Published online Feb 26, 2026. doi: 10.12998/wjcc.v14.i6.118545
Revised: January 24, 2026
Accepted: February 5, 2026
Published online: February 26, 2026
Processing time: 39 Days and 7.5 Hours
Acute submacular hemorrhage (SMH) secondary to choroidal neovascularization (CNV), most commonly in neovascular age-related macular degeneration, is a vision-threatening emergency. Thick, fovea-involving SMH can cause rapid photoreceptor injury, and timely intervention aimed at clot lysis and displace
We report a retrospective case series of three eyes with acute, fovea-involving CNV-related SMH treated with pars plana vitrectomy (PPV), subretinal tissue plasminogen activator (tPA), and expansile gas tamponade, with intravitreal anti-VEGF administered at the end of the procedure and continued postoperatively. Two cases had early intraocular pressure-related events (transient hypotony in one patient and transient ocular hypertension in another) requiring close post
PPV with subretinal tPA and gas tamponade is a practical surgical strategy for acute, thick, fovea-involving SMH secondary to CNV, particularly when rapid displacement is desired. Careful documentation of operative parameters, strict postoperative monitoring for pressure-related complications, and continued anti-VEGF therapy are essential components of care.
Core Tip: Acute submacular hemorrhage (SMH) secondary to choroidal neovascularization (CNV) is a vision-threatening emergency with no universal management consensus. This case series highlights the role of pars plana vitrectomy with subretinal tissue plasminogen activator, gas tamponade, and adjunct anti-vascular endothelial growth factor therapy for rapid foveal blood displacement. By integrating representative cases with contemporary evidence, we propose a practical, case-based treatment algorithm to guide timely surgical decision-making and postoperative management in severe CNV-related SMH.
- Citation: El Mollayess G, Jaroudi M, Tlaiss Y, Itaoui R, Abiad B. Surgical management of acute choroidal neovascularization related submacular hemorrhage: Three case reports. World J Clin Cases 2026; 14(6): 118545
- URL: https://www.wjgnet.com/2307-8960/full/v14/i6/118545.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i6.118545
Submacular hemorrhage (SMH) is a vision-threatening complication of choroidal neovascularization (CNV), most commonly in neovascular age-related macular degeneration (AMD)[1]. Acute SMH causes rapid photoreceptor damage via multiple mechanisms: It forms a physical barrier to nutrient diffusion, exerts fibrinous traction on the retina, and releases iron-related free radicals - all leading to irreversible photoreceptor loss if left untreated[1]. The natural history of large SMH is poor: In the submacular surgery trial observation arm, only 11% of eyes achieved better than 20/200 vision at 2 years[2]. Prompt intervention (ideally within 14 days of hemorrhage onset) is associated with significantly better visual outcomes, whereas delays beyond two weeks greatly diminish the chances of visual recovery[2].
A variety of treatment approaches for SMH have been described, ranging from less invasive therapies to vitreoretinal surgery. Small or thin subretinal hemorrhages may respond to intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections alone, as anti-VEGF can facilitate gradual blood resorption while treating the underlying CNV[3]. More extensive hemorrhages, however, often require active displacement of the clot from the fovea. Pneumatic displacement involves intravitreal injection of expansile gas (with or without intravitreal tissue plasminogen activator (tPA) to displace the submacular blood; this can be performed in the office and has shown favorable results in many cases[4]. Nonetheless, intravitreally delivered tPA may have limited ability to diffuse into the subretinal space, especially in dense clots[4]. For thick or refractory hemorrhages, surgical intervention with pars plana vitrectomy (PPV) and subretinal tPA injection allows direct clot lysis under the retina and, combined with gas tamponade, can achieve more complete blood dis
There is currently no universal consensus on the optimal management of SMH, and clinical practice varies. Recent evidence from randomized trials highlights the complexity: The TAPAS trial (factorial randomized trial of TPA vs sham and C3F8 gas vs sham, all with anti-VEGF) found that adding tPA significantly improved 3-month visual acuity, whereas adding gas did not confer a significant benefit[7]. Meanwhile, the French STAR trial (surgery vs pneumatic displacement for SMH) reported that both vitrectomy with subretinal tPA and pneumatic + tPA approaches yielded comparable vision gains (approximately 16 letters at 6 months) with no significant difference between them[8]. These studies suggest that less invasive therapy can suffice in many cases, although surgical treatment may achieve more complete and rapid anatomical displacement. Here we present a case series of three patients with acute CNV-related SMH who were treated with combined PPV, subretinal tPA injection, gas tamponade, and postoperative anti-VEGF therapy. We describe the surgical technique, clinical outcomes, and discuss the results in the context of current literature. We also propose a treatment algorithm to guide the use of subretinal tPA with pneumatic displacement for acute hemorrhagic CNV.
Case 1: Sudden marked vision loss in the right eye with central scotoma (3 days’ duration).
Case 2: Acute profound vision loss in the left eye.
Case 3: Acute vision loss in the left eye.
Case 1: Central scotoma for 3 days, then sudden marked right-eye vision loss in late November 2025 with best-corrected visual acuity (BCVA) decreased to counting fingers (CF) approximately 1 meter; exam and optical coherence tomography (OCT) confirmed acute fovea-involving SMH secondary to CNV. Surgery performed on November 25, 2025.
Case 2: In November 2025, developed acute profound left-eye vision loss with BCVA decreased to CF approximately 50 cm; exam and OCT confirmed acute fovea-involving SMH secondary to CNV.
Case 3: In November 2025, presented with acute left-eye vision loss with visual acuity (VA) light perception (LP); exam documented subretinal bleeding due to ruptured CNVM with retinal detachment.
Case 1: Diabetes mellitus. Baseline BCVA approximately 20/50 oculus uterque (OU); intraocular pressure (IOP) 10 mmHg OU. Ocular history notable for prior intravitreal anti-VEGF injections in the fellow eye.
Case 2: Hypertension; aspirin use; pseudophakia OU. Known nAMD oculus sinister (OS) treated with serial intravitreal bevacizumab and OCT monitoring. Baseline BCVA approximately 20/25 oculus dexter (OD) and approximately 20/50 OS.
Case 3: Hypertension; pseudophakia OU. nAMD OU treated with intravitreal aflibercept and OCT monitoring. Baseline VA approximately HM OD and 20/200 OS; IOP 12 mmHg OU.
Not reported in the manuscript.
Ophthalmic examination findings as documented.
Case 1: Subretinal hemorrhage consistent with acute fovea-involving SMH due to CNV; BCVA CF approximately 1 m.
Case 2: Subretinal hemorrhage consistent with acute fovea-involving SMH due to CNV; BCVA CF approximately 50 cm.
Case 3: Subretinal bleeding due to ruptured CNVM with retinal detachment; VA LP.
Not reported/not applicable in the manuscript.
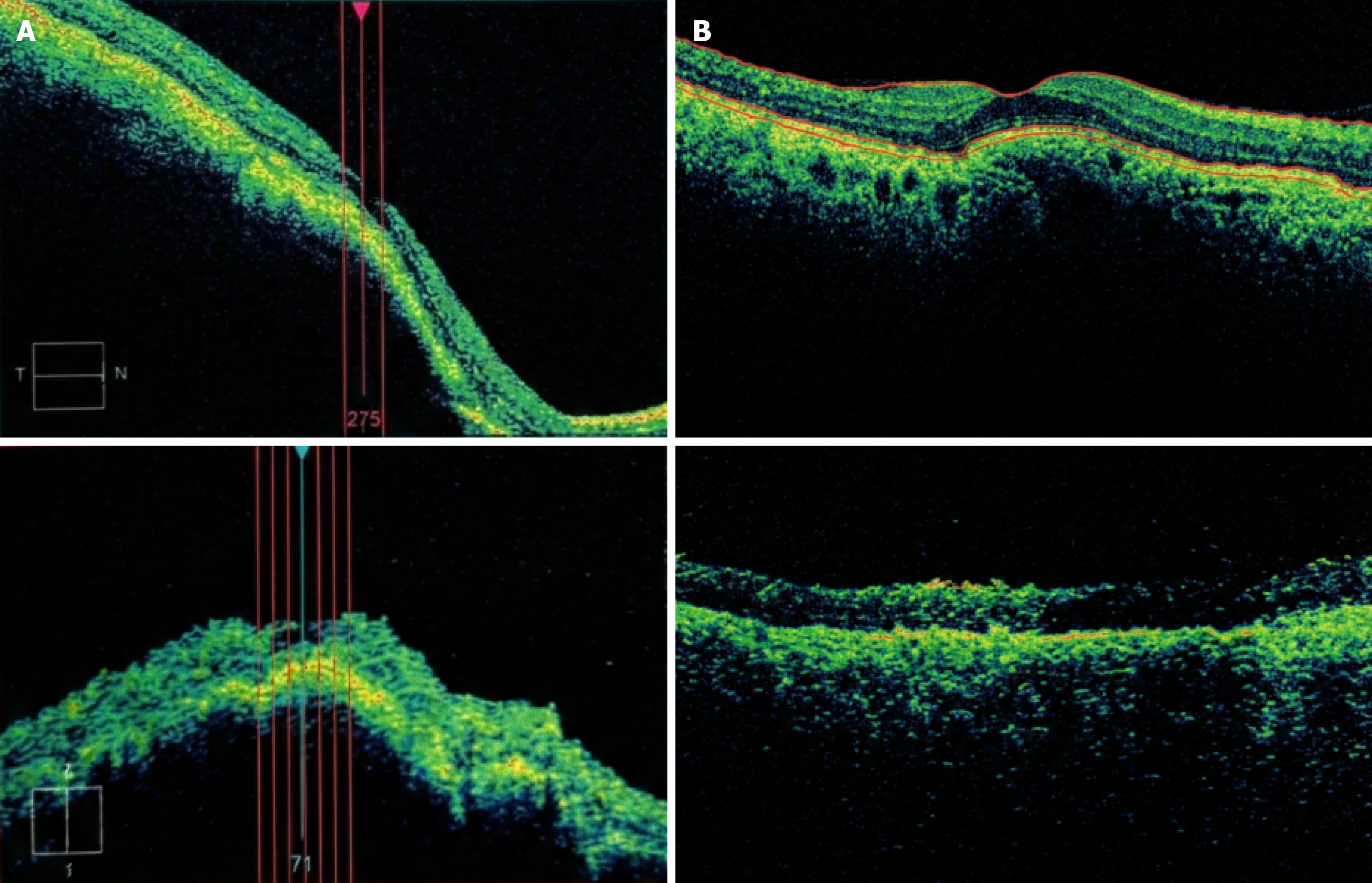
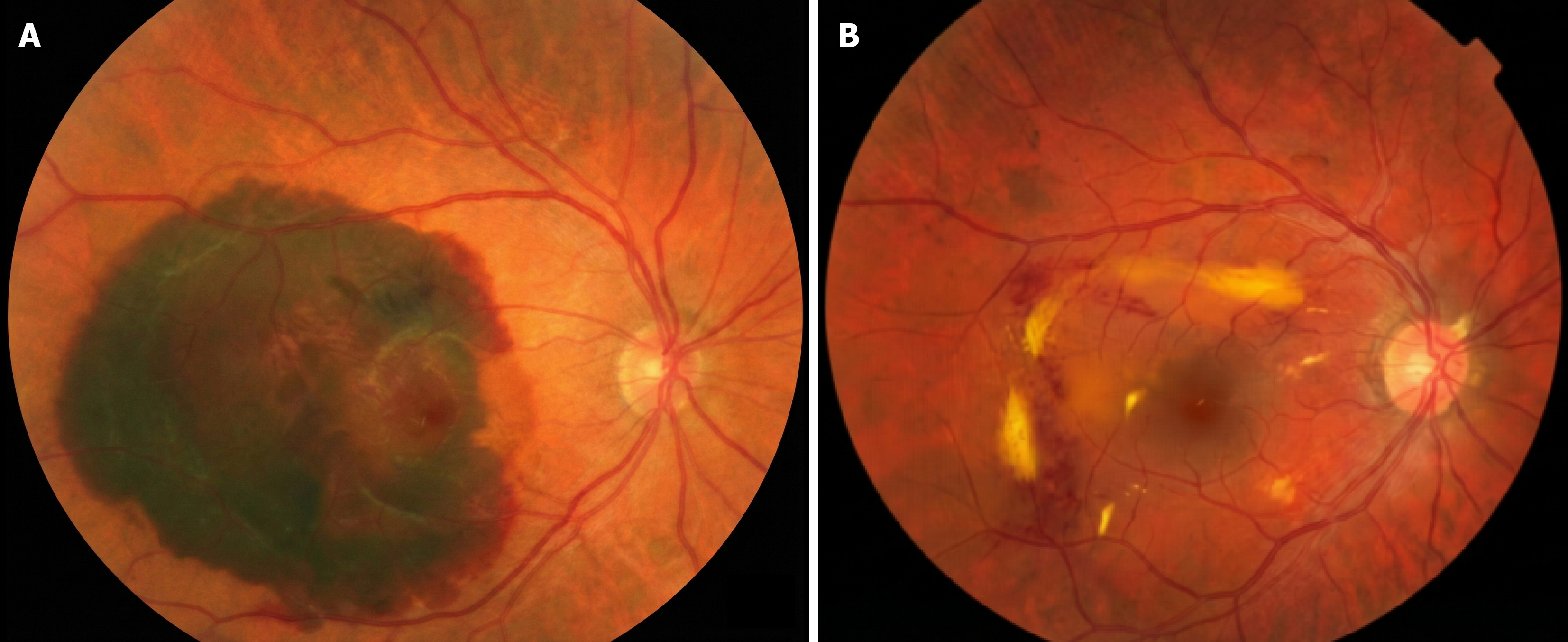
Case 1: OCT confirmed acute fovea-involving SMH secondary to CNV (baseline OCT referenced as Figure 1A; follow-up OCT as Figure 1B). Baseline and follow-up fundus photography provided (Figure 2).
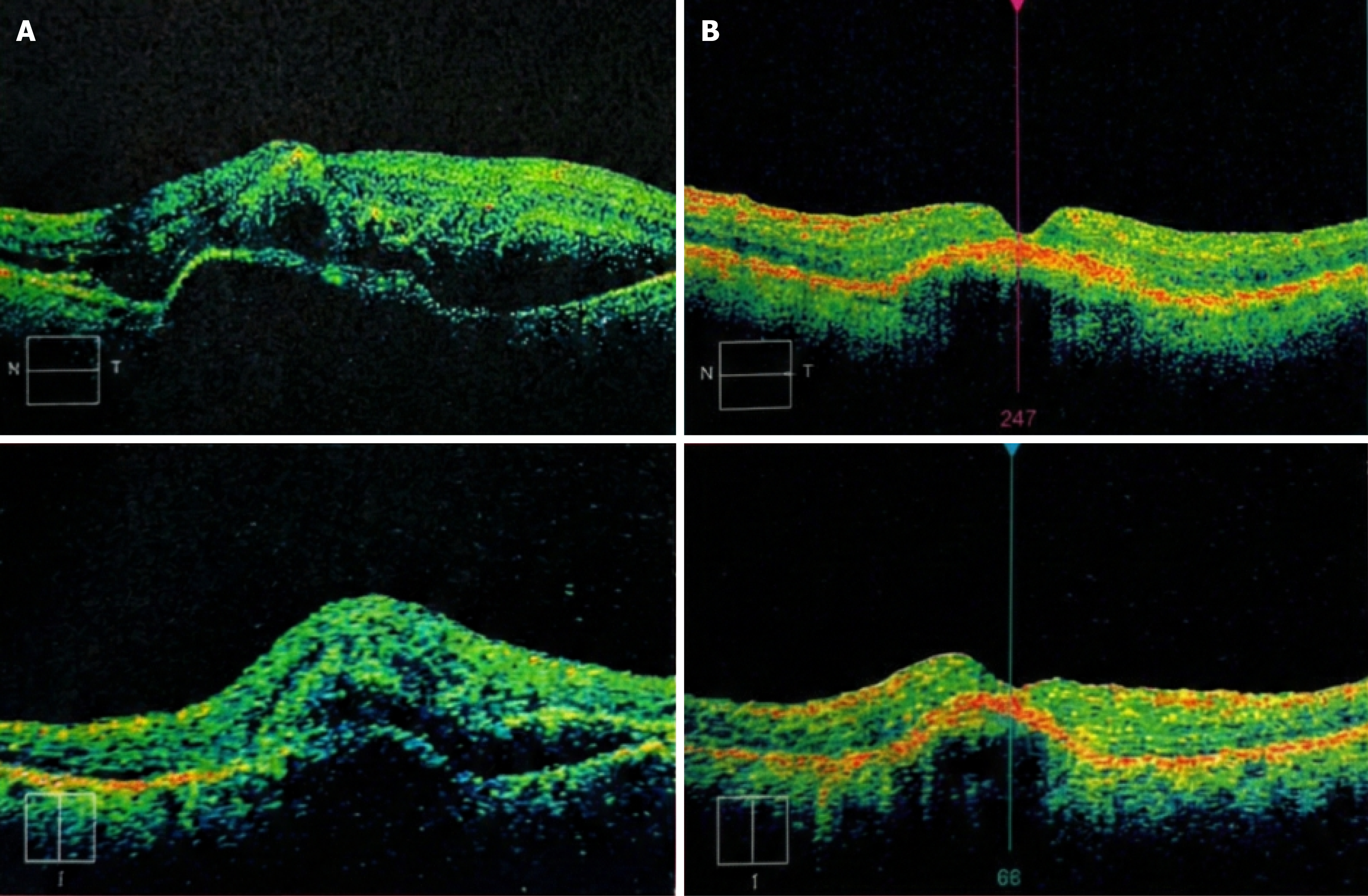
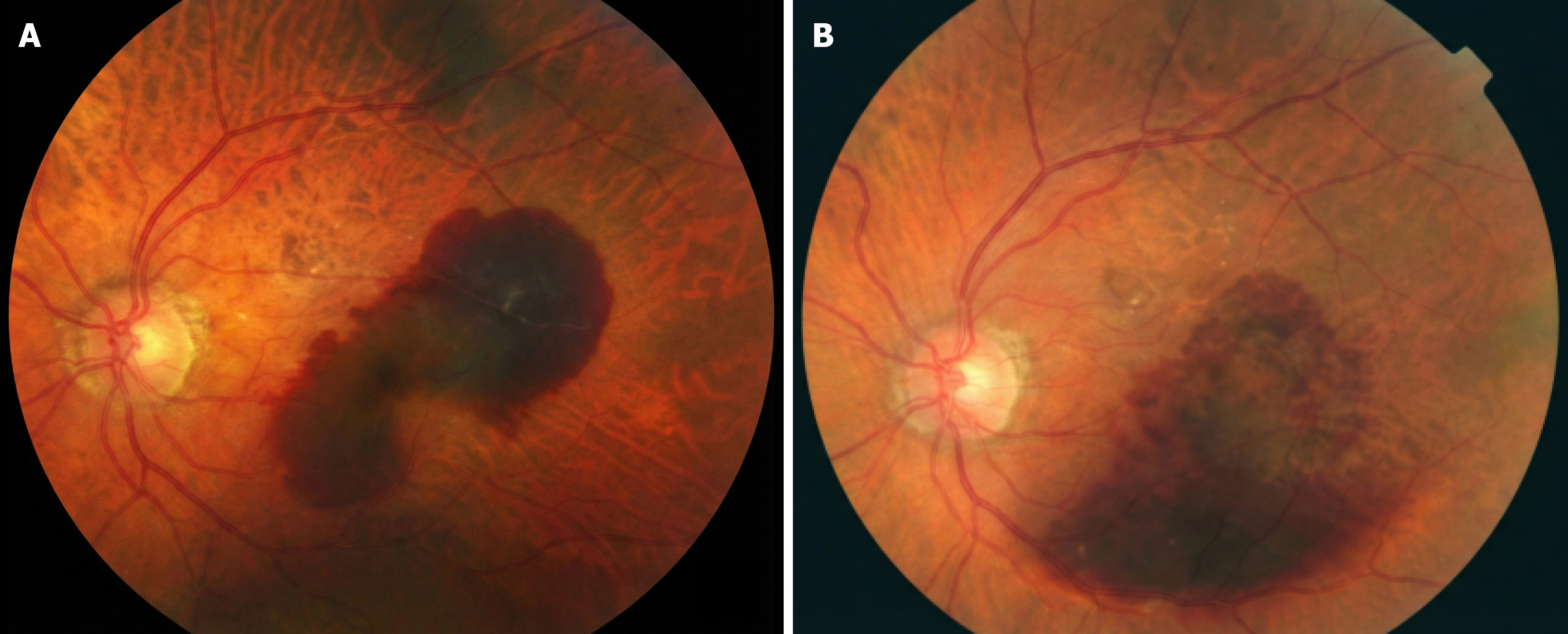
Case 2: OCT confirmed acute fovea-involving SMH secondary to CNV (baseline OCT referenced as Figure 3A; follow-up OCT as Figure 3B). Baseline and follow-up fundus photography provided (Figure 4).
Case 3: Imaging details beyond examination documentation are not provided in the current text.
Acute fovea-involving SMH secondary to CNV, with early postoperative hypotony.
Acute fovea-involving SMH secondary to CNV (nAMD context), with early postoperative ocular hypertension.
Ruptured CNVM with subretinal bleeding and retinal detachment, treated surgically.
All cases underwent 25-gauge PPV + subretinal tPA + gas tamponade, with intravitreal ranibizumab 0.5 mg at the end of surgery and postoperative positioning/anti-VEGF planning as described.
Operative technique (as written in your manuscript): 25-gauge 3-port PPV; no extensive internal limiting membrane peeling. 41-gauge flexible subretinal cannula; small retinotomy (approximately 200-300 µm) at superior edge of clot. tPA dose/volume: 25-50 µg in 0.05-0.1 mL BSS injected subretinally to create a localized bleb. Fluid-air exchange then gas tamponade: Case 1: 25% SF6; Case 2: 14% C3F8; finally: Ranibizumab 0.5 mg intravitreally. Positioning: Face-down 5 days. Postop anti-VEGF plan: 4-week intervals, 2 loading doses month 1 and 2, then PRN based on OCT/angiography.
All three cases were treated surgically and continued anti-VEGF therapy postoperatively, with early IOP-related complications highlighted (hypotony in Case 1, ocular hypertension in Case 2).
Case 1 (follow-up duration 6 months): VA course (early): Pre-op CF @ 1 m → POD1 CF @ 1 m → POW2 CF @ 1.5 m → POW3 HM. IOP: POD1 19 mmHg, POD2 2 mmHg with soft globe; repeat OCT and intensified topical therapy. Imaging follow-up: Fundus photo + OCT show postoperative evolution vs baseline (Figures 1B and 2B).
Case 2 (follow-up duration 12 months): VA course (early): Pre-op CF @ 50 cm → POD1 CF near face → POD10 CF < 1 m. IOP: POD1 27 mmHg treated with oral acetazolamide; POD10 14 mmHg with tapering drops and plan for continued anti-VEGF/OCT follow-up. Imaging follow-up: Fundus photo + OCT show postoperative evolution vs baseline (Figures 3B and 4B).
Case 3 (follow-up duration 6 months): VA course (early): Pre-op LP → POD1 CF @ 1 m → POD8 HM. IOP: POD1 11 mmHg, POD8 10 mmHg; drops tapered; further retinal imaging scheduled and injections to be resumed. A comparative summary of the three cases is provided in (Table 1).
| Variable | Case 1 | Case 2 | Case 3 |
| Age/sex | 73/M | 87/M | 89/M |
| Eye | OD | OS | OS |
| CNV context | nAMD suspected/treated | nAMD treated (serial IV bevacizumab) | nAMD treated (serial IV aflibercept) |
| Acute presentation (VA + finding) | CF @ approximately 1 m, subretinal bleed | CF @ approximately 50 cm, subretinal bleed | LP, subretinal bleed |
| Intervention | OR-based displacement procedure (PPV + subretinal tPA + gas per protocol) | PPV + subretinal tPA + gas | PPV + subretinal tPA + gas |
| VA course (early follow-up) | Pre-op: CF @ 1 m → POD1: CF @ 1 m → POW2: CF @ 1.5 m → POW3: HM | Pre-op: CF @ 50 cm → POD1: CF near face → POD10: CF < 1 m | Pre-op: LP → POD1: CF @ 1 m → POD8: HM |
| Early postop issue | Hypotony (IOP 2 on POD2) | Ocular hypertension (IOP 27 on POD1) | None |
| Anti-VEGF | Planned/ongoing | Continued/ongoing | Planned/ongoing |
| Follow-up duration | 6 months | 12 months | 6 months |
These cases illustrate real-world surgical management of acute SMH secondary to CNV using PPV with subretinal tPA and gas tamponade in the operating room, followed by continued anti-VEGF therapy to address the underlying neovascular lesion. Anti-VEGF therapy remains foundational in CNV care and is necessary to suppress exudation and reduce recurrent hemorrhage risk[9].
Surgical displacement with subretinal tPA aims to liquefy submacular clot to allow pneumatic displacement away from the fovea. In practice, case selection typically considers hemorrhage thickness and extent, symptom duration, and ability to posture. Postoperative complications may include pressure-related events, as observed here: Marked hypotony in Case 1 and transient ocular hypertension in Case 2. These events reinforce the importance of structured postoperative monitoring and prompt management of IOP abnormalities.
Timing of intervention is a crucial factor. Both of our cases were operated on within 7-10 days of hemorrhage onset. This likely contributed to the favorable outcomes, as experimental and clinical data indicate that retinal damage from submacular blood intensifies after about 1-2 weeks[10]. Ali Said et al[11] emphasize that subretinal tPA surgery should be considered only for hemorrhages that are relatively fresh (within approximately 2 weeks), since chronic hemorrhages that have turned fibrinous (“yellow” on exam) may not displace well and often have already caused retinal damage. Indeed, in chronic SMH cases or those with poor visual potential, conservative management (observation or anti-VEGF alone) may be more appropriate. In our patients, presenting within the ideal window allowed tPA to effectively liquefy the clot and likely limited phototoxic damage.
Another consideration is hemorrhage location and composition. Both patients primarily had subretinal hemorrhage (between retina and RPE) rather than sub-RPE hemorrhage. Sub-RPE blood is more difficult to displace and carries a high risk of causing an RPE tear if one tries to manipulate it; such cases often have worse visual prognosis. We intentionally confirmed via OCT that the bulk of the blood was in the subretinal space. If a large component of sub-RPE hemorrhage had been present, we might have tempered our expectations or selected a different strategy (for example, some might attempt pneumatic displacement knowing that sub-RPE blood often remains). Fortunately, neither case developed an RPE tear - a complication sometimes seen in hemorrhagic AMD, especially in eyes with pigment epithelial detachments.
Our results underscore that anti-VEGF therapy is a vital adjunct to any mechanical clot displacement. Without addressing the CNV, one risks recurrent bleeding or ongoing exudation that negates the benefits of the initial inter
In weighing surgery vs less invasive approaches, one should consider patient-specific factors. Advanced age, co-morbidities, and anti-coagulation status might favor an in-office pneumatic displacement to avoid surgical risks[11]. In our series, both patients were relatively healthy and were good surgical candidates. If a patient were unfit for surgery on short notice (e.g., due to systemic issues), we would attempt intravitreal tPA and gas as a first step. Conversely, if the hemorrhage is extremely large (e.g. > 5 disc diameters or causing a total hemorrhagic retinal detachment), primary vitrectomy with more extensive clot evacuation might be required, sometimes even using a retinectomy and silicone oil as described by some experts[11]. Those extreme cases are relatively rare; none of our cases fell into that category.
Proposed treatment algorithm: Based on our review of the literature and our experience, we suggest a practical algorithm for managing acute SMH secondary to CNV (Figure 2). The algorithm stratifies management by key factors such as hemorrhage size, thickness, location, and timing. In brief, small/thin hemorrhages without foveal involvement can be initially managed with anti-VEGF injections (and optional pneumatic displacement) since they often clear with medical therapy alone. For moderate-sized or thick hemorrhages involving the fovea (and presenting within approximately 14 days), prompt displacement is advised - either via intravitreal tPA with gas or via PPV with subretinal tPA if the hemorrhage is especially dense. If a pneumatic approach is chosen first and fails to displace the blood within a few days, one should escalate to vitrectomy. In cases of massive hemorrhage (> 5 disc areas or hemorrhagic detachment), vitrectomy with subretinal tPA is generally indicated as first-line, potentially combined with surgical clot extraction for the largest bleeds. Throughout all steps, anti-VEGF therapy is incorporated as soon as feasible. The table below summarizes when subretinal tPA + gas is most beneficial, highlighting that timing (acute hemorrhage) and extent (significant, fovea-threatening bleeds) are the scenarios to intervene aggressively.
Table 2 proposed algorithm for acute CNV-related submacular hemorrhage. Looking forward, there is ongoing evolution in SMH management. The use of intraoperative OCT to guide subretinal injections is an innovation that helps ensure tPA is delivered to the right plane. Fibrinolytic agents other than tPA (like recombinant plasmin) have been explored, though tPA remains the standard. Additionally, there is interest in adjunctive therapies for the CNV itself during surgery - for example, some surgeons perform photodynamic therapy (PDT) to a polypoidal lesion after clearing the hemorrhage[12]. In our PCV case, we did not do intraoperative PDT, but we acknowledge it as an option if polyps remain active. Finally, gene therapies and longer-acting anti-VEGFs on the horizon may reduce recurrence of hemorrhage by better controlling the neovascular disease.
| Hemorrhage characteristics | Recommended management |
| Small, thin hemorrhage (< 1-2 disc diameters, | Anti-VEGF therapy alone (intravitreal injections) is usually sufficient. Consider adding pneumatic displacement (gas injection) for faster clearance if hemorrhage overlies near-foveal area. Monitor closely on anti-VEGF; many will improve without surgery |
| Moderate hemorrhage (approximately 2-5 DD in area or 100-500 µm thick) with foveal involvement, onset < approximately 14 days, predominantly subretinal (not sub-RPE) | Displacement therapy indicated. If patient is a good candidate, perform PPV with subretinal tPA + gas tamponade for maximal clearance. Alternatively, an initial intravitreal tPA + gas (office procedure) may be attempted, especially if surgical timing is an issue. In either case, prompt anti-VEGF is given (at time of procedure or shortly after) to treat CNV |
| Large hemorrhage (> 5 DD, or any causing hemorrhagic retinal detachment), very thick | Pars plana vitrectomy is recommended. Use subretinal tPA to liquefy clot; expect to perform a larger retinotomy in cases of hemorrhagic detachment. Consider mechanically evacuating clot and CNV if it’s massive. Gas tamponade often used; for very extensive detachments, use longer-acting gas or even silicone oil if needed. Follow with intensive anti-VEGF therapy. Visual prognosis is guarded but intervention may still improve outcomes vs observation |
| Chronic or sub-RPE hemorrhage (duration | Conservative approach often advised. Surgical or pneumatic displacement is less effective once blood has organized (high fibrin). Treat underlying CNV with anti-VEGF; consider observing or applying photodynamic therapy (for PCV) if applicable. If visual potential is still significant and hemorrhage is thick, a case-by-case decision for surgery can be made, but counsel that visual improvement is less likely |
Acute SMH secondary to CNV (especially AMD) is an ophthalmic emergency in which timely intervention can preserve sight. This case series demonstrates that a combined surgical approach - PPV with subretinal tPA injection and gas tamponade, coupled with prompt anti-VEGF therapy - can effectively displace submacular clots and improve visual outcomes in patients with severe CNV-related hemorrhages. In our three cases, the treatment led to substantial anatomical clearing of the fovea and meaningful visual gains with no significant complications. Current evidence supports early treatment (preferably within 1-2 weeks of hemorrhage) and indicates that both less invasive (intravitreal tPA with gas) and surgical methods result in better vision than observation, with the choice of technique depending on hemorrhage characteristics and patient factors.
| 1. | Shaheen A, Mehra D, Ghalibafan S, Patel S, Buali F, Panneerselvam S, Perez N, Hoyek S, Flynn HW Jr, Patel N, Yannuzzi NA. Efficacy and Safety of Anti-VEGF Injections and Surgery for Age-Related Macular Degeneration-Related Submacular Hemorrhage: A Systematic Review and Meta-Analysis. Ophthalmol Retina. 2025;9:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 2. | Bressler NM, Bressler SB, Childs AL, Haller JA, Hawkins BS, Lewis H, MacCumber MW, Marsh MJ, Redford M, Sternberg P Jr, Thomas MA, Williams GA; Submacular Surgery Trials (SST) Research Group. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology. 2004;111:1993-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Ueda-Consolvo T, Takahashi S, Oiwake T, Nakamura T, Ishida M, Yanagisawa S, Hayashi A. Assessment of retinal pigment epithelium tears in eyes with submacular hemorrhage secondary to age-related macular degeneration. Sci Rep. 2025;15:3606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Handwerger BA, Blodi BA, Chandra SR, Olsen TW, Stevens TS. Treatment of submacular hemorrhage with low-dose intravitreal tissue plasminogen activator injection and pneumatic displacement. Arch Ophthalmol. 2001;119:28-32. [PubMed] |
| 5. | Haupert CL, McCuen BW 2nd, Jaffe GJ, Steuer ER, Cox TA, Toth CA, Fekrat S, Postel EA. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol. 2001;131:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Chotikkakamthorn P, Bhurayanontachai P, Jirarattanasopa P, Tsutsumi WD, Tantisarasart T, Ratanasukon M. Pneumatic displacement and intravitreal anti-vascular endothelial growth factor, with or without intravitreal tissue plasminogen activator, injection for submacular hemorrhage. Retina. 2025;45:394-401. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Murphy GSP, Saleh A, Ayis S, Cheema MR, Mehta A, Steel DH, Membrey L, Costen M, Jackson TL. Tissue Plasminogen Activator or Perfluoropropane for Submacular Hemorrhage in Age-Related Macular Degeneration: A Factorial Randomized Clinical Trial. JAMA Ophthalmol. 2024;142:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Barzelay A, Daniels A, Cohen GY, Barak A, Schwartz S, Katz G. Pneumatic displacement with intravitreal tPA injection versus vitrectomy with sub retinal tPA injection in small and medium sub macular hemorrhages- a multicenter comparative study. BMC Ophthalmol. 2024;24:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Kim JH, Chang YS, Kim JW, Kim CG, Yoo SJ, Cho HJ. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology. 2014;121:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Bakri SJ, Sears JE, Lewis H. Management of macular hole and submacular hemorrhage in the same eye. Graefes Arch Clin Exp Ophthalmol. 2007;245:609-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Ali Said Y, Dewilde E, Stalmans P. Visual Outcome after Vitrectomy with Subretinal tPA Injection to Treat Submacular Hemorrhage Secondary to Age-Related Macular Degeneration or Macroaneurysm. J Ophthalmol. 2021;2021:3160963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Gabrielle PH, Delyfer MN, Glacet-Bernard A, Conart JB, Uzzan J, Kodjikian L, Arndt C, Tadayoni R, Soudry-Faure A, Creuzot Garcher CP. Surgery, Tissue Plasminogen Activator, Antiangiogenic Agents, and Age-Related Macular Degeneration Study: A Randomized Controlled Trial for Submacular Hemorrhage Secondary to Age-Related Macular Degeneration. Ophthalmology. 2023;130:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |