-bp deletion mutation in exon 4 of the hepatocyte nuclear factor 1B, NM_000458: c.882_888del (p.V294fs), was identified by WES and confirmed by Sanger sequencing.
This novel mutation identified in a Chinese family with RCAD syndrome might be the molecular pathogenic basis of this disorder.
Core Tip: Renal cysts and diabetes (RCAD) syndrome is an autosomal dominant diabetic renal disease. Precise molecular diagnosis of RCAD syndrome has proven valuable for understanding its mechanism and selecting optimal therapy. A novel deletion mutation of hepatocyte nuclear factor 1B gene (NM_000458: c.882_888del, p.V294fs) was identified in a Chinese family with RCAD syndrome by whole exome sequencing and Sanger sequencing. Considering the gene function and the genotype-phenotype correlation, mutation location, and its conservativeness, this mutation is considered to play a pathogenic role in the development of RCAD syndrome.
- Citation: Xiao TL, Zhang J, Liu L, Zhang B. Hepatocyte nuclear factor 1B mutation in a Chinese family with renal cysts and diabetes syndrome: A case report. World J Clin Cases 2021; 9(28): 8461-8469
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8461.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8461
Renal cysts and diabetes (RCAD) syndrome (OMIM: 137920) is an autosomal dominant diabetic renal disease resulting from abnormal renal development. Highly variable phonotypes of the renal disease include renal cysts, glomerular tufts, aberrant nephrogenesis, primitive tubules, irregular collecting systems, oligomeganephronia, enlarged renal pelvises, abnormal calyces, small kidney, single kidney, horseshoe kidney, and hyperuricemic nephropathy[1]. The diabetic phonotypes of this disorder usually occur earlier than age 25 years and patients are thus diagnosed as having maturity-onset diabetes of the young type 5 (MODY5)[2]. Nevertheless, typical diabetic phonotypes may not occur in some cases.
At the molecular level, the RCAD syndrome is related to mutations of hepatocyte nuclear factor 1B (HNF1B). To date, more than 400 mutations of HNF1B gene have been identified in RCAD patients, and de novo mutations are encountered in up to 30%-50% of cases[3]. These mutations include missenses, nonsenses, frame shifts, splice site mutations, small indels, and large deletions. In fact, HNF1B-associated syndrome is much complicated, as this gene encodes a transcription factor of the homeodomain-containing superfamily that is expressed in multiple organs[4]. Most RCAD patients often present with renal cysts and renal function decline that precede the diabetes. Besides the phonotypes of diabetes and renal presentation, RCAD patients may also have anomalies of the organs such as the genital tract, including vaginal aplasia, rudimentary uterus, bicornuate uterus, epididymal cysts, and atresia of the vas deferens[5]. Thus, heterogeneous presentation of the multisystem phenotype is often found in HNF1B-associated syndrome. For example, a case study suggests that lack of HNF1B expression is related to chromophobe renal cell carcinoma, a rare renal cancer[6]. Notably, diabetes and renal cysts are not always present in HNF1B-mutated patients. Moreover, the phenotype of HNF1B mutant carriers is highly variable within and between families[7]. Recently, the clinical characteristics of HNF1B-related disorders in 33 patients were reported in a Japanese population[8]. Analysis of genotype-phenotype correlation showed that some clinical characteristics were significantly different between patients with heterozygous variant of HNF1B and those harboring a deletion of HNF1B. However, RCAD patients in the Chinese population are rarely reported. Here we report a frame shift mutation of HNF1B gene in a Chinese family with RCAD syndrome that has never been described previously.
A 24-year-old Chinese Han woman was admitted to our department of nephrology for sudden back pain and frequent micturition.
The patient also suffered from a temporary fever with the highest temperature of 41 °C.
The patient was hospitalized in the department of urology at our hospital 2 years ago, and diagnosed with bilateral multiple renal cysts. She was the sole child of her parents and denied the genetic history of kidney diseases.
The patient denied a family history of kidney diseases.
The patient’s temperature was 41 °C, heart rate was 98 bpm, respiratory rate was 20 breaths per minute, and blood pressure was 110/76 mmHg.
Laboratory test showed elevated levels of serum creatinine and uric acid (Table 1). Routine blood test showed normal white blood cell, neutrophil, and lymphocyte counts. Routine urine tests showed elevated levels of uric leucocytes and red cells, but without urine protein. The liver enzyme and magnesium levels were normal. Notably, the patient’s plasma glucose level was 6.88 mmol/L.
| Parameter | Proband | Mother | Father | Reference |
| Height (cm) | 160 | 155 | 168 | - |
| Weight (kg) | 44 | 54 | 72 | - |
| BMI (kg/m²) | 17.19 ↓ | 22.5 | 25.5 | 18.5-24 |
| WBC count, 109/L | 4.03 | 4.2 | 5.22 | 3.5-9.5 |
| NEUT% | 0.60 | 0.63 | 0.65 | 40-75 |
| LYM% | 32.8 | 35 | 34.0 | 20-50 |
| HGB, g/L | 129.0 | 122 | 140.0 | 115-150 |
| Platelet count,109/L | 254.0 | 267 | 289.0 | 125-350 |
| RBC count, 1012/L | 4.54 | 4.20 | 4.83 | 3.8-5.1 |
| Urine routine tests | ||||
| Specific gravity | 1.010 ↓ | 1.020 | 1.020 | 1.015-1.030 |
| Urine protein | - | - | - | - |
| Urinary occult blood | 3+ | - | - | - |
| Urine glucose | - | - | - | - |
| Urine ketone bodies | - | - | - | - |
| 24 h UPE, g/d | 0.09 | 0.01 | 0.01 | 0-0.12 |
| Immunoglobulin A, g/L | 1.36 | 1.58 | 2.04 | 0.7-4.0 |
| Immunoglobulin G, g/L | 13 | 10 | 12 | 7-15 |
| Immunoglobulin M, g/L | 1.36 | 1.52 | 1.47 | 0.4-2.6 |
| Complement C3, g/L | 0.64 ↓ | 2.5 | 2.1 | 0.9-2.1 |
| Complement C4, mg/dL | 12.70 ↓ | 22.10 | 24.50 | 16-38 |
| Lambda, mg/dL | 475.00 | 528.00 | 601.00 | 313-723 |
| Kappa, mg/dL | 975.00 | 876.00 | 930.00 | 629-1350 |
| Fasting plasma glucose | 6.88↑ | 7.1↑ | 5.8 | 3.9-6.1 |
| HBA1C, % | 6.7↑ | 5.4 | 5 | 4.0-6.0 |
| Albumin, g/L | 48.40 | 45.30 | 48.00 | 40-55 |
| Globulin, g/L | 28.40 | 25.17 | 26.09 | 20-40 |
| Serum creatinine, umol/L | 112.3↑ | 56.9 | 69 | 45-105 |
| eGFR (mL/min/1.73m²) | 59↓ | 110 | 110 | > 90 |
| Serum uric acid, umol/L | 535.7↑ | 362.5 | 378.6 | 140-420 |
| Cystatin-C, mg/L | 1.47↑ | 0.89 | 0.79 | 0-1.16 |
| PTH, pg/mL | 79.40↑ | 44 | 60 | 12-65 |
| Insulin autoantibody | ||||
| IAA | - | - | - | - |
| GADA | - | - | - | - |
| ICA-40KD | - | - | - | - |
| ICA-64KD | - | - | - | - |
| ICA-120KD | - | - | - | - |
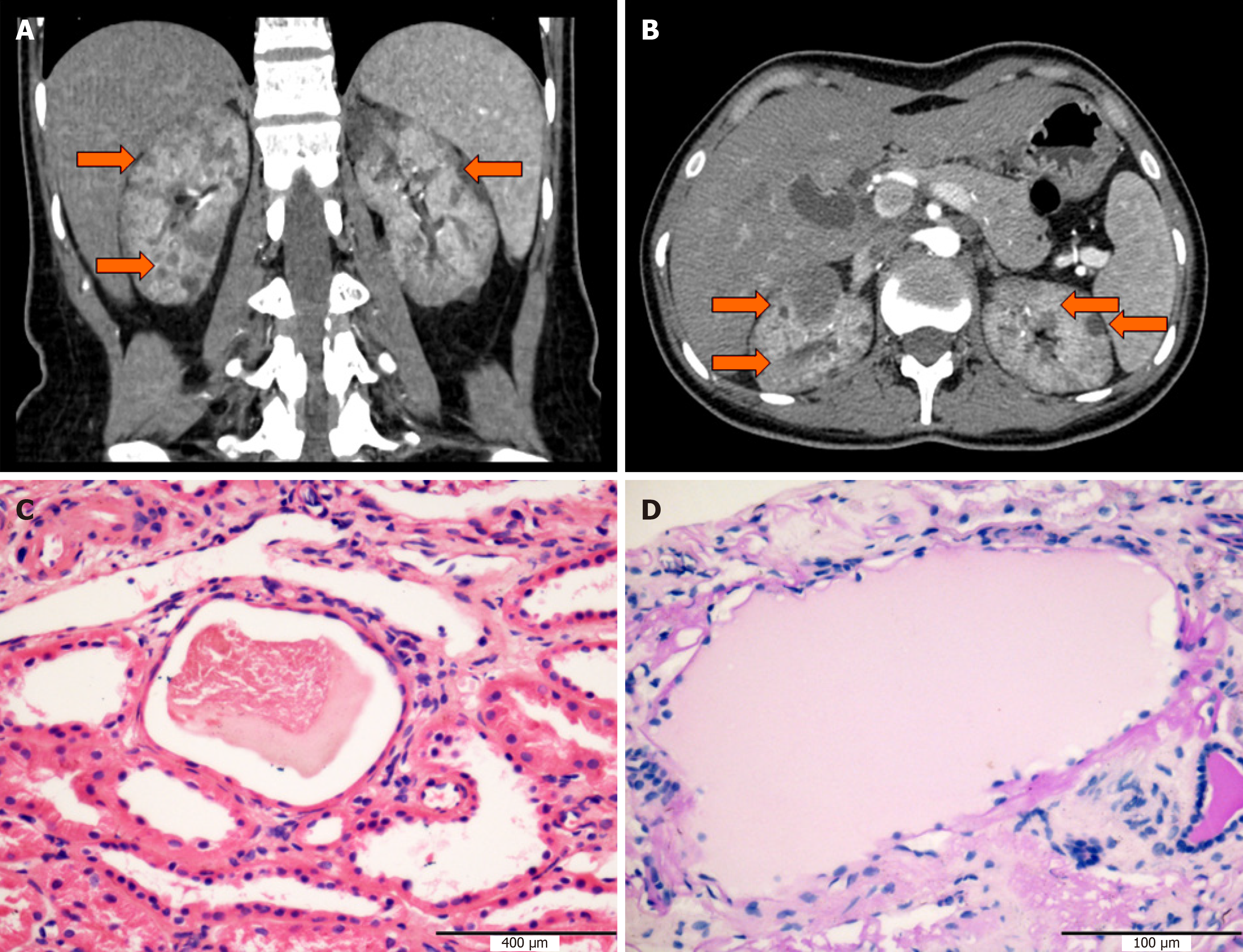
In order to confirm the previous diagnosis, abdominal ultrasound examination and computed tomography were performed. Result showed bilateral slight renal atrophy with hyperechogenicity and multiple renal cysts (Figure 1A and B). The diameter of the largest cysts in the left and right kidneys was 2.4 cm and 2.0 cm, respectively. No obvious structural anomalies were observed in other abdominal organs including the liver, spleen, pancreas, and gallbladder.
To further analyze the renal disease, histopathology study of renal biopsy was performed. A total of six glomeruli were observed, with one glomerulus having ischemic sclerosis. The volume of the ischemic glomerulus was increased, while the mesangial cells and matrix showed slight hyperplasia. The morphology of podocytes and the basements was normal. There was no obvious positive signal of Congo red staining and Masson staining. Granular degeneration of renal tubular epithelial cells with focal tubular atrophy was observed. The cystic structure with serous substances was visible in three tubular lumens (Figure 1C and D). Mild to moderate intimal and medial thickening was observed in arcuate and interlobular arteries. All the immunological staining including IgA, IgG, IgM, complement C3, C4, C1q, κ, and λ was negative. Electron microscopy showed renal interstitial fibrosis, tubular basement membrane shrinkage, matrix collagen fibrosis, and lymphatic and monocyte infiltration.
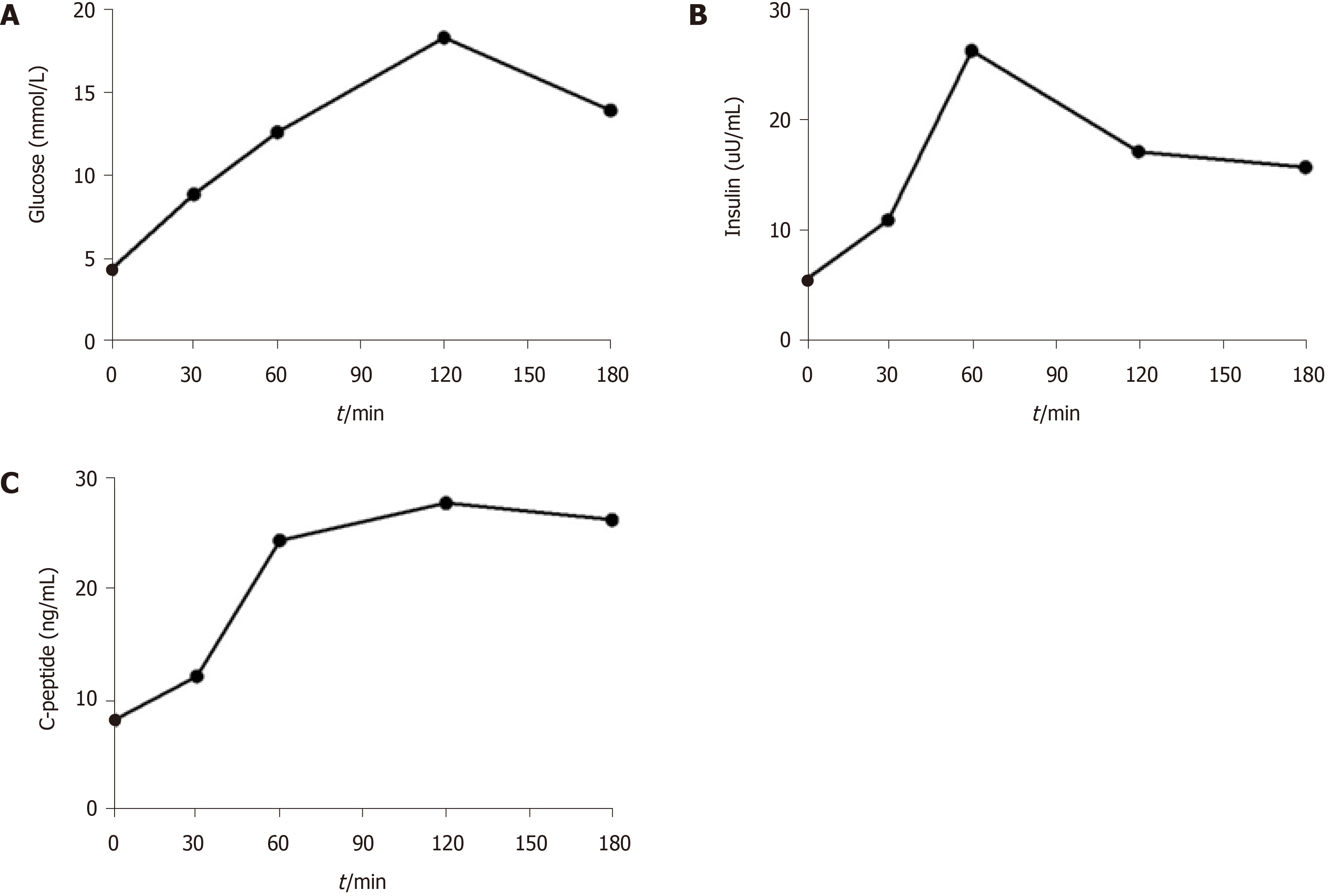
As her serum glucose level was higher than normal, we wondered whether islet function was impaired. Thus, the release of insulin and C-peptide was measured by oral glucose tolerance test. As shown in Figure 2, the concentration of serum glucose constantly increased until 2 h after oral administration of glucose, which indicated a deficiency of insulin. However, autoantibody against diabetes was negative.
Blood samples were collected from this patient and her parents for genomic DNA extraction using the CWBIO Blood Genomic DNA Mini Kit (CWBIO, Beijing, China). Whole exome sequencing (WES) was performed by Chigene (Beijing) Translational Medical Research Center Co. Ltd (Beijing, China).
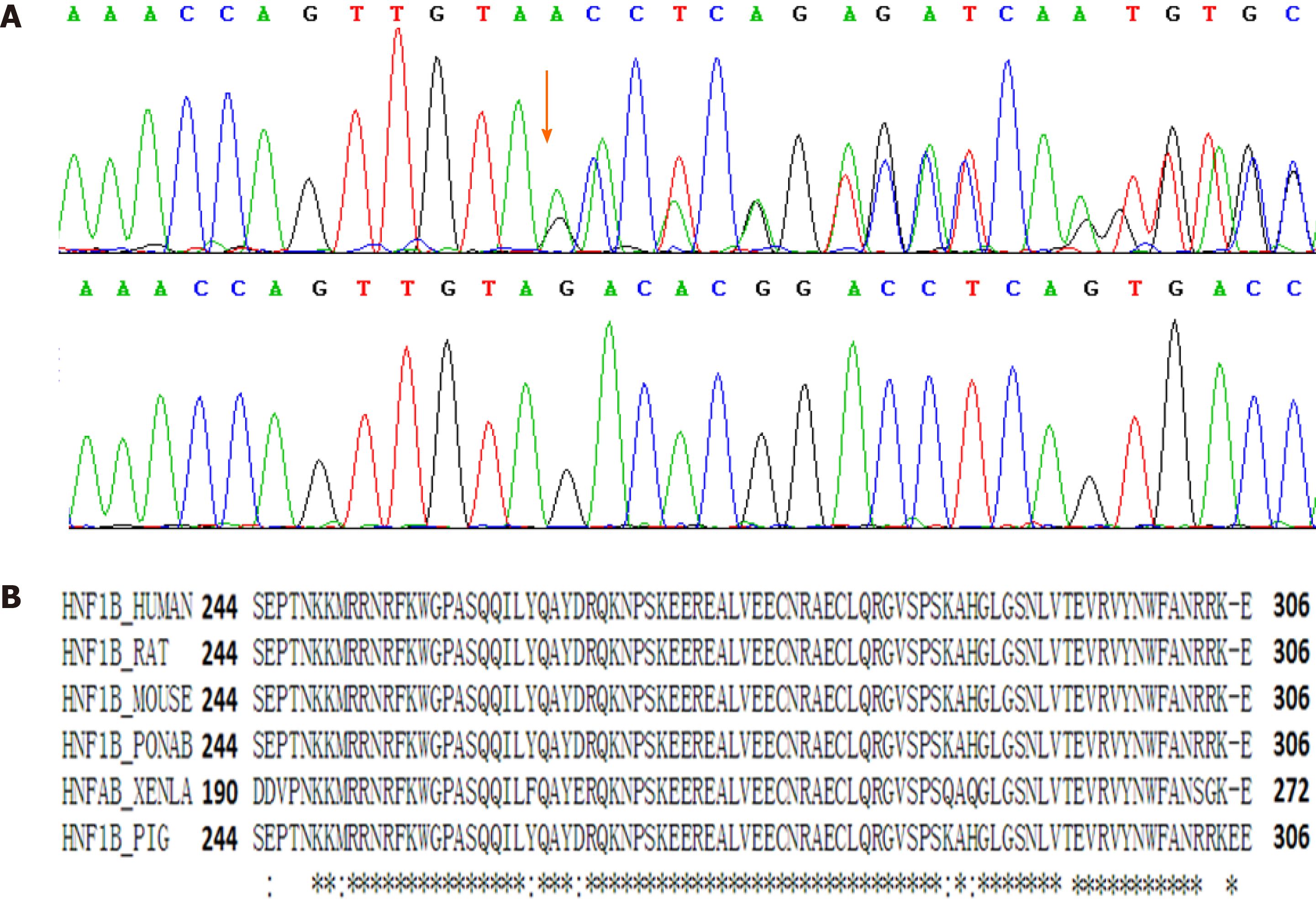
The sequence analysis revealed a novel heterozygous small deletion mutation, NM_000458: c.882_888del (p.V294fs), in exon 4 of the HNF1B gene. Sanger sequencing was performed to validate the identified variation (Figure 3A). The mutation was excluded from the Single Nucleotide Polymorphism database and the Human Genetic Variation Database. This de novo mutation was not found in her parents. The mutation was located in the DNA-binding domain of HNF1B, which contained about 60 amino acid residues and was highly conserved among species (Figure 3B). This variant can be classified as “pathogenic” (PS2+, PM2+, PM4) according to the American College of Medical Genetics and Genomics (ACMG) standards and guidelines[9]. After identifying the mutation in HNF1B gene, we also calculated the HNF1B score based on those items including antenatal discovery, family history, and the involved organs including the kidney, pancreas, liver, and genital tract. This tool provides a more rational approach to select patients for HNF1B screening[10]. The HNF1B score of this patient was 8, just the same as the optimal cutoff threshold for the negative predictive value.
The final diagnosis of the presented case was RCAD syndrome.
The patient received metformin to control blood sugar, and renin-angiotensin-aldosterone system blockade to delay the progression of kidney disease. She was recommended to have a high-quality protein, low-salt (< 6 g/d) diabetes diet.
After 2 year of follow-up, the patient's blood glucose and renal function were relatively stable.
We report a novel small deletion mutation in exon 4 of the HNF1B gene in a RCAD patient from a Chinese family. As RCAD syndrome is an autosomal dominant disorder, the de novo mutation might occur at the somatic level, as the genotypes of parents are normal.
HNF1B is a developmentally regulated transcription factor required for tissue-specific gene expression in mouse epithelial cells[11]. It activates or represses transcription of target genes through binding of its specific domain. A previous study showed that mice with renal-specific inactivation of HNF1B developed polycystic kidney disease, and renal cyst formation was accompanied by a drastic defect in the transcriptional activation of UMOD, Pkhd1, and Pkd2 genes[12]. Recently, cell experiment also showed that ablation of HNF1B in proximal tubule cells led to a shift from oxidative phosphorylation to glycolysis[13]. Such evidence suggests that the HNF1B gene is vital for mouse renal development and function. In humans, mutations of the HNF1B gene are found in patients with inherited and sporadic malformations of the kidney and genitourinary tract. Recently, the mutant spectrum of HNF1B gene was analyzed in a Japanese population and their finding of genotype-phenotype correlation was interesting[8]. The clinical characteristics of HNF1Bassociated syndrome include renal phenotype, diabetes, pancreatic phenotype, and reproductive organ phenotype[8]. A study in 2018 found that kidney anomalies including bilateral cystic dysplasia and bilateral hyperechogenic kidneys were the most frequent[14]. In addition, more than one-third of the patients with HNF1B mutations developed moderate to severe chronic kidney disease. Our patient was diagnosed as having bilateral multiple renal cysts at the age of 22 years. Her renal manifestation included multiple renal cysts and hyperechogenicity. Histopathology study of renal biopsy confirmed the kidney anomalies in glomeruli and tubules. Analysis of HNF1B score suggested that her molecular basis of the disease might be associated with mutation of HNF1B gene.
The second frequent clinical characteristic of HNF1B-associated syndrome is diabetes. More than half of the patients with HNF1B mutations presented diabetes or prediabetes. Although no obvious structural anomalies were observed in other abdominal organs including the liver, spleen, pancreas, and gallbladder, a functional test showed that the patient’s islet function was impaired, which suggested the existence of diabetes. This is also in line with the fact that RCAD patients often present with renal phenotype preceding the diabetes, as diabetes in these cases usually appears in the second and third decades of life[15]. Another characteristic of HNF1B-associated syndrome is hypomagnesaemia. However, this was absent in our patient. Comparing all the clinical characteristics of our patient with those in literature, we speculated that this patient was just at the early stage of RCAD syndrome.
Currently, more than 400 mutations of HNF1B gene have been recorded in the ClinVar database. Among the records in the ClinVar, 303 records of mutation are classified as “pathogenic” and “likely pathogenic”. As this gene encodes a transcription factor highly conserved among species, small indels and point mutations of HNF1B gene are found to be pathogenic. Nevertheless, copy number variations (CNVs) including microdeletion and microduplication of HNF1B gene can also be pathogenic. Fu et al[16] found that CNVs of HNF1B region were revealed by chromosome microarray analysis testing in fetal multicystic dysplastic kidneys. The encoded protein has three domains. The N-terminal domain (8-173) contains a dimerization sequence and an acidic region that may mediate the formation of HNF-1B homodimers or heterodimers with the related protein HNF-1α[17]. The homeodomain (240-305) is the DNA-binding domain involved in the transcriptional regulation of key eukaryotic developmental processes, and its crystal structure has already been determined[18]. The C-terminal domain (314-550) is responsible for the activation of transcription. The homeodomain is the most conservative region (Figure 3). The 7-bp deletion mutation of HNF1B gene leads to a frame shift mutation and the mutated protein lacks the C-terminal domain. Based on the gene function and the genotype-phenotype correlation in this family, the mutation was classified as “pathogenic” according to the ACMG guidelines. Moreover, point mutations of this motif are also classified as “pathogenic” in the ClinVar database.
Here, we summarize the previously reported HNF1B mutations in the Chinese population (Table 2). Most studies of the mutations in HNF1B gene in the Chinese population are concerned with MODY5. For example, Wang et al[19] found a substitution of S36F in an MODY family. Amazingly, the phenotype of mutation carriers in this family was different: One had early onset diabetes, renal function impairment, and renal cyst, while the other had impaired glucose tolerance only. Similarly, a case report by Wang et al[20] showed that a missense mutation (c.1007A>G, p.H336R) in the HNF1B gene was found in a Chinese family of MODY with diabetic kidney disease. However, in these cases, the diabetes phenotype occurred earlier than renal phenotype. These findings suggest that the phenotype of HNF1B-related disorders might be relative to ethnic region.
| S/N | Sex | Age at diagnosis | Amino acid change | Clinical symptoms | Ref. |
| 1 | Male | 37 | p.ES36F | Diabetes mellitus, mild renal dysfunction; one cyst in the left kidney; impaired concentration function of the renal tubules | Wang et al[19] |
| 2 | Female | 30 | p.H336R | Diabetes mellitus; albuminuria; diabetic nephropthy, peripheral neuropathy, and diabetic retinopathy; lipid metabolism disorder; kidney stones | Wang et al[20] |
| 3 | Male | 39 | p.D221V | Diabetes mellitus and mild renal dysfunction; microalbuminuria; epididymal cysts and bilateral hydrocele testis | Wang et al[21] |
| 4 | Female | 11 | p.R165H | Low birth weight, diabetes mellitus, and microalbuminuria; renal structural abnormalities; pancreatic hypoplasia (lack of pancreatic body and tail); liver cysts | Wang et al[21] |
| 5 | Female | 65 | p.E260D | Diabetes mellitus; microalbuminuria; renal structural abnormalities; pancreatic hypoplasia (lack of pancreatic body and tail); liver cysts | Wang et al[21] |
| 6 | Female | 11 | p.G239E | Low birth weight and diabetes mellitus; elevated levels of serum creatinine, urea, liver enzymes, and uric acid; hyperosmolality; atrophy of the pancreas; agenesis of the left kidney combined with hydronephrosis, and multiple cysts in the right kidney | Luo et al[22] |
A novel deletion mutation of HNF1B gene (NM_000458: c.882_888del, p.V294fs) was identified in a Chinese family with RCAD syndrome by using WES and Sanger sequencing. Considering the gene function and the genotype-phenotype correlation, mutation location, and its conservativeness, this mutation is considered to play a pathogenic role in the development of RCAD syndrome.
| 1. | Bohn S, Thomas H, Turan G, Ellard S, Bingham C, Hattersley AT, Ryffel GU. Distinct molecular and morphogenetic properties of mutations in the human HNF1beta gene that lead to defective kidney development. J Am Soc Nephrol. 2003;14:2033-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Peixoto-Barbosa R, Reis AF, Giuffrida FMA. Update on clinical screening of maturity-onset diabetes of the young (MODY). Diabetol Metab Syndr. 2020;12:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Ferrè S, Igarashi P. New insights into the role of HNF-1β in kidney (patho)physiology. Pediatr Nephrol. 2019;34:1325-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Igarashi P, Shao X, McNally BT, Hiesberger T. Roles of HNF-1beta in kidney development and congenital cystic diseases. Kidney Int. 2005;68:1944-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Bockenhauer D, Jaureguiberry G. HNF1B-associated clinical phenotypes: the kidney and beyond. Pediatr Nephrol. 2016;31:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Wang CC, Mao TL, Yang WC, Jeng YM. Underexpression of hepatocyte nuclear factor-1β in chromophobe renal cell carcinoma. Histopathology. 2013;62:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Decramer S, Parant O, Beaufils S, Clauin S, Guillou C, Kessler S, Aziza J, Bandin F, Schanstra JP, Bellanné-Chantelot C. Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol. 2007;18:923-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Nagano C, Morisada N, Nozu K, Kamei K, Tanaka R, Kanda S, Shiona S, Araki Y, Ohara S, Matsumura C, Kasahara K, Mori Y, Seo A, Miura K, Washiyama M, Sugimoto K, Harada R, Tazoe S, Kourakata H, Enseki M, Aotani D, Yamada T, Sakakibara N, Yamamura T, Minamikawa S, Ishikura K, Ito S, Hattori M, Iijima K. Clinical characteristics of HNF1B-related disorders in a Japanese population. Clin Exp Nephrol. 2019;23:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26738] [Cited by in RCA: 24689] [Article Influence: 2244.5] [Reference Citation Analysis (0)] |
| 10. | Faguer S, Chassaing N, Bandin F, Prouheze C, Garnier A, Casemayou A, Huart A, Schanstra JP, Calvas P, Decramer S, Chauveau D. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Ott MO, Rey-Campos J, Cereghini S, Yaniv M. vHNF1 is expressed in epithelial cells of distinct embryonic origin during development and precedes HNF1 expression. Mech Dev. 1991;36:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23:1657-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 259] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Casemayou A, Fournel A, Bagattin A, Schanstra J, Belliere J, Decramer S, Marsal D, Gillet M, Chassaing N, Huart A, Pontoglio M, Knauf C, Bascands JL, Chauveau D, Faguer S. Hepatocyte Nuclear Factor-1β Controls Mitochondrial Respiration in Renal Tubular Cells. J Am Soc Nephrol. 2017;28:3205-3217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Madariaga L, García-Castaño A, Ariceta G, Martínez-Salazar R, Aguayo A, Castaño L; Spanish group for the study of HNF1B mutations. Variable phenotype in HNF1B mutations: extrarenal manifestations distinguish affected individuals from the population with congenital anomalies of the kidney and urinary tract. Clin Kidney J. 2019;12:373-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Chen YZ, Gao Q, Zhao XZ, Chen YZ, Bennett CL, Xiong XS, Mei CL, Shi YQ, Chen XM. Systematic review of TCF2 anomalies in renal cysts and diabetes syndrome/maturity onset diabetes of the young type 5. Chin Med J (Engl). 2010;123:3326-3333. [PubMed] |
| 16. | Fu F, Chen F, Li R, Zhang Y, Pan M, Li D, Liao C. Prenatal diagnosis of fetal multicystic dysplastic kidney via high-resolution whole-genome array. Nephrol Dial Transplant. 2016;31:1693-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Saji T, Kikuchi R, Kusuhara H, Kim I, Gonzalez FJ, Sugiyama Y. Transcriptional regulation of human and mouse organic anion transporter 1 by hepatocyte nuclear factor 1 alpha/beta. J Pharmacol Exp Ther. 2008;324:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Lu P, Rha GB, Chi YI. Structural basis of disease-causing mutations in hepatocyte nuclear factor 1beta. Biochemistry. 2007;46:12071-12080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Wang C, Fang Q, Zhang R, Lin X, Xiang K. Scanning for MODY5 gene mutations in Chinese early onset or multiple affected diabetes pedigrees. Acta Diabetol. 2004;41:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Zhao Y, Zhang J, Yang Y, Liu F. A case of a novel mutation in HNF1β-related maturity-onset diabetes of the young type 5 with diabetic kidney disease complication in a Chinese family. J Diabetes Complications. 2017;31:1243-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Wang C, Zhang R, Lu J, Jiang F, Hu C, Zhou J, Liu F, Zhang F, Qin W, Li M, Ma X, Yan J, Bao Y, Xiang K, Jia W. Phenotypic heterogeneity in Chinese patients with hepatocyte nuclear factor-1β mutations. Diabetes Res Clin Pract. 2012;95:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Luo Y, Dai Z, Li L, Shan X, Wu C. Hepatocyte nuclear factor 1β maturity-onset diabetes of the young in a Chinese child presenting with hyperglycemic hyperosmolar state. Acta Diabetol. 2017;54:969-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sultana N S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Li JH