Published online Jul 26, 2020. doi: 10.12998/wjcc.v8.i14.3064
Peer-review started: March 19, 2020
First decision: April 22, 2020
Revised: May 1, 2020
Accepted: June 25, 2020
Article in press: June 25, 2020
Published online: July 26, 2020
Processing time: 126 Days and 21.9 Hours
Renal cell carcinomas are usually unilateral. However, they are bilateral in 2% to 4% of sporadic cases and is considerably more common in familial cases. Synchronous sporadic bilateral multiple chromophobe renal cell carcinoma (CHRCC) with different subtypes is rare.
In this case report, we describe a case of synchronous bilateral CHRCC with two histological variants, accompanied by a clear cell carcinoma and a cyst in a 50-year-old male. The patient underwent retroperitoneal laparoscopic bilateral nephron-sparing surgery and there was no serious postoperative renal dysfunction.
We report a rare case of synchronous bilateral CHRCC with two histological variants associated with a clear cell carcinoma and a cyst.
Core tip: Synchronous sporadic bilateral multiple chromophobe renal cell carcinoma (CHRCC) is rare. We present an incidentally detected case of synchronous occurrence of bilateral CHRCC. This case had two concomitant different subtypes of CHRCC in each kidney (classic type and eosinophilic type), accompanied by a clear cell carcinoma and a cyst. The patient underwent retroperitoneal laparoscopic bilateral nephron-sparing surgery and there was no serious postoperative renal dysfunction.
- Citation: Yang F, Zhao ZC, Hu AJ, Sun PF, Zhang B, Yu MC, Wang J. Synchronous sporadic bilateral multiple chromophobe renal cell carcinoma accompanied by a clear cell carcinoma and a cyst: A case report. World J Clin Cases 2020; 8(14): 3064-3073
- URL: https://www.wjgnet.com/2307-8960/full/v8/i14/3064.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i14.3064
Renal cell carcinomas (RCCs) are usually unilateral, but bilateral synchronous or metachronous have been found in 2% to 4% of reported sporadic cases. However, the incidence is higher among patients suffering from hereditary renal cancer such as Von Hippel-Lindau disease, tuberous sclerosis, and Birt-Hogg-Dube syndrome[1]. Synchronous sporadic bilateral multiple chromophobe renal cell carcinoma (CHRCC) is rare.
Surgical excision is considered the standard treatment modality for RCC. In this case report, we describe a case of bilateral simultaneous CHRCC with two histological variants, accompanied by a clear cell carcinoma and a cyst that were all treated by retroperitoneal laparoscopic partial nephrectomy.
A 50-year-old male patient was admitted to our department because of multiple renal tumors bilaterally, incidentally discovered during abdominal ultrasonography for screening purposes.
The patient had a free previous medical history.
The patient had no previous medical history.
His family history was unremarkable.
The patient’s temperature was 36.7°C, heart rate was 78 beats per minute, respiratory rate was 16 breaths per minute, blood pressure was 128/88 mmHg, and oxygen saturation in room air was 96%. Clinical physical examination revealed no abnormalities.
The results of routine blood tests, routine urine tests and urinary sediment examination, routine fecal tests and occult blood test, blood biochemistry, immune indexes, and infection indexes were in the normal range. Electrocardiogram, chest X-ray, and arterial blood gas were also normal. Preoperative blood urea nitrogen (BUN) was 4.4 mmol/L and serum creatinine value was 86 μmol/L. Preoperative glomerular filtration rate (GFR) was 36.2 mL/min in the left kidney and 37.86 mL/min in the right kidney.
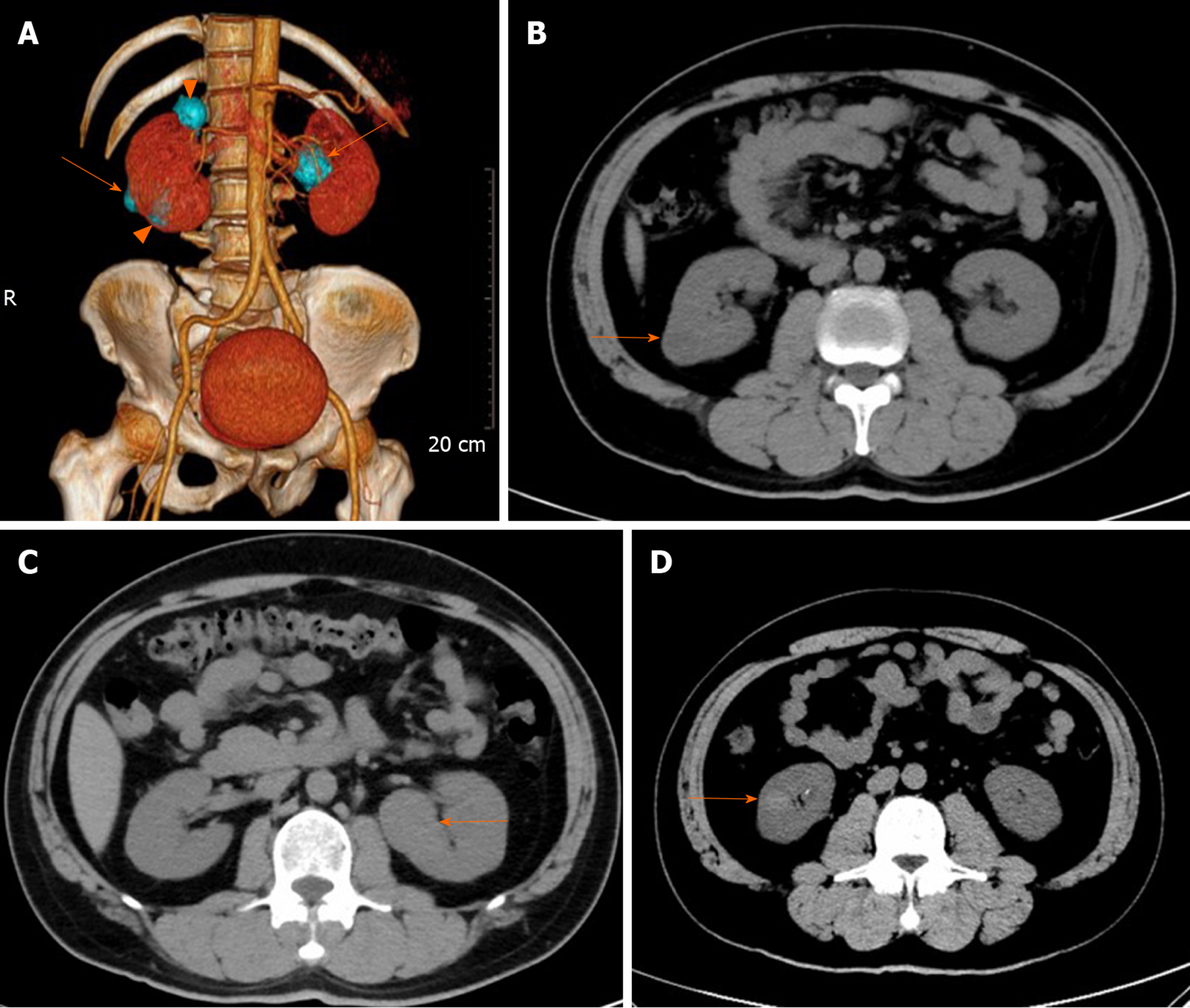
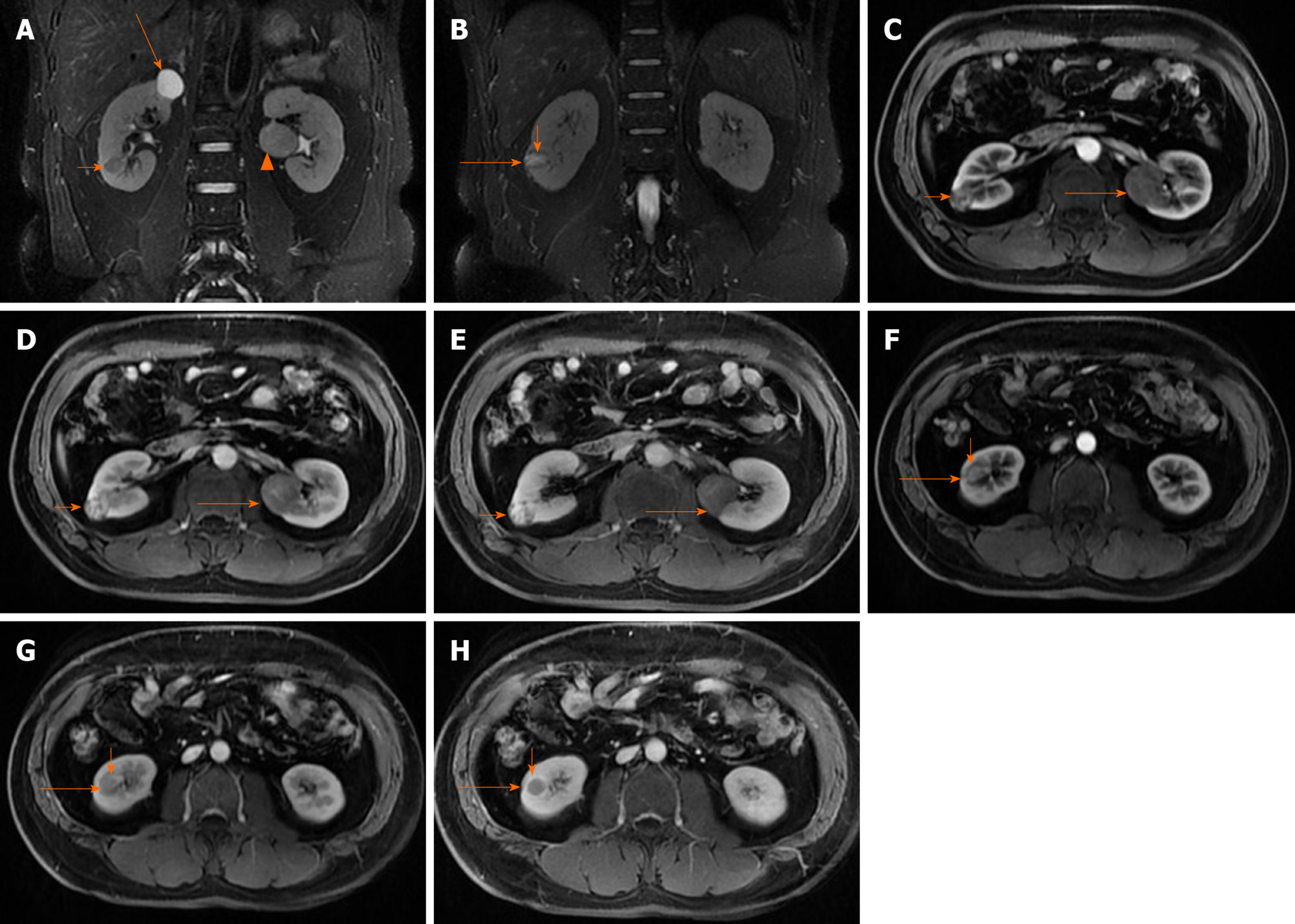
To investigate the findings of bilateral renal tumors, the patient underwent contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) examination. Contrast CT/MRI scan showed the following. (1) A 2.0 cm × 1.9 cm × 1.9 cm in size, solid mass at the lower pole of the right kidney, well-defined margins, and exophytic. MRI, T2-weighted image identifying expansile, heterogeneous signal intensity, with a distinct pseudocapsule, heterogeneous delay enhancement,and perirenal fat invasion were not identified. (2) A 1.4 cm × 1.3 cm × 1.1 cm in size, solid mass at the lower pole of the right kidney, with circumscribed enhancing lesions. MRI, T2-weighted image presented a homogeneous intense hyposignal that was considerably less intense than the renal cortex. (3) A 2.2 cm × 1.9 cm × 2.4 cm in size, cyst mass at the upper pole of the right kidney, well-defined margins, and exophytic. And (4) A 3.3 cm × 3.2 cm × 2.9 cm in size, solid mass in the middle of the left kidney, well-defined margins, exophytic, T2-weighted image showing intermediate signal intensity enhanced to the same degree as the renal cortex, slight homogeneous delayed enhancement (Figure 1 and 2). There were no abnormally enlarged retroperitoneal lymph nodes, without perinephric tissues or renal vein invasion.
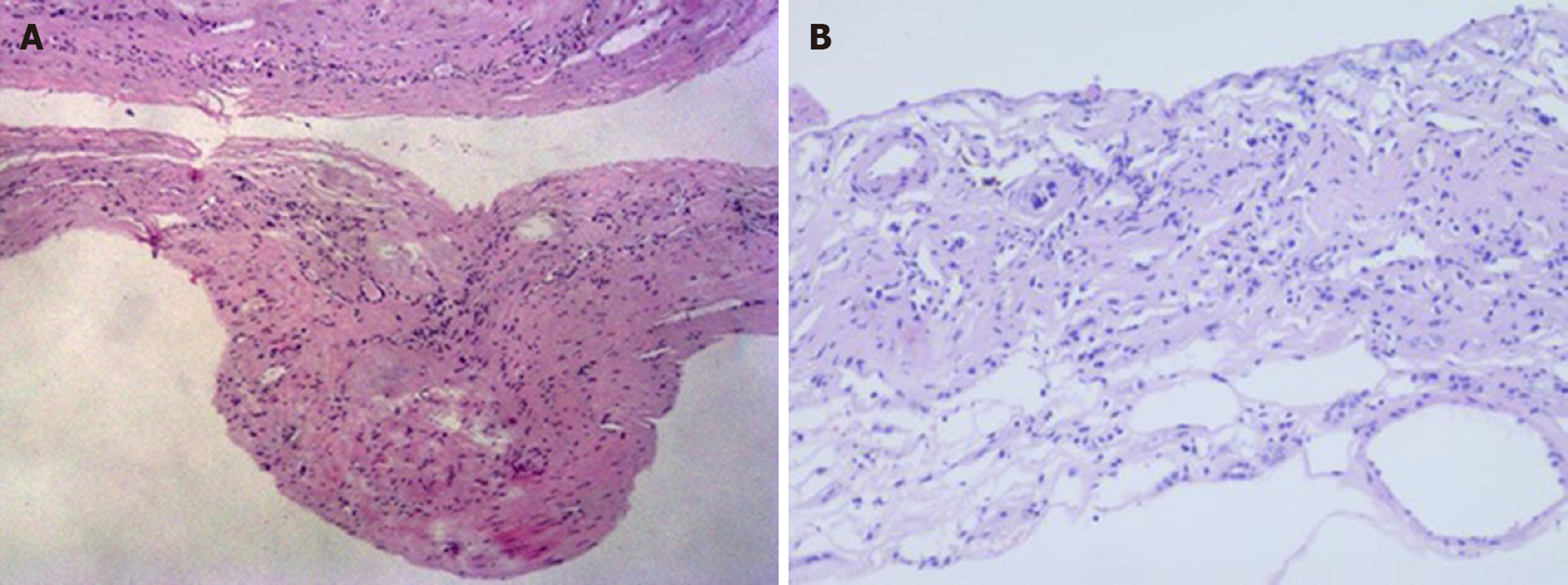
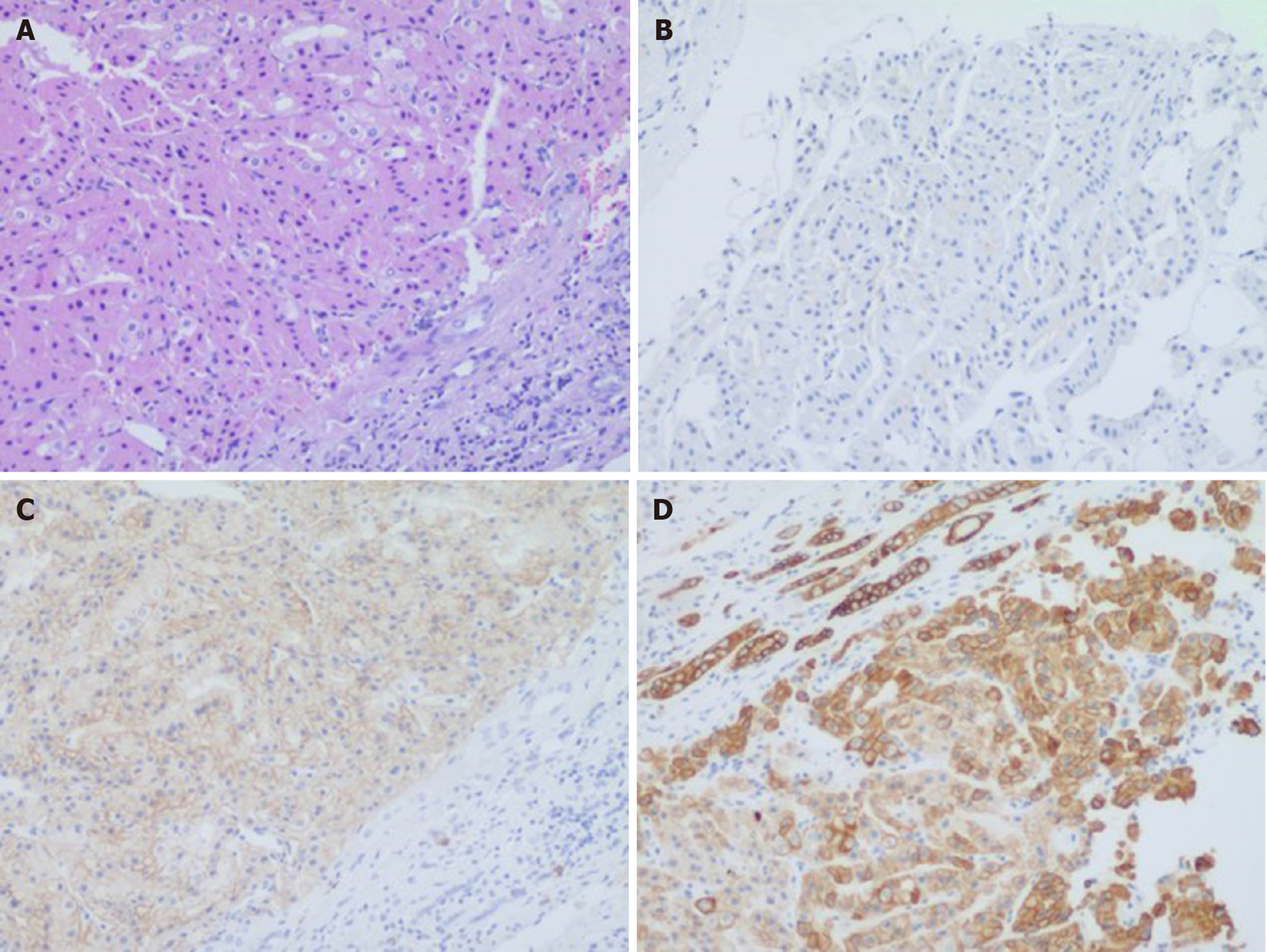
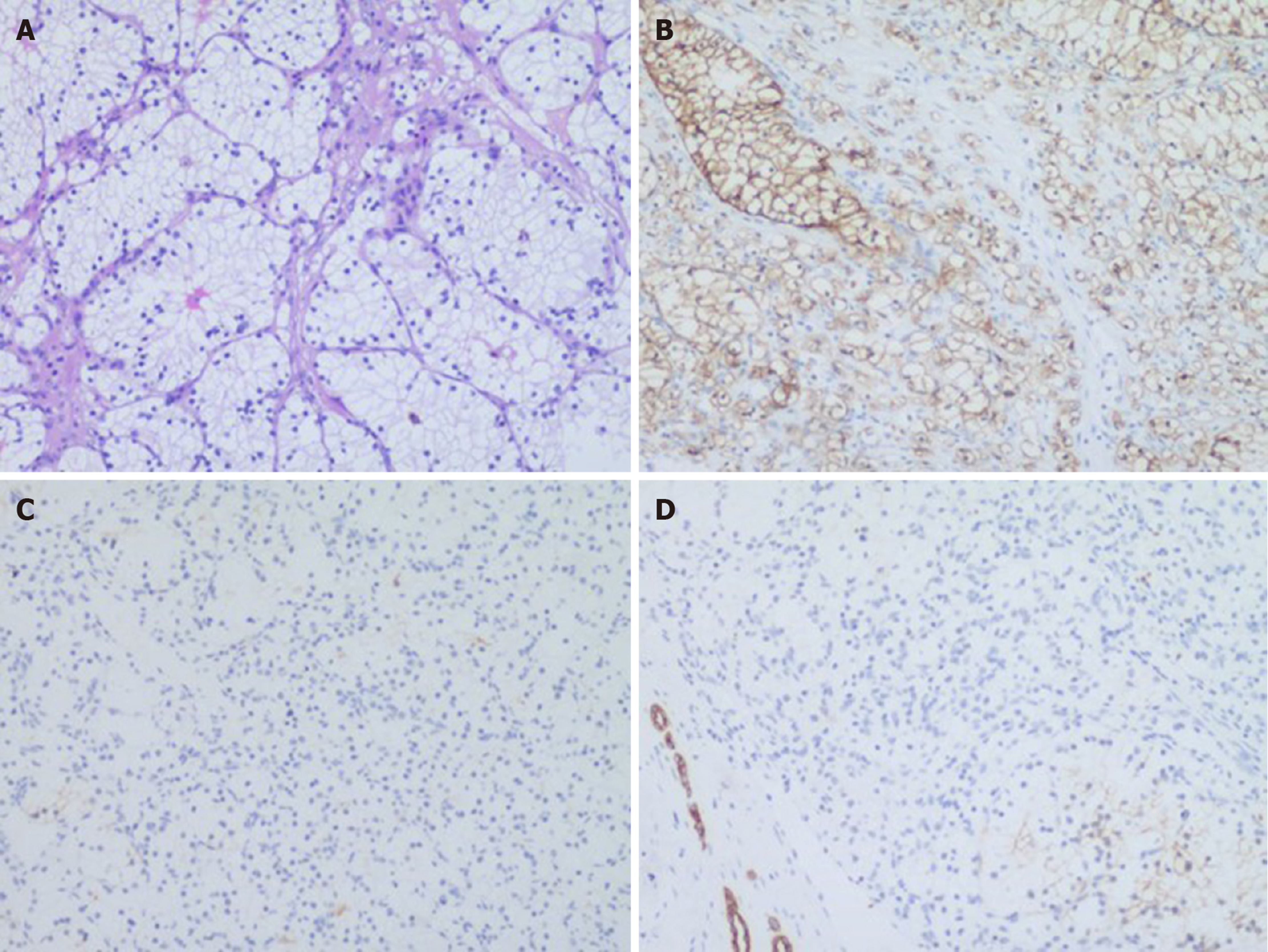
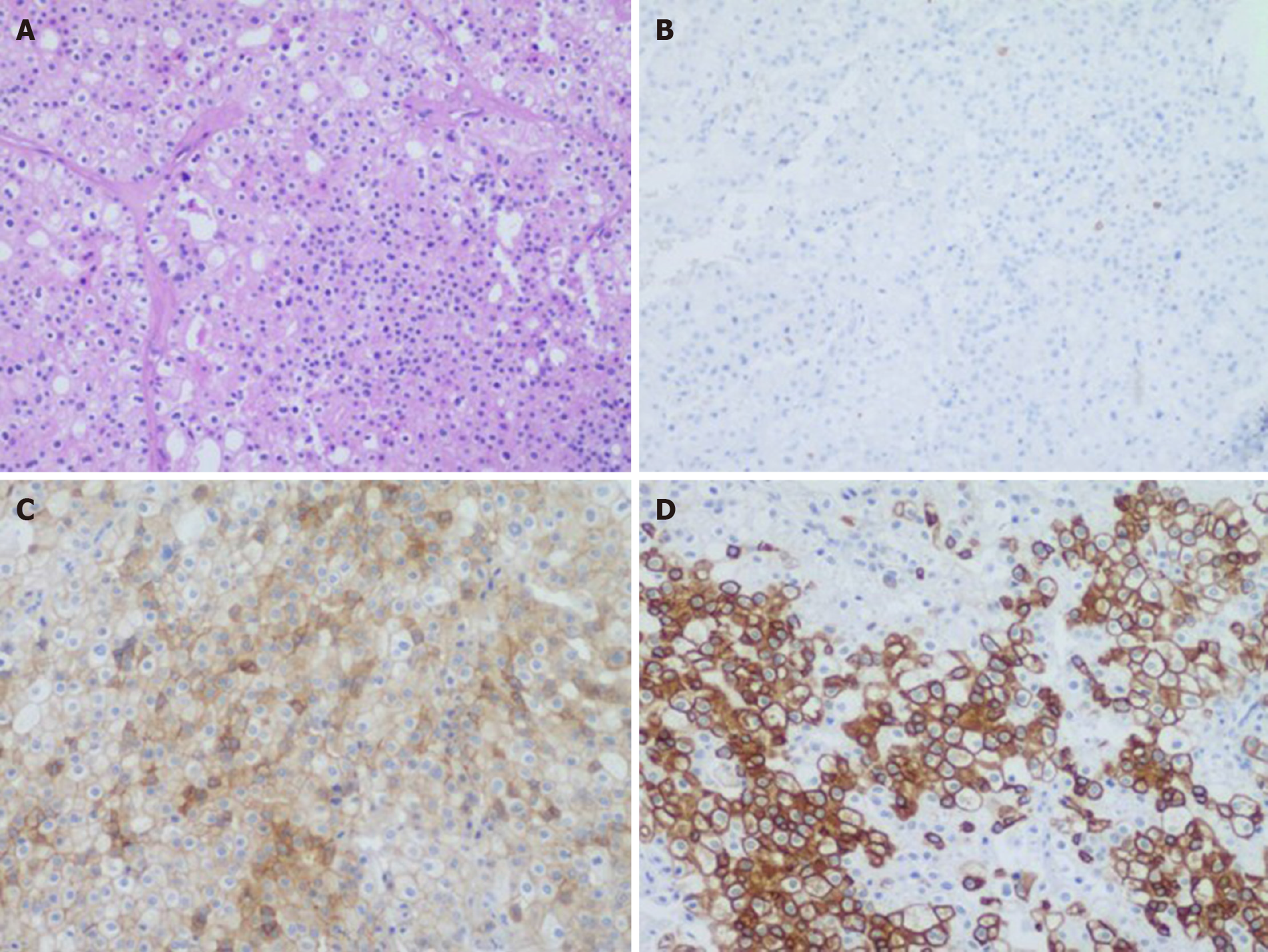
There were two different tumors and a cyst (Figure 3) on the right kidney. The bigger tumor was diagnosed as oncocytic variant of CHRCC on the lower pole with clear surgical margins. Immunohistochemically, the tumor showed positivity for paired box gene 8 (PAX-8) (+), carbonic anhydrase 7 (CA7)x2 (+), cluster of differentiation (CD117)x2 (+), succinate dehydrogenase iron-sulfur subunit (+), cytokeratin 20 (CK20) (+), but negativity for CAIXx2 (-), vimentin (-), transcription factor binding to IGHM enhancer 3 (TFE3) (-), and alpha-methylacryl-CoA racemase (P504S) (-) (Figure 4). The smaller one was diagnosed as clear cell carcinoma with clear surgical margins. Immunohistochemically, the tumor showed positivity for PAX-8 (+), CAIX (+), vimentin (part+), and CK7 (part+), but negativity for mucin 1 (-) and TFE3 (-) (Figure 5). The left tumor was diagnosed as CHRCC (classical subtype), with clear surgical margins. In immunocytochemical staining, the tumor cells showed a positive reaction for epithelial membrane antigen (+), CD117 (+), CK7 (part+), and Ki-67 (about 5%+). However, CD10 (-), RCCa (-), vimentin (-), and CAIX (-) were completely negative (Figure 6). Bilateral synchronous RCC was diagnosed according to its imaging manifestation and pathological results.
The patient was subjected to retroperitoneal laparoscopic nephron-sparing surgery (LNSS) of the right kidney under general anesthesia. There were two different tumors and a cyst on the right kidney. The cyst underwent unroofing. He was discharged on post-operative day 7, without any complications. At 10 wk postoperatively, the patient was readmitted to undergo surgery to remove the tumors of the left kidney. BUN was 3.56 mmol/L and the serum creatinine value was 119.8 mg/dL. GFR was 37.23 mL/min in the left kidney and 18.3 mL/min in right kidney. The patient underwent a second retroperitoneal laparoscopic nephron-sparing surgery under general anesthesia. He was discharged on postoperative day 12.
Six months postoperatively, there was no recurrence on CT urography. BUN was 5.11 mmol/L and the serum creatinine value was 111.2 μmol/L. There was no evidence of recurrence or lymphadenopathy at 12 mo after surgery. He was continuously followed up.
The incidence of bilateral RCC is relatively low, accounting for 1%-5% of all renal cancers[2,3]. Typical symptoms are abdominal pain, gross hematuria, and palpable mass, but most bilateral RCC has no symptoms. There was no difference in manifestation between bilateral multiple RCC and single RCC. As a screening tool, ultrasonography plays an important role in the detection of kidney tumors. Our case was first detected by ultrasonography. Whether there is a family history, bilateral RCC is divided into sporadic and hereditary. Bilateral RCC can occur synchronously or metachronously. Bilateral synchronous RCC accounts for 3.0%-4.2% of RCC, and metachronously bilateral RCC accounts for 0.4% of RCC[4].
At present, the etiology and pathogenesis of bilateral synchronous RCC are still unclear, especially for those with different histological subtypes. Synchronous tumors may arise from similar embryologic processes affected by abnormal factors such as carcinogens and hormones. Cancer stem cells that follow a dissimilar differentiation pathway regulated by tissue microenvironmental interactions might lead to different renal tumors[5]. In the published literature, the rate of pathologic concordance of sporadic synchronous bilateral RCC ranged from 84% to 95%, and the surveillance epidemiology and end results revealed a histology concordance rate of 93%, and nuclear grade concordance was 85%[6].
To date, the origin of bilateral RCC has not been definitively established. As limited studies on the genetics of bilateral tumors exist, defining the clinical behavior of these lesions remains important[7]. According to the literature, the synchronous presentation of RCC of diverse histology is a rare phenomenon. CCRCC and papillary renal cell carcinoma (PRCC) are the main histologic subtypes in bilateral RCC[8,9]. To the best of our knowledge, only six cases of synchronous bilateral CHRCC were reported by searching PubMed[10-15]. Tsutsumi et al[10] reported “Multiple chromophobe renal cell carcinoma: A case report” in 2010. The patient underwent left partial nephrectomy, and all of the excised tumors were determined to be CHRCC. Hidai et al[11] reported a case of a 42-year-old male with bilateral chromophobe cell RCC diagnosed with tuberous sclerosis complex and polycystic kidney disease in 1997. Yakout et al[12] reported a case of “synchronous bilateral chromophobe renal cell carcinoma” in a 71-year-old female in 2001. Mukai et al[13] reported “Synchronous bilateral chromophobe renal cell carcinoma: A case report” in 2009. Radopoulos et al[14] reported a case of a 57-year-old male patient with two bilateral synchronous chromophobe RCCs accompanied by an oncocytoma and an angiomyolipoma. The patient was treated with open partial nephrectomy. Li et al[15] reported a case of “synchronous bilateral multiple chromophobe renal cell carcinoma complicated with right renal cysts” in 2012. In our case, the patient had bilateral simultaneous CHRCC with two histological variants (classic type and eosinophilic type), accompanied by clear cell renal cell carcinoma (CCRCC) and a cyst. This suggests that both renal tumors may be primary, and further proves that there are different pathological types of bilateral renal tumors in the same body.
Most bilateral RCC has no typical symptoms in the early phase, and is usually discovered incidentally. CT and MRI remain the most widely available and effective modality for the detection and staging of RCC. It is quite important to accurately diagnose and distinguish RCC before surgery, especially bilateral RCC. Recently, some studies have demonstrated that imaging methods can differentiate CCRCC from the papillary and chromophobe histological types, which are the second and third most common RCC types, respectively[16]. CCRCC usually presents with intense contrast uptake in the corticomedullary phase and typical washout in the nephrographic phase. PRCC and CHRCC appear hypovascular compared with the adjacent renal parenchyma. These tumors tend to present with progressive uptake in contrast-enhanced CT/MRI[17,18], with maximum enhancement occurring during the renal parenchyma phase[19]. CHRCC is less intense than the CCRCC, and more intense than PRCC. Most CHRCC tends to be more homogeneous than CCRCC on CT/MRI, and CHRCC can exhibit a central scar and segmental enhancement inversion in some cases. CT/MRI findings correlate closely with the histopathology characteristics. The stellate scar observed on imaging corresponds to the coalescent central bands of fibrosis and compressed blood vessels that have been described as histologic findings in CHRCC[20]. In addition, microscopic lipid, calcification, necrosis and hemorrhage are uncommon. A central stellate scar appears to be an exclusive feature of CHRCC, although it may also be found in oncocytoma, because they originate from a common progenitor cell in the kidney and have overlapping histologic features[21,22]. However, histologic examination remains necessary to determine if a lesion is CHRCC or oncocytoma.
CHRCC originates from intercalated cells of the collecting duct system. CHRCC is a rare neoplasm that is less aggressive and has the best prognosis among RCCs. CHRCC tends to be solid, well-defined lesions. It is a heterogeneous group including classic type, eosinophilic type, and mixed type[23]. The simultaneous occurrence of the two subtypes of CHRCC is rare. This case had two concomitant different subtypes of CHRCC in each kidney (classic type and eosinophilic type), accompanied by a CCRCC.
Surgery, whether nephron-sparing or radical nephrectomy, is considered to be the ideal treatment for RCC. However, the choice of simultaneous or staged surgery is still controversial[24]. The surgical approach is determined by performance status and comorbidity of the patient, and tumor characteristics (size, location, and growth pattern). Balancing the complete eradication of potentially malignant tissue with minimizing treatment-related loss of renal function is challenging. Staging surgery can determine the histological type and related risk factors of the primary tumor in a timely manner, providing a reference point for management of the secondary contralateral renal tumor and renal reserve for possible secondary interventions. Nephron-sparing surgery is the treatment standard for patients with bilateral RCCs[25]. Our patient was treated with retroperitoneal LNSS. The malignancy of CHRCC is lower than that of CCRCC with less metastasis. In combination with imaging data, to ensure that one kidney was working normally during the two surgeries and to reduce the risk of perioperative acute renal failure, our case underwent right LNSS followed by left LNSS. Compared with open surgery, LNSS has advantages of less operative time, decreased blood loss, shorter ischemia time, and fewer complications, but it has a higher technical requirement for surgeons. These advantages are more fully reflected in the treatment of bilateral RCC. The interval time for the two surgeries was 10 wk. The patients still recovered and were discharged smoothly after two major operations.
Prior studies have reported a statistically significantly decreased 5-year survival rate in patients with bilateral RCC compared to patients with unilateral RCC[26,27]. However, recent studies have demonstrated that the prognosis of patients with bilateral RCC is similar to that of patients with unilateral RCC[2,28,29]. Treatment timing, surgical options, and individual differences may contribute to these differences. Follow-up in patients at regular intervals is necessary to exclude local recurrence and systemic progression, and should include physical examination, chest X ray, CT, and GFR based on the blood test of kidney function. The case was followed up without obvious signs of tumor recurrence and metastasis.
This was a rare case of bilateral simultaneous CHRCC with two histological variants, accompanied by a CCRCC and a cyst. Balancing complete eradication of potentially malignant tissue with minimizing treatment-related loss of renal function is a challenge. Our case report may serve as a reference for further studies.
| 1. | Diaz de Leon A, Pedrosa I. Imaging and Screening of Kidney Cancer. Radiol Clin North Am. 2017;55:1235-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 2. | Pahernik S, Cudovic D, Roos F, Melchior SW, Thüroff JW. Bilateral synchronous sporadic renal cell carcinoma: surgical management, oncological and functional outcomes. BJU Int. 2007;100:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 3. | Wang B, Gong H, Zhang X, Li H, Ma X, Song E, Gao J, Dong J. Bilateral Synchronous Sporadic Renal Cell Carcinoma: Retroperitoneoscopic Strategies and Intermediate Outcomes of 60 Patients. PLoS One. 2016;11:e0154578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 4. | Hu XY, Xu L, Guo JM, Wang H. Surgical strategy of bilateral synchronous sporadic renal cell carcinoma-experience of a Chinese university hospital. World J Surg Oncol. 2017;15:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 5. | Qi N, Li T, Ning X, Peng X, Cai L, Gong K. Clinicopathologic Features and Prognosis of Sporadic Bilateral Renal Cell Carcinoma: A Series of 148 Cases. Clin Genitourin Cancer. 2017;15:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Rothman J, Crispen PL, Wong YN, Al-Saleem T, Fox E, Uzzo RG. Pathologic concordance of sporadic synchronous bilateral renal masses. Urology. 2008;72:138-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (2)] |
| 7. | Boorjian SA, Crispen PL, Lohse CM, Leibovich BC, Blute ML. The impact of temporal presentation on clinical and pathological outcomes for patients with sporadic bilateral renal masses. Eur Urol. 2008;54:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 8. | Patel AR, Lee BH, Campbell SC, Zhou M, Fergany AF. Bilateral synchronous sporadic renal tumors: pathologic concordance and clinical implications. Urology. 2011;78:1095-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 9. | Guo J, Ma J, Sun Y, Qin S, Ye D, Zhou F, He Z, Sheng X, Bi F, Cao D, Chen Y, Huang Y, Liang H, Liang J, Liu J, Liu W, Pan Y, Shu Y, Song X, Wang W, Wang X, Wu X, Xie X, Yao X, Yu S, Zhang Y, Zhou A; written; CSCO Renal Cell Carcinoma Committee. Chinese guidelines on the management of renal cell carcinoma (2015 edition). Ann Transl Med. 2015;3:279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (2)] |
| 10. | Tsutsumi N, Soda T, Shimizu T, Watanabe J, Yoshimura K, Kamba T, Kanematsu A, Nakamura E, Nishiyama H, Ito N, Kamoto T, Ogawa O. [Multiple chromophobe renal cell carcinoma: a case report]. Hinyokika Kiyo. 2010;56:319-321. [PubMed] |
| 11. | Hidai H, Chiba T, Takagi Y, Taki A, Nagashima Y, Kuroko K. Bilateral chromophobe cell renal carcinoma in tuberous sclerosis complex. Int J Urol. 1997;4:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (2)] |
| 12. | Yakout HH, Bissada NK, Fahmy W, Creasman W, Fraig M, Hull GW. Synchronous bilateral chromophobe cell renal carcinoma. J Urol. 2001;166:1826. [PubMed] |
| 13. | Mukai M, Imamura R, Takayama H, Nishimura K, Nonomura N, Okuyama A. [Synchronous bilateral chromophobe cell renal carcinoma: a case report]. Hinyokika Kiyo. 2009;55:567-569. [PubMed] |
| 14. | Radopoulos D, Tahmatzopoulos A, Kalinderis N, Dimitriadis G. Bilateral synchronous occurrence of three different histological types of renal tumor: a case report. J Med Case Rep. 2009;3:6798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (2)] |
| 15. | Li XG, Cui XG, Zhang DX, Xu DF, Gao Y, Yin L, Li YL, Chen M. Synchronous bilateral multiple chromophobe cell renal carcinoma complicated with right kidney cyst: a case report. Journal of Medical Colleges of PLA. 2012;27:58-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 16. | Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol. 2016;8:484-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (10)] |
| 17. | Galia M, Albano D, Bruno A, Agrusa A, Romano G, Di Buono G, Agnello F, Salvaggio G, La Grutta L, Midiri M, Lagalla R. Imaging features of solid renal masses. Br J Radiol. 2017;90:20170077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | He J, Huan Y, Qiao Q, Zhang J, Zhang JS. Renal carcinomas associated with Xp11.2 translocations: are CT findings suggestive of the diagnosis? Clin Radiol. 2014;69:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 19. | Bhatnagar A, Rowe SP, Gorin MA, Pomper MG, Fishman EK, Allaf ME. Computed Tomography Appearance of Renal Hybrid Oncocytic/Chromophobe Tumors. J Comput Assist Tomogr. 2016;40:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Rosenkrantz AB, Hindman N, Fitzgerald EF, Niver BE, Melamed J, Babb JS. MRI features of renal oncocytoma and chromophobe renal cell carcinoma. AJR Am J Roentgenol. 2010;195:W421-W427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Abrahams NA, Tamboli P. Oncocytic renal neoplasms: diagnostic considerations. Clin Lab Med. 2005;25:317-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 22. | Chao DH, Zisman A, Pantuck AJ, Freedland SJ, Said JW, Belldegrun AS. Changing concepts in the management of renal oncocytoma. Urology. 2002;59:635-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (2)] |
| 23. | Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 2160] [Article Influence: 216.0] [Reference Citation Analysis (2)] |
| 24. | Blute ML, Itano NB, Cheville JC, Weaver AL, Lohse CM, Zincke H. The effect of bilaterality, pathological features and surgical outcome in nonhereditary renal cell carcinoma. J Urol. 2003;169:1276-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 25. | Jang HA, Kim JW, Byun SS, Hong SH, Kim YJ, Park YH, Yang KS, Cho S, Cheon J, Kang SH. Oncologic and Functional Outcomes after Partial Nephrectomy Versus Radical Nephrectomy in T1b Renal Cell Carcinoma: A Multicenter, Matched Case-Control Study in Korean Patients. Cancer Res Treat. 2016;48:612-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 26. | Novick AC, Streem S, Montie JE, Pontes JE, Siegel S, Montague DK, Goormastic M. Conservative surgery for renal cell carcinoma: a single-center experience with 100 patients. J Urol. 1989;141:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 180] [Article Influence: 4.9] [Reference Citation Analysis (2)] |
| 27. | Marberger M, Pugh RC, Auvert J, Bertermann H, Costantini A, Gammelgaard PA, Petterson S, Wickham JE. Conservation surgery of renal carcinoma: the EIRSS experience. Br J Urol. 1981;53:528-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 28. | Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1998] [Article Influence: 222.0] [Reference Citation Analysis (1)] |
| 29. | Lowrance WT, Yee DS, Maschino AC, Cronin AM, Bernstein M, Thompson RH, Russo P. Developments in the surgical management of sporadic synchronous bilateral renal tumours. BJU Int. 2010;105:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mogulkoc R, Mukherjee S S-Editor: Zhang L L-Editor: Filipodia E-Editor: Liu JH