Published online Jul 26, 2020. doi: 10.12998/wjcc.v8.i14.3057
Peer-review started: April 1, 2020
First decision: April 24, 2020
Revised: April 30, 2020
Accepted: July 14, 2020
Article in press: July 14, 2020
Published online: July 26, 2020
Processing time: 113 Days and 4.2 Hours
Compared with colorectal adenocarcinoma, basaloid squamous cell carcinomas (BSCCs) arising in the colorectum are rare and have very poor prognosis. To date, only nine cases have been reported. Most BSCCs are extensively involved in metastasis to the lymph node, liver, and lung at diagnosis. Despite many clinicians attempting to effectively treat BSCCs, therapeutic consensus has not been established due to lack of information.
A 58-year-old woman presented with abdominal pain, diarrhea, fever, and hematochezia. She was referred from a department of gynecology and was diagnosed with a suspicious leiomyosarcoma of the rectum or a pedunculated myoma of the uterus. An exophytic growing mass at the right lateral wall of the rectum with an internal cystic portion and hemorrhage was observed on magnetic resonance imaging. The patient underwent low anterior resection and total hysterectomy with bilateral salphingo-oophorectomy. Histopathological findings revealed a cellular mass with a solid growth pattern and few glandular structures, many foci of intratumoral necrosis, and a palisading pattern. The pathologist diagnosed tumor as a BSCC, and the patient received chemotherapy with fluorouracil/leucovorin without radiotherapy. The patient is currently alive 8 years after the surgery with no manifestations of metastatic colon cancer.
Our case suggest that curative resection and chemotherapy play important roles in improving survival, and radiotherapy may be an option to avoid radiation-associated enteritis.
Core tip: Basaloid squamous cell carcinoma in the colorectum is extremely rare and has poor prognosis. Here, we present a rare case of the successful treatment of basaloid squamous cell carcinoma in the rectosigmoid colon. The patient with this rare tumor has the longest survival, and thus, curative resection and chemotherapy may play important roles in improving survival. To avoid radiation-associated enteritis, postoperative radiotherapy may be an option.
- Citation: Lee TG, Yoon SM, Kim MJ. Successful treatment of basaloid squamous cell carcinoma in the rectosigmoid colon: A case report and review of literature. World J Clin Cases 2020; 8(14): 3057-3063
- URL: https://www.wjgnet.com/2307-8960/full/v8/i14/3057.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i14.3057
Most basaloid squamous cell carcinomas (BSCCs) are located in the transitional cloacogenic zone of the anus (cloacogenic carcinoma)[1], the incidence of which varies between 2% and 3% among anorectal neoplasms[2]. BSCC occurring in the colorectum is very rare, and only nine cases of primary BSCC in the colorectum have been reported. Histopathological analysis is focused on its origin and differential diagnosis based on microscopic findings and immunohistochemical characterization. BSCCs are often misdiagnosed because they have several histological patterns such as mucoepidermoid carcinoma-like and adenoid cystic carcinoma-like patterns[3]. Immunohistochemistry can be performed for differential diagnosis of neuroendocrine tumors, gastrointestinal stromal tumor, and poorly differentiated anal and metastatic carcinomas from the lungs and genitourinary cancers[4]. To date, most BSCCs in the colorectum have been reported to arise from a totipotent basal cell in the colonic mucosa because the cloaca remnant and mucosal dysplasia or metaplasia has not been found[5].
Although some case reports have confirmed survival, most cases were associated with histopathological and immunological diagnoses. Survival was not sufficiently long as judged based on the efficacy of the treatment; hence, a therapeutic consensus regarding BSCC in the colorectum has not been reached. Here, we report a case of BSCC in the rectosigmoid colon that was successfully treated following curative resection and a postoperative chemotherapy with 5-fluorouracil (5-FU) and leucovorin (LV) regimen without radiotherapy.
A 58-year-old woman presented with lower abdominal pain from the deep pelvis and frequent failure of flatulence for 4 mo.
She was referred from a department of gynecology and diagnosed with a suspicious leiomyosarcoma of the rectum or a pedunculated myoma of the uterus after colonoscopy and abdominopelvic computed tomography (CT) in a previous hospital. She visited the outpatient clinic after 10 d and was transferred to the emergency center because of severe lower left abdominal pain on 2 d before her scheduled admission. She had nausea, vomiting, and non-whirling dizziness. She was diagnosed with colonic cancer perforation with an abscess and was admitted for antibiotic treatment. We ensured that the acute septic condition was stabilized, and the patient underwent operation.
She had no co-morbid disease.
On digital rectal examination, a circumferential fungating mass with a central ulceration was palpated 8 cm from the anal verge, and it was fixed in the pelvic cavity. In the emergency center, the patient’s body temperature was 38.9 °C. The systolic and diastolic blood pressures were 110 mmHg and 70 mmHg respectively, pulse rate was 78 beats per minute, and respiratory rate was 20 breaths per minute.
Laboratory results were as follows: White blood cell count (23900/µL; 93.9% of neutrophil), hemoglobin (11.6 g/dL), platelet count (416000/µL), C-reactive protein (16.14 mg/dL), lipase (9 IU/L), albumin (3.2 g/dL), alkaline phosphatase (265 IU/L), sodium (132 mEq/L), chloride (93 mEq/L), and prothrombin time percentage (73.2%).
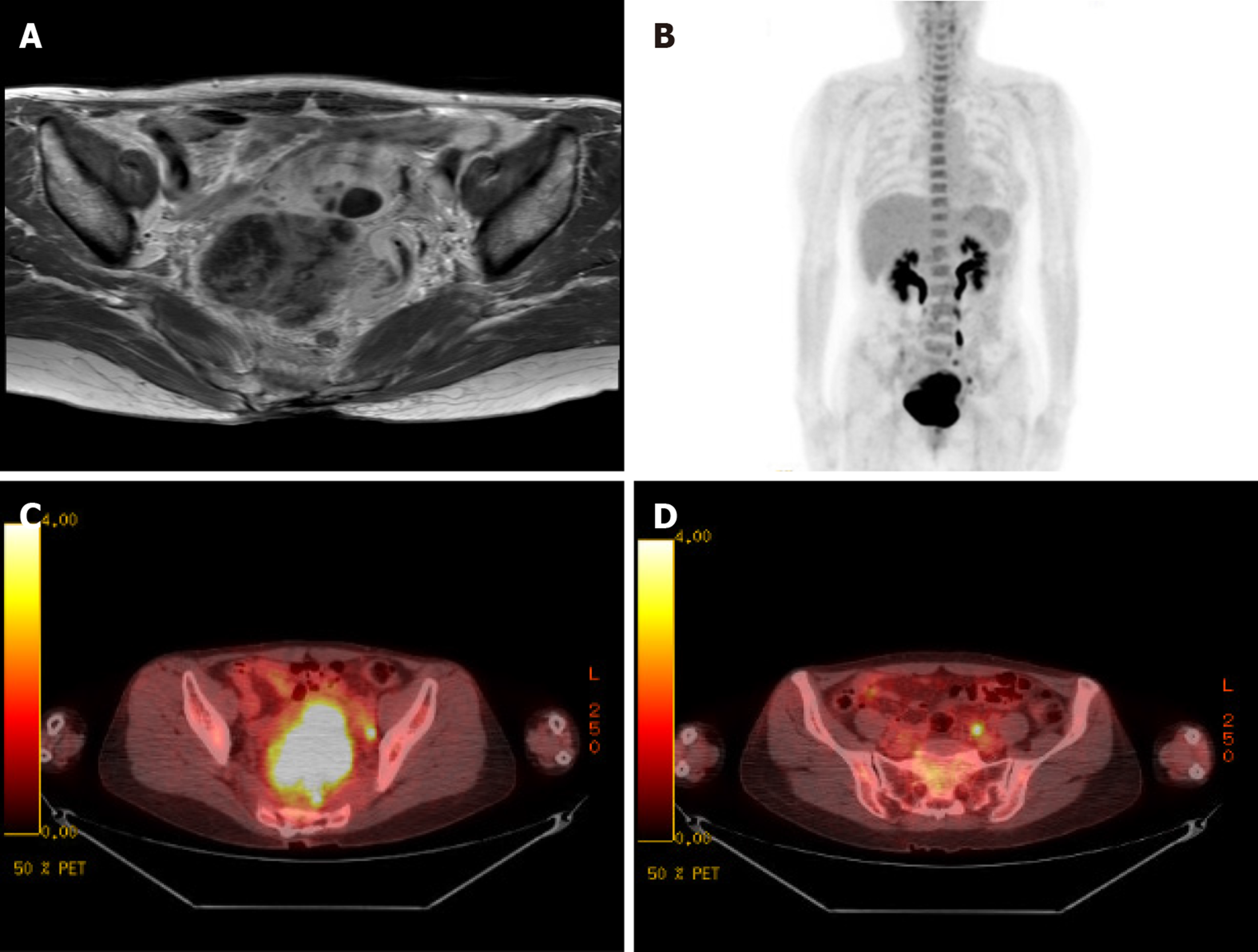
Chest X-ray showed a nodule in the right upper lung field. Besides this, there was no active lesion in the lungs. Colonoscopy revealed a huge submucosal mass-like lesion with focal ulceration located 8 to 15 cm from the anal verge. Abdominopelvic CT showed a poorly marginated mass measuring 7.4 cm × 6.3 cm × 5.5 cm in size located between the rectum and uterus and separated from the uterus by fat tissues. The mass had focal non-enhancing lesions without lymph node enlargement. To rule out the origin of the mass, the patient underwent pelvic magnetic resonance imaging (MRI) before the operation. MRI revealed an exophytic growing mass with a maximum diameter of 7.2 cm at the right lateral wall of the rectum with an internal cystic portion and hemorrhage, multiple lymph node enlargement at the iliac bifurcations, obturator, and presacral areas, and a 2.2-cm-sized multiseptated cystic mass in the fundus of uterus (Figure 1A). Positron emission tomography-CT revealed a high possibility of a malignant tissue with a hypermetabolic mass (SUVmax = 15.1) and cystic changes in the rectum, and this lesion was considered to directly invade the adjacent uterus. Multifocal FDG uptake (SUVmax = 4.0) was observed in the left common iliac, perirectal area, and presacral area (Figure 1B).
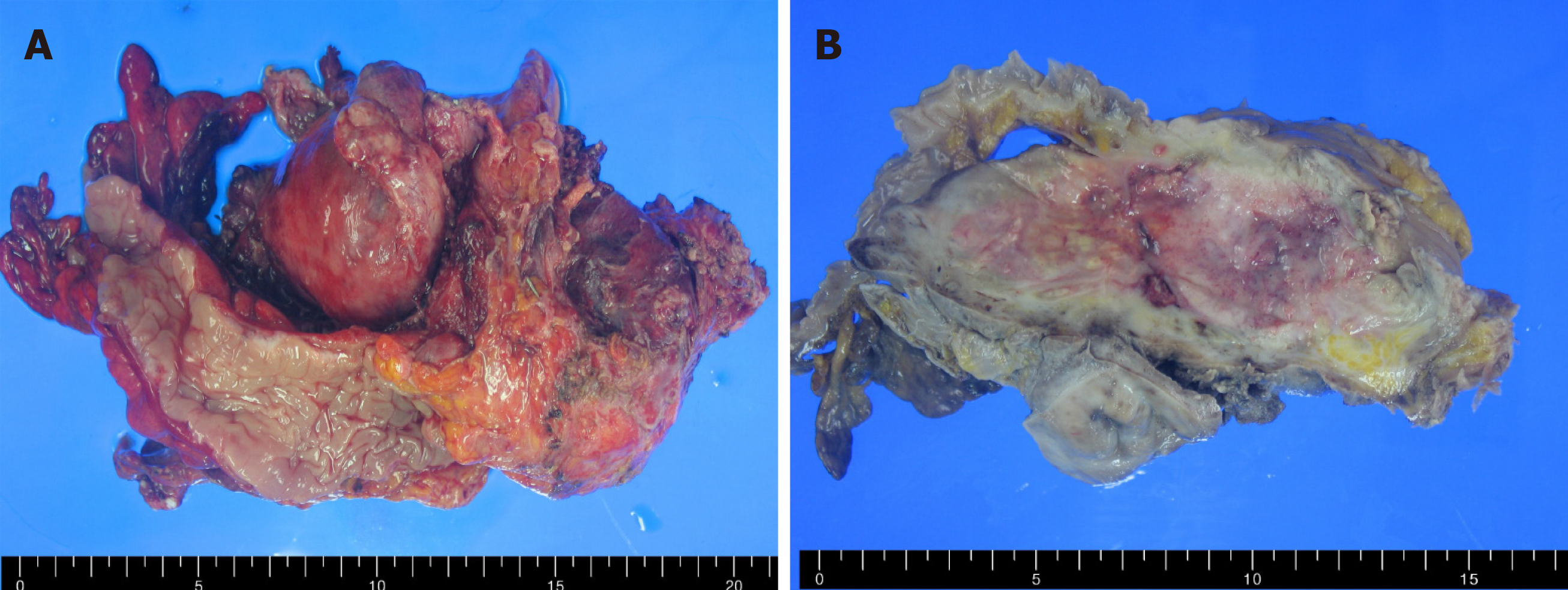
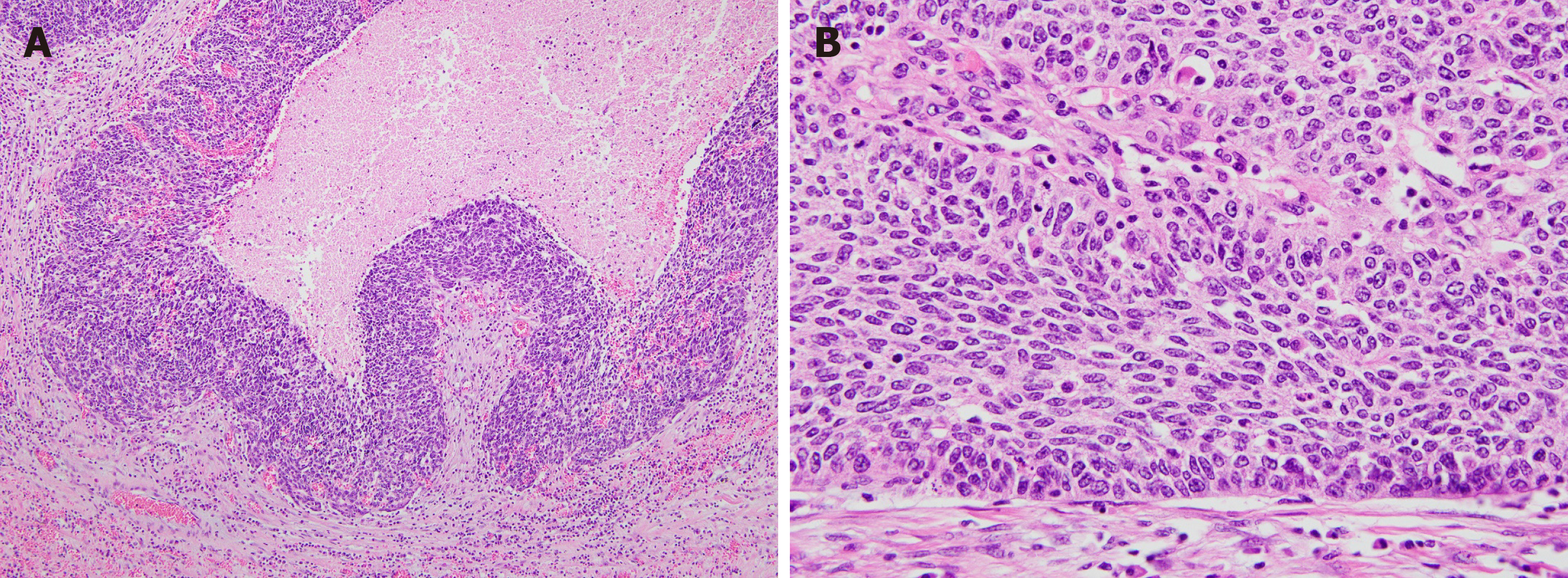
The tumor was a polypoid mass measuring 10 cm × 4 cm × 4 cm in size, and it invaded left ovary and salpinx via the serosal membrane (Figure 2A and B). No lymph node metastasis, lymphovascular invasion, or perineural invasion was observed. Microscopically, the tumor was a cellular mass that had a solid growth pattern with few glandular structures and many foci of intratumoral necrosis. Tumor cells had elongated nuclei and scant cytoplasm. Mitotic figures, including atypical ones, were also frequently observed. At the boundary between the tumor and normal tissue, tumor cells were arranged in a palisading pattern, resembling basal cell carcinomas of the skin (Figure 3A and B). Immunohistochemical examinations revealed that the tumor cells were focally positive for cytokeratin (CK) 7, p63, and Pan-CK and were negative for CK20; neuroendocrine markers, such as synaptophysin and CD56; and mesenchymal cell markers, including CD34, CD117, desmin, S100 protein, and α-smooth muscle actin. Additionally, the tumor cells were negative for vimentin, WT-1, calretinin, CD99, TTF-1, and CDX2.
The final diagnosis of the presented case was BSCC in the rectosigmoid colon.
The patient underwent low anterior resection, lateral lymph node dissection, total hysterectomy with bilateral salphingo-oophorectomy, and diverting ileostomy on May 2, 2012. Adjuvant chemotherapy comprising 5-FU and LV was initiated 3 wk after the curative resection. Postoperative pelvic radiotherapy was not performed to avoid radiation exposure to the small bowel because the tumor was located above the peritoneal reflexion.
The patient is currently alive 8 years after the surgery and has no evidence of recurrence of a metastatic colon cancer.
BSCC is a rare subtype of squamous cell carcinoma (SCC), which often occurs in the skin, uterus, bladder, anus, and upper aerodigestive tract, such as the tongue base, hypopharynx, sinus, tonsil, and larynx[6]. BSCC is more common in the anus and esophagus than in other parts of the digestive tract. BSCC of the anus is known as cloacogenic carcinoma because it originates from a persistent remnant of the cloacogenic membrane[5]. Most BSCCs occur in the head and neck, and only nine cases of BSCCs outside the anal canal have been reported since Strate et al[7] first published their report in 1977[5,7-14]. Seven of the nine cases were located in the rectum and sigmoid colon. One case reported BSCC in the descending colon and splenic flexure (Table 1).
| Ref. | Age/gender | Tumor location | Tumor size, cm | Operation methods | Depth of invasion | Lymph node metastasis | Systemic metastasis | Treatment after surgery | Survival, mo | Immune positive | Immune negative |
| Strate et al[7], 1977 | 59/F | Sigmoid | 7 × 1.5 | palliating colectomy, colostomy | Subserosa | Yes | Liver | NA | 0 | PTH, ACTH | NA |
| Hall-Craggs et al[8], 1982 | 69/F | Sigmoid | 4 × 2 | excised colon | Serosa | No | Liver | NA | NA | NA | NA |
| Indinnimeo et al[9], 1998 | 61/F | Rectum | 6 × 5 × 7 | APR | Serosa | No | No | Radiotherapy Chemotherapy (cisplatin plus 5-FU) | 19 | ki67, CD31 | NA |
| Newell et al[5], 2001 | 54/M | Splenic flexure | 10 | palliative resection | Serosa | Yes | Liver | NA | 0.3 | Low/high-molecular weight keratin, CK 5/6, EMA, NSE | CK 7, CK 20, CEA, vimentin, desmin, SMA, chromogranin, synaptophysin, serotonin, PTH, ACTH, calcitonin, gastrin, glucagon, VIP, PP, CD34, somatostatin |
| Garcia-higuera et al[12], 2002 | 67/M | Recto-sigmoid | 3.5 × 3.2 | Anterior resection | Serosa | Yes | Liver, lung | Radiotherapy Chemotherapy (cisplatin plus 5-FU) | 6 | NA | NA |
| Jaswal et al[13], 2002 | 24/F | Descending colon | 3 × 2.5 | Left hemicolectomy | Serosa | Yes | Liver, para-aortic and iliac lymph nodes | NA | 3 | NA | NA |
| Akbulut et al[10], 2009 | 23/M | Recto-sigmoid | 8 × 8.5 | Hartmann`s operation | Serosa | NA | NA | Radiotherapy Chemotherapy (irinotecan) | 5 | High-molecular weight keratin, p63 | NA |
| Ha et al[14], 2013 | 70/M | Recto-sigmoid | 6.0 × 7.0 | Low anterior resection | Serosa | NA | Liver, lung | NA | Loss the follow up | NA | NA |
| Gurzu et al[11], 2014 | 61/F | Sigmoid | 3.5 × 2.5 | Anterior resection | Subserosa | No | Liver | Chemotherapy (FOLFOX plus bevacizumab) | 4 | Pan-CK, CK 5/6, EMA, NSE, bcl-2, VEGF, CD105, p53 | CK 7, CK 20, CEA, chromogranin, p63, c-KIT, maspin |
| Our case | 58/F | Recto-sigmoid | 10 × 4 × 4 | Low anterior resection, PLND, diverting ileostomy, TAH with BSO | Serosa | No | No | Chemotherapy (Mitomycin C plus 5-FU) | 96 | CK7, p63, Pan-CK | CK20, CD34. CD117, Vimentin, CD56, WT-1, Calretinin, SMA, CD99, Synaptophysin, TTF-1, CDX2, desmin. S-100 protein |
Distinguishing BSCC from other poorly differentiated carcinoma is difficult based on histopathological morphology because BSCC has several histological patterns such as mucoepidermoid and adenoid cystic carcinomas[3,15,16]. To improve histological differential diagnosis of BSCC, the most frequent morphologic pattern, including neoplastic cells arranged in trabeculae with a peripheral palisade, and focal squamous differentiation are useful. Previous reports revealed that although the degree of squamous differentiation was variable, all cases had histopathological characteristics of basaloid carcinoma[3,17]. In our case, the histological criteria for BSCC, such as palisading pattern, elongated nuclei, and scant cytoplasm, were observed, and immunohistochemical staining was helpful for differential diagnosis. Banks et al[15] reported that head and neck BSCCs are positive for pan-CK (AE1/AE3; approximately 80%) and S100 protein (approximately 39%) and are negative for synaptophysin, chromogranin, and muscle-specific actin[15]. A study of the differential diagnosis between BSCC and SCC showed that p63 and CK7 were often positive in BSCC. They also found that p63-positive staining in SCC was limited to peripheral and basal layers, but diffuse positivity was recorded in BSCC in the nucleus and peripheral layers. P63 stain has been used for discriminating poorly differentiated SCC from small cell carcinoma or adenocarcinoma[16]. Graham et al[3] reported that BSCC of the anus was negative for chromogranin, synaptophysin, S100 protein, Melan A, and KIT, but the tumor cells were positive for the squamous marker CK5/6. In our case, immunohistochemical staining was performed to distinguish gastrointestinal stromal tumor and poorly differentiated metastatic carcinoma from primary colon cancers and cancers of the genitourinary tract or lungs. Neuroendocrine markers, such as CD56 and synaptophysin, and gastrointestinal stromal tumor markers, including CD34 and CD117(c-KIT), were negatively stained in tumor cells. CDX2, a diagnostic marker for gastrointestinal differentiation (especially colorectal), was negative. The tumor was negative for TTF-1 and CK20 to distinguish primary colorectal cancer and metastatic carcinoma from cancers of the genitourinary or lungs and positive for CK7, p63, and pan-CK (AE1/AE3). Furthermore, P63-positive staining located nucleus. In our case, experienced pathologists confirmed diagnosis as BSCC.
Clinically, BSCC rapidly deteriorates because it is a more aggressive variant of SCC, is often presented and diagnosed at an advanced stage, and has earlier metastasis. BSCCs in the colorectum also have a poor prognosis and rapid progression based on the histological characteristics of BSCC[5,7-14]. As shown in Table 1, two of the nine patients died early after operation due to postoperative hepatic failure, coagulopathy, and renal failure[5,7]. Seven patients currently have metastasis to the lungs, liver, and para-aortic lymph nodes[5,7,8,11-14]. Patients with cancers occurring in the colon had short survival than those with same staged colorectal adenocarcinomas between 5 wk and 19 mo. The patient who did not undergo curative resection died 5 mo after receiving palliative radiotherapy combined with chemotherapy and irinotecan (3 cycles)[10]. Indinnimeo et al[9] reported that the longest survival in case reports was 19 mo of disease-free survival, with the patient being treated with radiation and chemotherapy comprising cisplatin and 5-FU. No consensus has been reached regarding the treatment for BSCC in the colorectum. Despite different oncological characteristics between BSCC and colorectal adenocarcinoma, four of the nine patients were administered chemotherapeutic regimen for colorectal or anal cancer[9-12]. Three of the four patients underwent radiotherapy and chemotherapy with 5FU and LV combined with cisplatin or irinotecan[9,10,12]. One patient received palliative chemotherapy with FOLFOX (oxaliplatin with 5-FU and folinic acid) plus bevacizumab[11]. In our case, pathologists recommend treatment with cisplatin or etoposide based on small cell lung cancer because tumor growth rate and invasiveness are very aggressive. However, the patient received a 5-FU, mitomycin, and LV regimen because she did not recover sufficiently to tolerate recommended regimen. We did not perform radiotherapy to avoid radiation exposure. The radiation field could include a large portion of the ileum because the main mass was located above the peritoneal reflexion. The patient is currently alive 8 years post-surgery with no manifestations of metastatic colon cancer.
To the best of our knowledge, we report the longest survival period of a patient with BSCC in the colorectum. Our case findings show that curative resection may play important roles in improving survival, and radiotherapy may be an option to avoid radiation-associated enteritis. Thus, the selection of an appropriate chemotherapy regimen is important because BSCCs have poor prognosis and rapid progression.
| 1. | Serota AI, Weil M, Williams RA, Wollman JS, Wilson SE. Anal cloacogenic carcinoma: classification and clinical behavior. Arch Surg. 1981;116:456-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Schechterman L. Transitional cloacogenic carcinoma. Am J Proctol. 1960;11:212-232. [PubMed] |
| 3. | Graham RP, Arnold CA, Naini BV, Lam-Himlin DM. Basaloid Squamous Cell Carcinoma of the Anus Revisited. Am J Surg Pathol. 2016;40:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 4. | Bahrami A, Truong LD, Ro JY. Undifferentiated tumor: true identity by immunohistochemistry. Arch Pathol Lab Med. 2008;132:326-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Newell KJ, Penswick JL, Driman DK. Basaloid carcinoma of the colon arising at the splenic flexure. Histopathology. 2001;38:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Hum Pathol. 1986;17:1158-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 315] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Strate RW, Richardson JD, Bannayan GA. Basosquamous (transitional cloacogenic) carcinoma of the sigmoid colon. Cancer. 1977;40:1234-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Hall-Craggs M, Toker C. Basaloid tumor of the sigmoid colon. Hum Pathol. 1982;13:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Indinnimeo M, Cicchini C, Stazi A, Limiti MR, Ghini C. An unusual location of cloacogenic carcinoma. Int Surg. 1998;83:343-346. [PubMed] |
| 10. | Akbulut S, Cakabay B, Sezgin A, Ozmen CA. Basaloid (Cloacogenic) Carcinoma Mimicking Intraabdominal Abscess: Report of a Case and Review of the Literature. Case Rep Gastroenterol. 2009;3:248-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Gurzu S, Szentirmay Z, Bara T, Bara T, Iurcsuk O, Jung I. Molecular and immunohistochemical profile of a basaloid (cloacogenic) carcinoma of the sigmoid colon: possible predictive value for clinical outcomes. Eur J Gastroenterol Hepatol. 2014;26:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | García-higuera I, Echevarría-iturbe C, Remón-garijo ML. Carcinoma basalioide (cloacogénico) de recto-sigma : a propósito de un caso y revisión de la literatura. Rev Esp Patol. 2002;35:213-216. |
| 13. | Jaswal TS, Gupta S, Singh S, Marwah N, Marwah S, Arora B. Basaloid carcinoma of descending colon. Indian J Gastroenterol. 2002;21:159-160. [PubMed] |
| 14. | Ha TH, Jeon TJ, Park JY, Jang YH, Kim DH, Ryu MJ, Sinn DH, Oh TH. [A case of basaloid squamous cell carcinoma of rectosigmoid colon]. Korean J Gastroenterol. 2013;62:375-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Banks ER, Frierson HF, Mills SE, George E, Zarbo RJ, Swanson PE. Basaloid squamous cell carcinoma of the head and neck. A clinicopathologic and immunohistochemical study of 40 cases. Am J Surg Pathol. 1992;16:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 154] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Emanuel P, Wang B, Wu M, Burstein DE. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod Pathol. 2005;18:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 17. | Fisher ER. The basal cell nature of the so-called transitional cloacogenic carcinoma of anus as revealed by electron microscopy. Cancer. 1969;24:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership(s) in Professional Societies: The Korean Society of Gastroenterology, No. 1-08-1162.
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordonaro M S-Editor: Gong ZM L-Editor: A E-Editor: Xing YX