Published online Feb 6, 2026. doi: 10.12998/wjcc.v14.i4.117314
Revised: December 19, 2025
Accepted: January 19, 2026
Published online: February 6, 2026
Processing time: 63 Days and 6.9 Hours
Chromovitrectomy using vital dyes has become integral to internal limiting membrane (ILM) peeling in macular surgery. Although brilliant blue G (BBG) is considered safer than earlier dyes, emerging clinical and experimental evidence suggests that dye-light interactions during surgery may result in retinal photo
To describe the retinal phototoxicity features associated with BBG dye in patients undergoing ILM peeling for macular surgeries.
This observational, comparative, retrospective case series analyzed 9 eyes of 9 patients demonstrating retinal phototoxicity after uneventful BBG-assisted ILM peeling. Surgical indications included full-thickness macular hole (MH) in 4 eyes, epiretinal membrane associated retinal traction in 4 eyes, and vitreomacular tr
In group A, all cases showed foveal phototoxic damage along with macular involvement despite successful MH closure. Two eyes experienced worsened VA, while the other two maintained baseline VA. In group B, pho
BBG-related retinal phototoxicity may occur following ILM peeling, with eyes undergoing MH surgery appearing more vulnerable, possibly due to the absence of protective foveal neurosensory tissue. Awareness of this potential risk may encourage surgeons to consider minimizing illumination intensity and dye exposure, and to adopt pro
Core Tip: This case series identifies retinal architecture as a key determinant of brilliant blue G (BBG)-related toxicity during internal limiting membrane peeling. Phototoxicity was more severe in macular hole (MH) cases where the exposed retinal pigment epithelium allowed direct BBG-light interaction, leading to foveal damage and poor visual recovery. Non-MH eyes, including epiretinal membrane and vitreomacular traction, retained foveal protection and demonstrated better postoperative vision. The study reinforces careful control of illumination and BBG exposure in MH surgery.
- Citation: Venkatesh R, James E, Hande P, Raj P, Tendulkar K, Prabhu V. Retinal architecture as a determinant of brilliant blue G phototoxicity during internal limiting membrane peeling: A case series. World J Clin Cases 2026; 14(4): 117314
- URL: https://www.wjgnet.com/2307-8960/full/v14/i4/117314.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i4.117314
The primary objective of surgery for vitreomacular interface disorders is to relieve anteroposterior traction through pars plana vitrectomy with posterior vitreous detachment induction, and tangential traction through epiretinal membrane (ERM) and internal limiting membrane (ILM) peeling, thereby restoring normal foveal architecture and improving visual acuity[1,2]. ILM peeling is now a widely established technique in the management of macular holes (MHs), vitreomacular traction (VMT), macular puckers, ERMs, tractional and selected non-tractional diabetic macular edema, myopic tractional maculopathy (MTM), optic disc pit maculopathy (ODPM), and sub-ILM hemorrhages such as those seen in Valsalva retinopathy and Terson’s syndrome[3].
Vital dyes including indocyanine green (ICG), brilliant blue G (BBG), and bromophenol blue have been routinely used to facilitate ILM visualization during surgery[4-6]. However, toxic effects on photoreceptors and the retinal pigment epithelium (RPE) have been documented, particularly with ICG, with both experimental and clinical evidence de
Although the surgical steps involved in managing vitreomacular interface disorders are largely similar, the underlying foveal anatomy varies substantially across disease entities. Eyes with full-thickness or lamellar MHs are characterized by focal loss of neurosensory foveal tissue, whereas conditions such as ERM, VMT, MTM, and ODPM typically retain in
To explore this, we conducted a retrospective analysis of BBG-associated retinal phototoxicity in eyes undergoing ILM peeling across various macular surgery indications.
This retrospective case series included patients diagnosed with BBG-related retinal phototoxicity following ILM peeling for various macular pathologies between 2017 and 2022. Phototoxicity was defined as outer retinal and/or RPE damage detected during postoperative follow-up. Diagnosis was based on clinical observation of pigmentary changes corresponding to the ILM-peeled area and confirmed using multimodal imaging, including mottled hyper- and hypo-autofluorescence on fundus autofluorescence (FAF) and loss of outer retinal layers with irregular RPE alterations on optical coherence tomography (OCT). The study adhered to the tenets of the Declaration of Helsinki. Institutional ethics approval was obtained (EC Ref. No: C/2019/03/04) from the Narayana Nethralaya Ethics Committee, Bangalore. Owing to the retrospective design, informed consent was waived.
Following identification of eligible cases, clinical records were reviewed to extract demographic details, preoperative clinical and imaging characteristics, intraoperative parameters, and postoperative outcomes. Demographics included age and sex. Preoperative variables included eye laterality, best-corrected visual acuity, surgical indication, OCT assessment of the outer retina and RPE, and central foveal thickness. Central macular thickness was measured from the ILM to the inner RPE boundary using built-in callipers on the OCT device.
All patients underwent standard 25-gauge three-port pars plana vitrectomy using the Alcon CONSTELLATION® Vision System (TX, United States). Posterior vitreous detachment was induced in all cases using intravitreal triamcinolone acetonide. ILM peeling was performed after staining with BBG. Intraoperative details recorded included whether BBG (Ocublue plus 0.05% w/v, Aurolab, Madurai, India) staining was single or repeated, staining under air or balanced salt solution (BSS), duration of intraocular dye exposure, total surgical time, and endotamponade type. All procedures were performed by a single experienced surgeon.
Postoperative assessment was limited to clinical examination, optical coherence tomography, and FAF imaging, as additional functional and microvascular investigations were not routinely performed during the study period. Post
To evaluate whether anatomical substrate influenced phototoxic patterns and visual outcomes, cases were categorized into two groups based on indication: (A) MH; and (B) non-MH. Given the retrospective design of the study and the rarity of clinically evident BBG-related phototoxicity, the inclusion of a matched control group was not feasible.
Data analysis was conducted using XLSTAT software (Lumivero, Denver, CO, United States version 2023.3.1.1416). Data distribution was summarized as medians with interquartile ranges for continuous variables, while categorical variables were presented as n (%).
The study identified 9 eyes (9 cases) with outer retinal layer and RPE damage secondary to BBG-related retinal pho
| Case No. | Age | Sex | Eye | Reason for ILM peeling | Study group | Preop VA | Pre-op CMT | Pre-op ORL status | Pre-op RPE layer healthy | BBG exposure time (minute) | BBG stain under | Stain | Tamponade agent | Total surgery time (minute) | Time gap post-surgery after which retinal damage was noted (weeks) | Retinal toxicity involving the fovea | Surgical result | Early post op VA | Last visit VA | F/U time (months) |
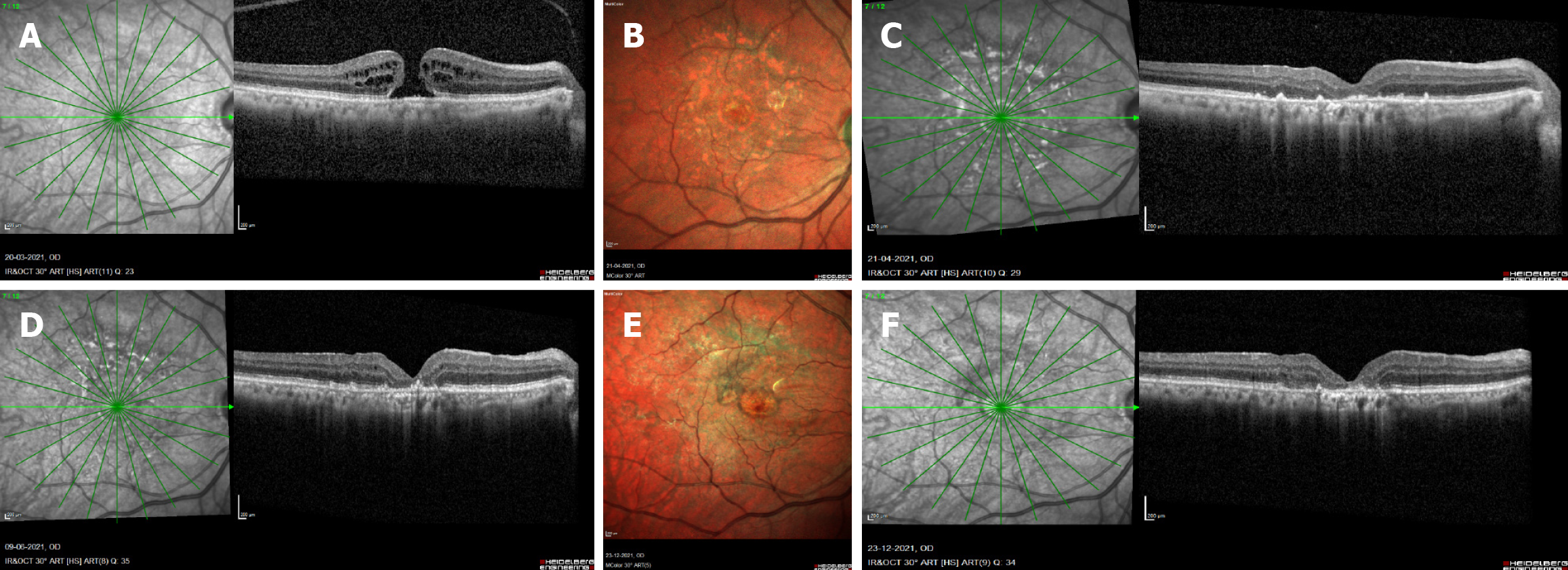
| 1 | 69 | F | RE | Idiopathic MH | A | 6/36 | 0 | CBA | Yes | 2 | AIR | SINGLE | SF6 | 90 | 1 | Yes | Closed MH | 6/36 | 6/36 | 60 |
| 2 | 75 | M | RE | Idiopathic MH | A | 6/24 | 0 | CBA | Yes | 2 | AIR | SINGLE | SF6 | 75 | 3 | Yes | Closed MH | 6/36 | 6/36 | 9 |
| 3 | 77 | M | LE | Idiopathic MH | A | 6/120 | 0 | CBA | Yes | 2 | AIR | SINGLE | SF6 | 45 | 2 | Yes | Closed MH | 6/120 | 6/120 | 60 |
| 4 | 66 | F | LE | Idiopathic MH | A | 6/15 | 0 | CBA | Yes | 2 | BSS | SINGLE | C3f8 | 65 | 4 | Yes | Closed MH | 6/24 | 6/24 | 8 |
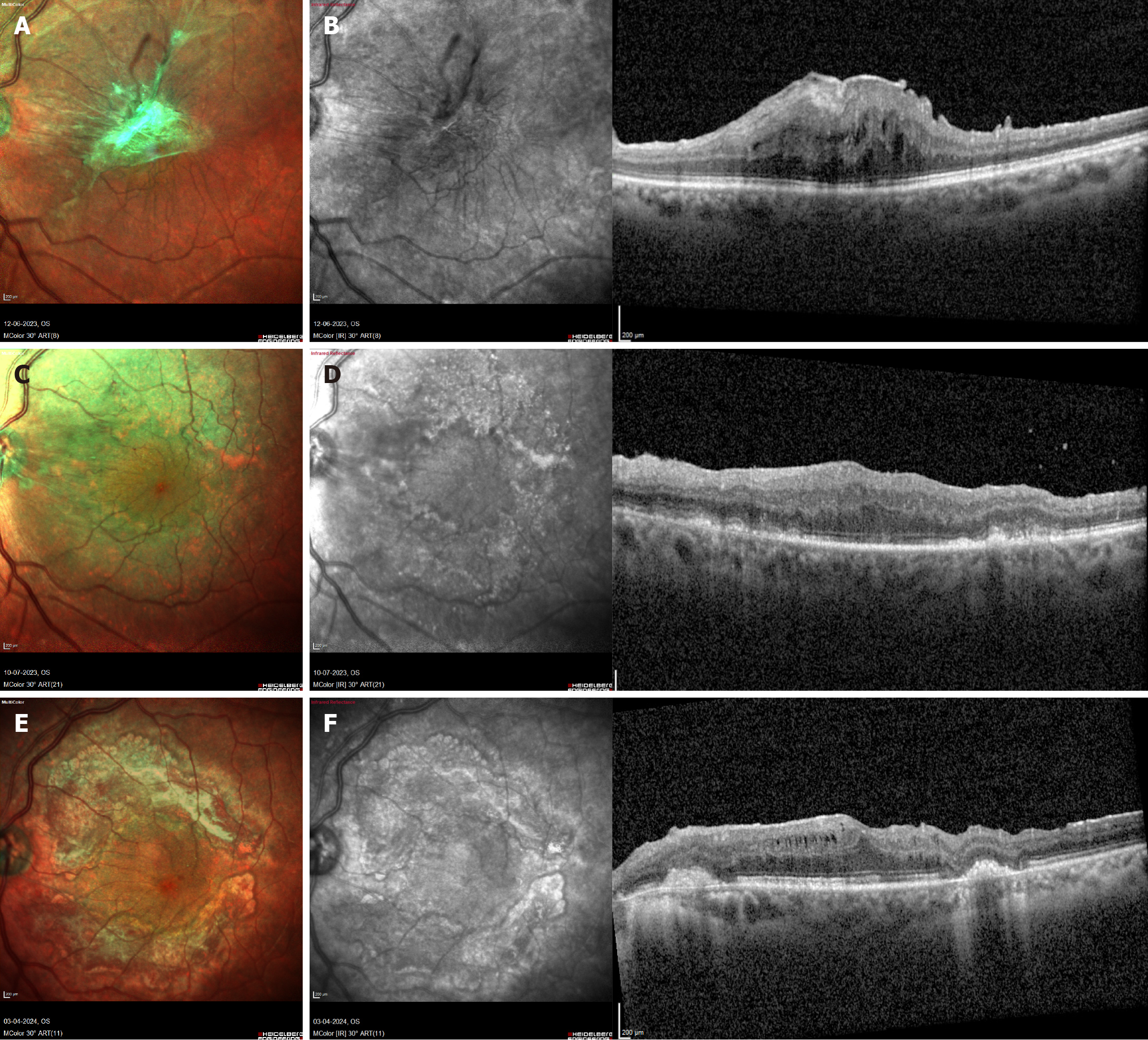
| 5 | 86 | F | LE | ERM with increased retinal traction | B | 6/120 | 515 | N | Yes | 2 | AIR | DOUBLE | SF6 | 90 | 1 | No | Retinal thickness reduced | 6/60 | 6/12 | 14 |
| 6 | 77 | M | RE | ERM with increased retinal traction | B | 6/60 | 518 | N | Yes | 2 | AIR | DOUBLE | SF6 | 60 | 1 | No | Retinal thickness reduced | 6/60 | 6/60 | 48 |
| 7 | 52 | F | RE | ERM with increased retinal traction | B | 6/30 | 957 | N | Yes | 2 | AIR | DOUBLE | AIR | 80 | 4 | No | Retinal thickness reduced | 6/30 | 6/30 | 22 |
| 8 | 67 | F | LE | ERM with increased retinal traction | B | 6/30 | 590 | N | Yes | 3.5 | AIR | DOUBLE | BSS | 110 | 1 | No | Retinal thickness reduced | 6/24 | 6/8 | 10 |
| 9 | 65 | F | LE | Vitreofoveal traction with increased retina thickness | B | 6/18 | 468 | N | Yes | 2 | BSS | SINGLE | C3f8 | 65 | 4 | No | Normal foveal contour | 6/9 | 6/9 | 5 |
Group A included four eyes (44%). Preoperative best-corrected visual acuity ranged from 20/50 to 20/400. Preoperative assessment of central macular thickness and outer retinal integrity was not feasible due to the presence of a MH; how
Group B comprised five eyes (56%). Preoperative best-corrected visual acuity ranged from 20/60 to 20/400, and central macular thickness measured 468-957 µm. The outer retinal layers and RPE reflectivity were intact on preoperative OCT in all cases. ILM staining with BBG was performed under air in four eyes (80%) and under BSS in one eye (20%). Staining was performed twice in four eyes (80%) and once in one eye (20%). The duration of dye exposure was 2 minutes in four cases (80%) and 3.5 minutes in one case (20%). Endotamponade selection included SF6 in two eyes (40%) and C3F8, BSS, and air in one eye each (20%). The median surgical duration was 80 minutes (range: 60-110 minutes). All cases de
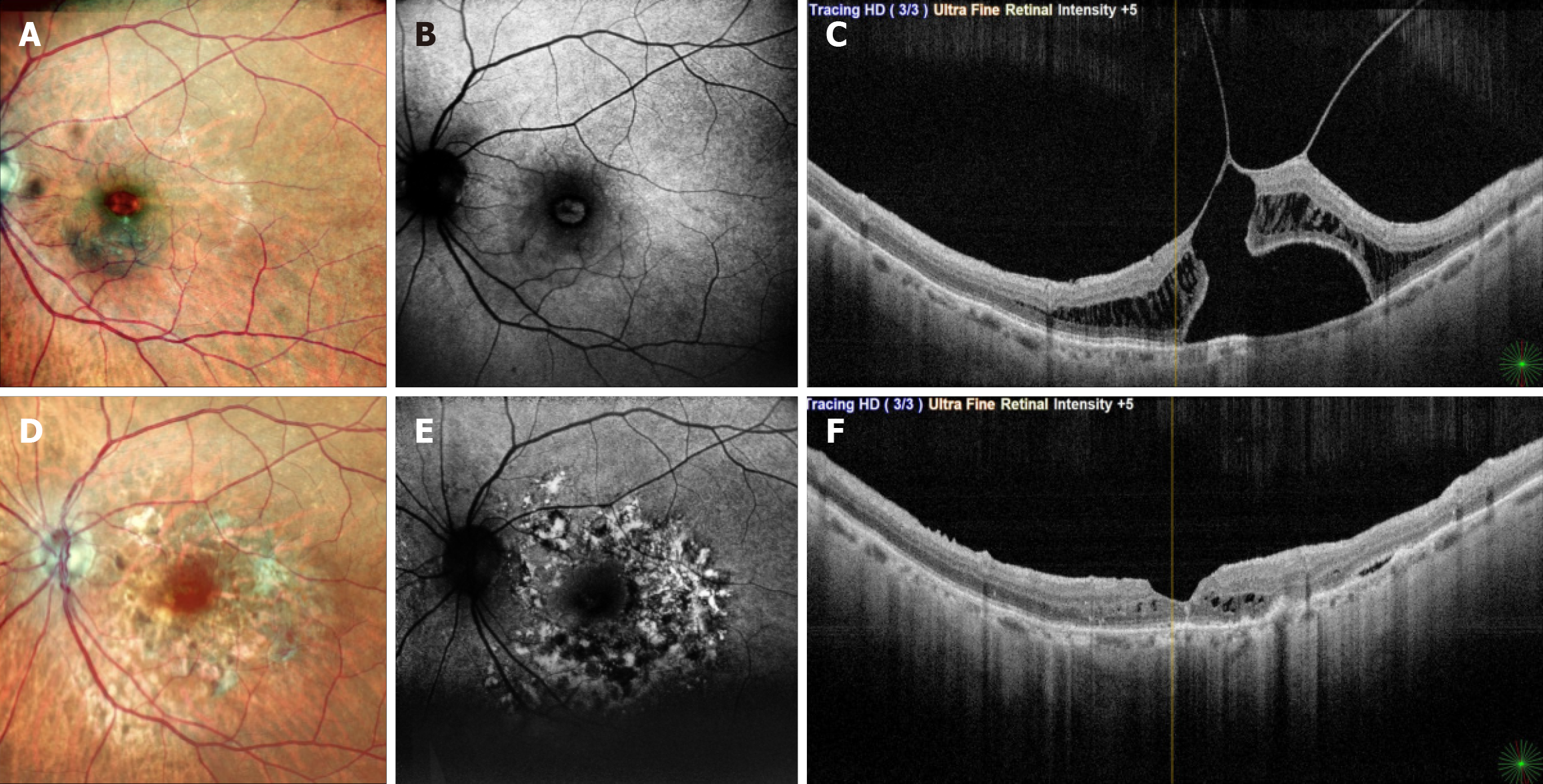
This study identified nine cases of BBG-related retinal phototoxicity following vitreoretinal surgery for macular disease. Among eyes undergoing MH repair, phototoxic damage involved the fovea in addition to surrounding macular areas, whereas foveal involvement was not observed in non-MH cases. This pattern is unexpected, as the fovea representing the thinnest retinal region with taller and densely pigmented RPE cells would typically be anticipated to demonstrate greater susceptibility to phototoxic injury.
Previous research has examined the relationship between BBG concentration, exposure duration, and retinal toxicity. In an in vitro study, Penha et al[15] demonstrated that BBG concentrations below 0.25 mg/mL were non-toxic to RPE cells and may even confer a protective effect through upregulation of the anti-apoptotic protein Bcl-2[15,16]. These findings suggest that BBG alone, at concentrations routinely used in macular surgery, is unlikely to induce retinal toxicity. Also, our study findings demonstrated that BBG-related retinal toxicity was not influenced by specific ILM staining techniques and indicated that repeated or prolonged BBG staining, regardless of the medium, was not solely responsible for retinal damage.
The main contributors to BBG-induced retinal toxicity appear to be the characteristics of endoillumination, particularly the intensity and duration of light exposure, as well as the proximity of the endoilluminator to the retinal surface during surgery[11,12,17]. In all cases included in the current series, the total surgical duration was prolonged, ranging from 45 minutes to 110 minutes. The cumulative photoreceptor and RPE injury observed may be attributable to prolonged focal high-intensity endoillumination during extended surgical time. The phototoxic effects of BBG can be attributed to its light absorption properties and emission spectrum, which ranges from 260 nm to 900 nm depending on the solvent used. All BBG solutions exhibit a double-peak absorption curve, with the first peak observed between 260 nm and 280 nm, and the second peak occurring between 540 nm and 680 nm[8]. Endoilluminator emitting light with wavelengths ≥ 400 nm is known to induce phototoxic effects, particularly in the RPE. The CONSTELLATION® Vision System from Alcon (TX, United States), which utilizes xenon light for endoillumination during vitreoretinal surgeries, has a peak wavelength of 450 nm, with a broader range from 420 nm to 700 nm[16]. Xenon light’s interaction with BBG plays a critical role in phototoxicity. The increased absorption of xenon light by BBG, coupled with alterations in BBG’s emission spectrum, leads to the production of these reactive oxygen species and toxic free radicals, which in turn cause damage to RPE cells and photoreceptors. In vitro studies using human RPE cells (ARPE-19) have demonstrated that BBG exposure sig
In this study, fovea-involving toxicity occurred exclusively in MH cases, whereas BBG-related toxic foveal damage was not observed in cases of ERM, MTM, ODPM, or MH-associated retinal detachment, despite routine ILM peeling with BBG in these conditions. This pattern suggests that greater retinal thickness may confer a protective effect against BBG-induced phototoxic injury, contributing to more positive visual outcomes. This observation may be explained by two mechanisms: (1) ERMs demonstrate poor affinity for BBG staining, reducing the amount of dye adhering to the foveal surface; and (2) Increased retinal thickness acts as a physical buffer, shielding the RPE from BBG-enhanced phototoxic stress. Conversely, in MH cases, the absence of foveal tissue leaves the BBG-coated RPE directly exposed to endoillumination, reactive oxygen species, and free radical formation, resulting in greater foveal damage and poorer functional recovery. Collectively, these findings highlight the protective role of retinal thickness and structural architecture in modulating foveal susceptibility to BBG-induced phototoxicity during vitreoretinal surgery.
Although BBG-related injury results in immediate cellular damage to photoreceptors and the RPE with corresponding functional impact, structural changes become clinically apparent only later and may be influenced by the type of endotamponade used[15,19]. In most macular procedures involving BBG-assisted ILM peeling, gas endotamponade is employed, which obscures early postoperative visualization and delays recognition of dye-related toxicity[20]. Structural alterations are more readily detected on OCT and FAF when the damage is parafoveal. In cases with fovea-involving toxicity, OCT proved more sensitive than FAF in detecting early structural disruption. This reduced FAF sensitivity likely reflects difficulty distinguishing true hypoautofluoroscence from damaged RPE vs the physiologic hypoautofluoroscence produced by the densely pigmented foveal RPE.
Additional assessment modalities such as OCT angiography, microperimetry, chromatic perimetry, and focal ele
This study is limited by its retrospective design, small sample size, and absence of a control group. Functional ass
BBG-related retinal phototoxicity may occur following ILM peeling, with eyes undergoing MH surgery appearing more vulnerable due to the absence of protective foveal neurosensory tissue. These findings suggest that underlying retinal architecture may influence susceptibility to dye- and light-related injury. Given the retrospective nature of this case series, the observations should be considered hypothesis-generating, and prospective studies incorporating functional and microvascular assessments are required to confirm these associations. Awareness of this potential risk may help guide surgeons toward adopting protective intraoperative strategies to preserve foveal integrity and optimize visual outcomes.
| 1. | Almony A, Nudleman E, Shah GK, Blinder KJ, Eliott DB, Mittra RA, Tewari A. Techniques, rationale, and outcomes of internal limiting membrane peeling. Retina. 2012;32:877-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Abdelkader E, Lois N. Internal limiting membrane peeling in vitreo-retinal surgery. Surv Ophthalmol. 2008;53:368-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Walia HS, Shah GK, Hariprasad SM. ILM peeling a vital intervention for many vitreoretinal disorders. Ophthalmic Surg Lasers Imaging Retina. 2014;45:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Hernández F, Alpizar-Alvarez N, Wu L. Chromovitrectomy: an update. J Ophthalmic Vis Res. 2014;9:251-259. [PubMed] |
| 5. | Bergamo VC, Caiado RR, Maia A, Magalhães O Jr, Moraes NSB, Rodrigues EB, Farah ME, Maia M. Role of Vital Dyes in Chromovitrectomy. Asia Pac J Ophthalmol (Phila). 2020;10:26-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Musat O, Stefan C, Boariu AM, Colta D, Cernat C, Alexandru L, Georgescu RD, Patoni IS, Timaru CM, De Algerino S. Chromovitrectomy. Rom J Ophthalmol. 2016;60:59-62. [PubMed] |
| 7. | Gandorfer A, Haritoglou C, Kampik A. Toxicity of indocyanine green in vitreoretinal surgery. Dev Ophthalmol. 2008;42:69-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Balaiya S, Sambhav K, Cook WB, Chalam KV. Osmolarity and spectrophotometric property of brilliant blue green define the degree of toxicity on retinal pigment epithelial cells exposed to surgical endoilluminator. Clin Ophthalmol. 2016;10:1543-1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Baba T, Hagiwara A, Sato E, Arai M, Oshitari T, Yamamoto S. Comparison of vitrectomy with brilliant blue G or indocyanine green on retinal microstructure and function of eyes with macular hole. Ophthalmology. 2012;119:2609-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Williamson TH, Lee E. Idiopathic macular hole: analysis of visual outcomes and the use of indocyanine green or brilliant blue for internal limiting membrane peel. Graefes Arch Clin Exp Ophthalmol. 2014;252:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Venkatesh R, Aseem A, Jain K, Yadav NK. Combined brilliant blue G and xenon light induced outer retinal layer damage following macular hole surgery. Indian J Ophthalmol. 2020;68:247-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Venkatesh R, Gupta A, Yadav NK, Chhablani J. Presumed Combined Brilliant Blue G and Endolight-Induced Macular Damage following Epiretinal Membrane Removal Surgery. J Curr Ophthalmol. 2022;34:267-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Venkatesh R, Mangla R, Sharief S, Chhablani J. Long-term effects of combined brilliant blue G and xenon light induced retinal toxicity following macular hole repair surgery. BMC Ophthalmol. 2023;23:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Ambiya V, Goud A, Khodani M, Chhablani J. Inner retinal thinning after Brilliant Blue G-assisted internal limiting membrane peeling for vitreoretinal interface disorders. Int Ophthalmol. 2017;37:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Penha FM, Pons M, Costa EF, Barros NM, Rodrigues EB, Cardoso EB, Dib E, Maia M, Marin-Castaño ME, Farah ME. Retinal pigmented epithelial cells cytotoxicity and apoptosis through activation of the mitochondrial intrinsic pathway: role of indocyanine green, brilliant blue and implications for chromovitrectomy. PLoS One. 2013;8:e64094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Costa Ede P, Rodrigues EB, Farah ME, Dib E, Penha F, Magalhães O Jr, Furlani BA, Lima Filho AA, de Miranda A, Maia M. Vital dyes and light sources for chromovitrectomy: comparative assessment of osmolarity, pH, and spectrophotometry. Invest Ophthalmol Vis Sci. 2009;50:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Soni A, Parameswarappa DC, Tyagi M, Sahoo NK, Dogra A, Pappuru RR, Chhablani J. Brilliant Blue G toxicity in macular hole surgeries: A report on combined phototoxicity and dye-induced macular damage. Semin Ophthalmol. 2022;37:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Gorgels TG, van Norren D. Ultraviolet and green light cause different types of damage in rat retina. Invest Ophthalmol Vis Sci. 1995;36:851-863. [PubMed] |
| 19. | Yang S, Zhou J, Li D. Functions and Diseases of the Retinal Pigment Epithelium. Front Pharmacol. 2021;12:727870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 20. | Engelmann K, Herbrig E. [Different endotamponade agents and their clinical indications]. Klin Monbl Augenheilkd. 2008;225:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/