Published online Feb 6, 2026. doi: 10.12998/wjcc.v14.i4.117226
Revised: January 6, 2026
Accepted: January 19, 2026
Published online: February 6, 2026
Processing time: 65 Days and 20.5 Hours
Conjunctival melanoma is rare and prone to local recurrence, and optimal sur
A middle-aged patient with a history of conservatively treated left Co-M was referred for suspected local recurrence, reporting a new palpable subcutaneous lump near the lateral orbital rim and a pigmented lesion at the medial canthus of the same eye. Clinical examination confirmed a periocular nodule suggestive of relapse. Cervical lymph node ultrasound showed no suspicious lymphadenopathy. UHFUS (48 MHz) with Doppler of the periocular region revealed epi
UHFUS can non-invasively detect and map recurrent Co-M, improving local staging and guiding multidisciplinary follow-up.
Core Tip: This case report describes recurrent conjunctival melanoma in a previously treated eye, evaluated with ultra-high-frequency ultrasound (UHFUS) (48 MHz). UHFUS accurately depicted subtle periocular epidermal thickening and multiple subepidermal nodules not fully appreciated on clinical examination, while confirming the absence of suspicious cervical lymph nodes. Integrating UHFUS into multidisciplinary follow-up refined local staging in a patient on anti-programmed cell death 1 therapy and supported an imaging-based, organ-preserving management strategy.
- Citation: Russo A, Patanè V, Pezzella MC, Troiani T, Argenziano G, Reginelli A. Ultra-high-frequency ultrasound in the detection of recurrent conjunctival melanoma: A case report. World J Clin Cases 2026; 14(4): 117226
- URL: https://www.wjgnet.com/2307-8960/full/v14/i4/117226.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i4.117226
Conjunctival melanoma (Co-M) is a rare but highly aggressive ocular surface malignancy arising from melanocytes within the conjunctival epithelium[1-4]. Although it accounts for only a small fraction of all melanomas, Co-M is associated with high rates of local recurrence, regional lymph node metastasis, and distant spread, leading to substantial disease-specific mortality[5-7]. These features underscore the need for accurate local staging and long-term surveillance, particularly after conservative, eye-preserving treatments.
Current follow-up of Co-M relies mainly on slit-lamp biomicroscopy, anterior segment optical coherence tomography, and conventional B-scan ultrasonography[8,9]. However, these tools may be suboptimal for detecting small, subepidermal periocular nodules or satellite lesions after prior treatments and in the context of systemic immunotherapy[10-12]. Only limited reports have described the use of high-frequency ultrasound (HFUS) for periocular tumors, and its role in mapping recurrent Co-M remains poorly characterized[13].
Here, we report the case of a patient with previously treated left-eye Co-M who developed a suspected periocular recurrence while on anti-programmed cell death 1 (anti-PD-1) therapy and underwent 48-MHz HFUS evaluation. Our aim was to explore whether HFUS could identify clinically occult periocular recurrences, refine local staging, and inform multidisciplinary management. We hypothesized that HFUS would detect additional subepidermal nodules beyond clinical examination, thereby addressing a specific and increasingly relevant clinical problem: Surveillance of recurrent Co-M in the era of immunotherapy.
A patient with a history of conservatively treated Co-M of the left eye, previously managed at another institution, was referred to our high-frequency ultrasound unit at the University Hospital of Campania “Luigi Vanvitelli” for evaluation of a palpable subcutaneous nodule in the left periocular region, clinically suspicious for local recurrence.
The patient was receiving first-line systemic immunotherapy with an anti-PD-1 monoclonal antibody for the management of Co-M according to oncologic indications.
The most relevant past medical history was the previously treated left-eye Co-M, managed with eye-preserving, conservative treatment. Apart from ongoing anti-PD-1 immunotherapy, no other systemic therapies, depot injections, or herbal or over-the-counter medications were reported as influencing the clinical course. There was no history of drug allergies or previous adverse drug reactions relevant to the current presentation.
No additional prior ocular tumors, hereditary cancer syndromes, or familial ocular diseases were reported as contributing to the current condition. Details of other systemic comorbidities, social habits, dietary history, and family history were not contributory to the present case.
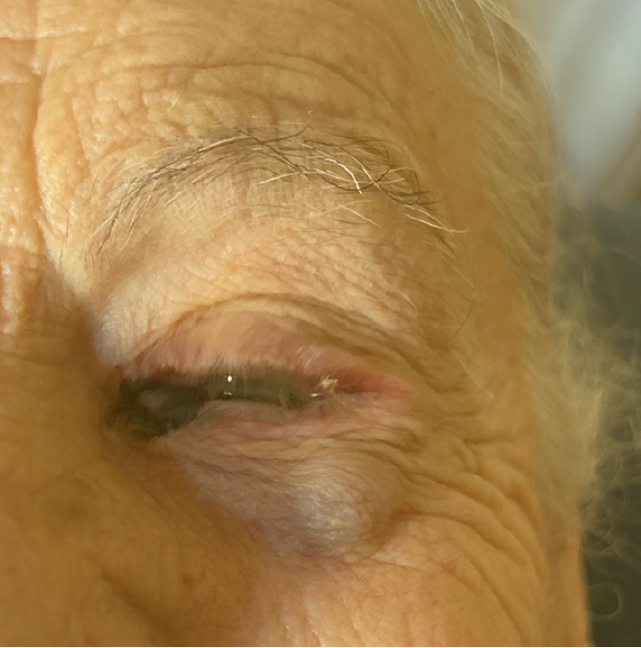
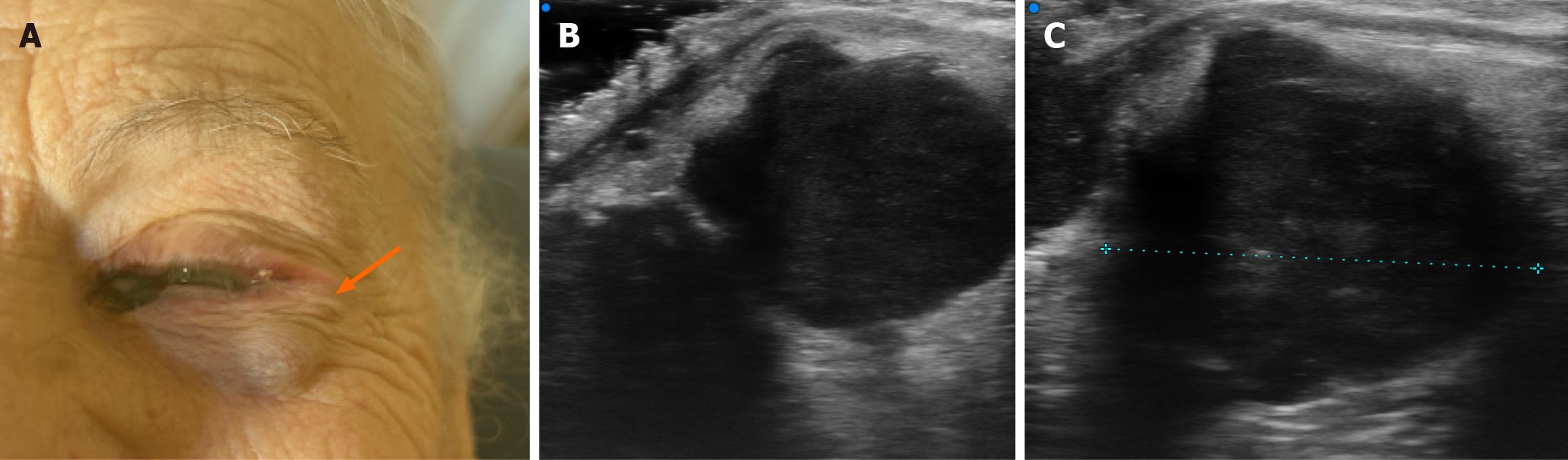
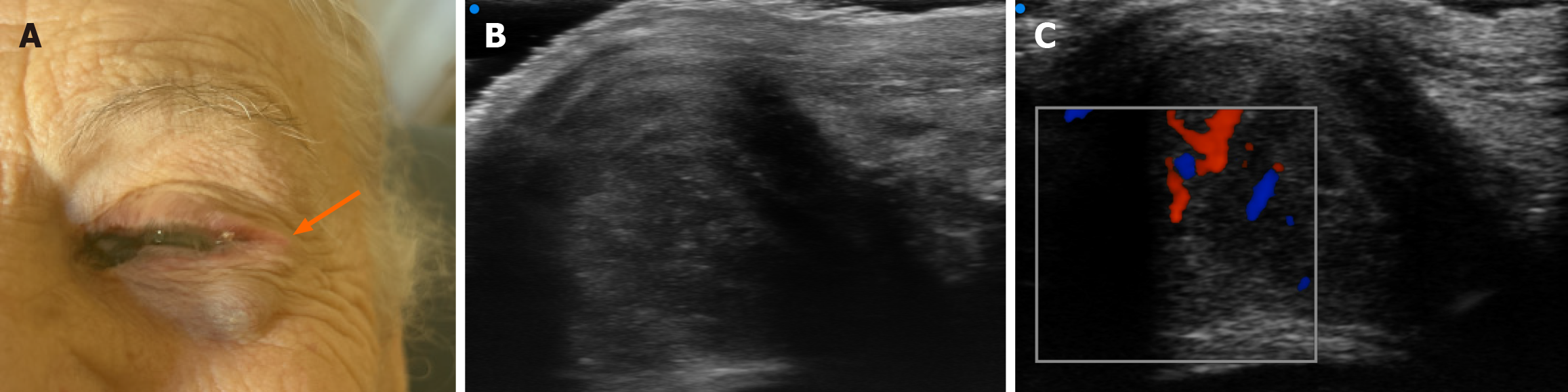
At referral, ophthalmologic examination revealed a pigmented lesion at the medial conjunctival canthus of the left eye associated with a clearly visible and palpable subcutaneous nodule along the lateral orbital rim, raising suspicion of local relapse (Figure 1).
Routine laboratory tests, including hematologic and biochemical parameters, did not reveal abnormalities that impacted diagnostic or therapeutic decision-making.
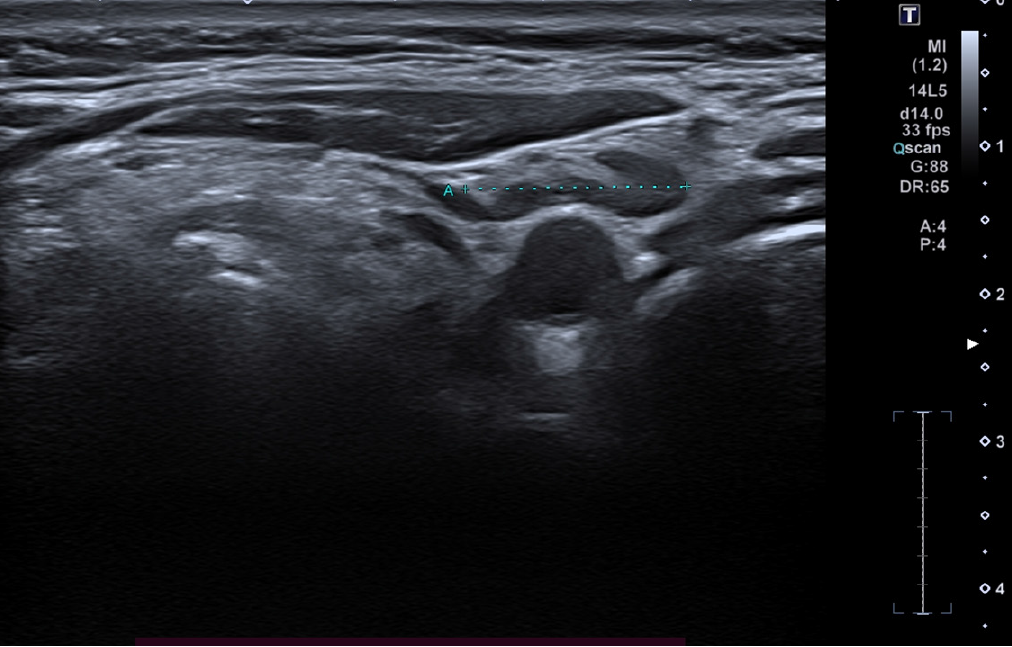
As part of the restaging work-up, high-frequency ultrasound (24 MHz) of the cervical lymph node stations was per
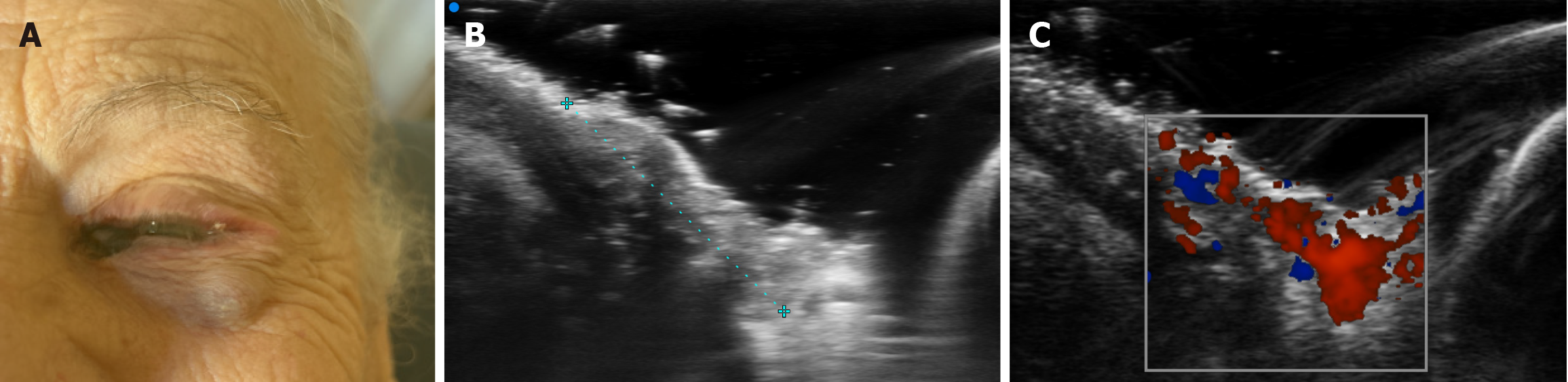
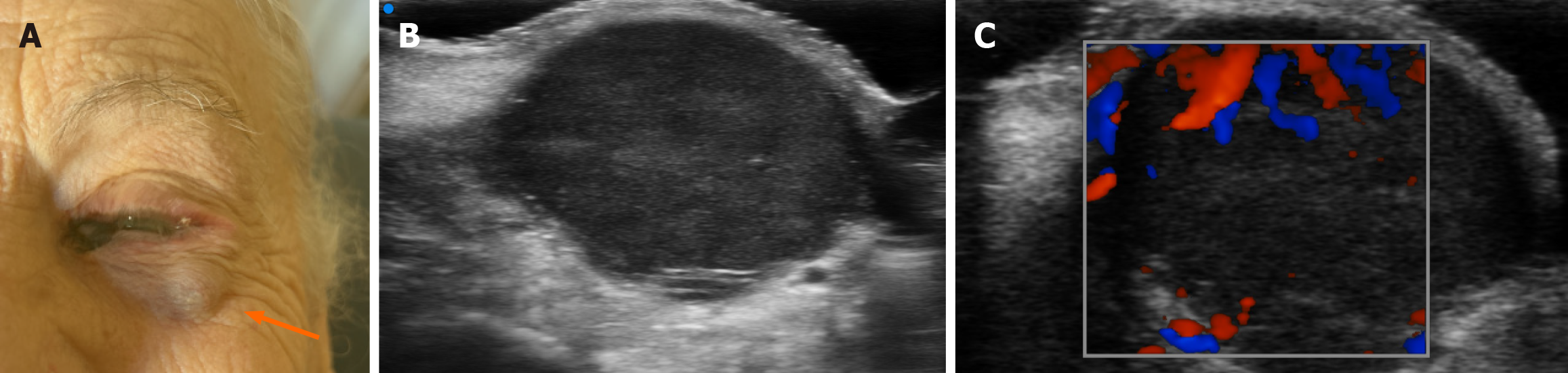
At the medial canthus/epicanthus region, UHFUS showed epidermal thickening extending into the hypodermis with disorganized echotexture and irregular margins, superficial to the lacrimal canal, with increased Doppler signal suggestive of neovascularization (Figure 3). In correspondence with the palpable nodule of the lower eyelid, a well-demarcated, subepidermal hypoechoic lesion measuring approximately 9 mm × 8 mm with scattered hyperechoic foci was visualized, consistent with a suspected conjunctival satellite metastasis; color Doppler demonstrated increased and accelerated perilesional vascularization (Figure 4). A second hypoechoic nodule with internal hyperechoic spots, located deeper in the subcutaneous tissue adjacent to the first satellite lesion, was identified, with a maximum diameter of about 12 mm × 10 mm (Figure 5). A third lesion at the lateral canthus appeared as a subepidermal nodule with irregular margins and slightly higher echogenicity than the other nodules, showing predominantly intralesional vascularization on Doppler evaluation (Figure 6). Taken together, UHFUS with color Doppler demonstrated multiple subepidermal and conjunctival nodules, including clinically occult lesions, with morphologic and vascular features highly suggestive of recurrent Co-M.
The cervical lymph node ultrasound and subsequent 48 MHz UHFUS examinations of the periocular region were requested as part of the restaging work-up under ongoing immunotherapy, with the aim of better defining local and regional disease extent and supporting multidisciplinary therapeutic planning.
Recurrent Co-M of the left eye with multiple periocular subepidermal satellite nodules demonstrated by UHFUS, without ultrasonographic evidence of cervical lymph node metastasis in a patient on anti-PD-1 immunotherapy.
At the time of referral, the patient was already undergoing first-line systemic therapy with an anti-PD-1 monoclonal antibody for Co-M. This case report focuses on the imaging-based assessment and mapping of recurrent lesions; detailed description of subsequent local therapeutic interventions (such as surgery or radiotherapy), if any, is beyond the scope of the present report.
Cervical lymph node ultrasound confirmed the absence of sonographic signs of nodal metastasis, while UHFUS (48 MHz) of the periocular region demonstrated multiple subepidermal nodules with morphologic and vascular features highly suggestive of recurrent Co-M, including clinically occult satellite lesions. These findings refined local and regional staging and supported multidisciplinary planning of further management while the patient was receiving ongoing anti-PD-1 immunotherapy. The present report focuses on the diagnostic contribution of UHFUS; detailed long-term clinical outcomes and extended follow-up imaging are not reported.
Co-M is a rare but aggressive ocular surface malignancy with substantial risks of local recurrence, lymphatic spread, and melanoma-related mortality. Reported local recurrence rates remain high, ranging from approximately 20% in more recent large series to over 50%-60% in older cohorts, despite advances in surgery and adjuvant therapies[14]. These data support the need for meticulous long-term surveillance, particularly in patients treated with eye-preserving strategies and in those receiving systemic checkpoint inhibitors, in whom patterns of local and in-transit recurrence may be complex[15].
In current practice, follow-up of Co-M relies mainly on slit-lamp biomicroscopy, anterior segment optical coherence tomography, ultrasound biomicroscopy (UBM), and cross-sectional imaging when indicated. High-frequency ultrasound (HFUS, approximately 20-50 MHz) and UBM have been used to measure conjunctival tumor thickness, assess scleral or intraocular invasion, and guide surgical planning[16]. However, their role in systematically mapping small, subepidermal periocular satellites or recurrent nodules-especially in previously treated, complex anatomical areas such as the canthi and eyelid skin-has been only sparsely addressed[17,18]. In parallel, dermatologic literature has shown that HFUS and UHFUS (≥ 40 MHz) can non-invasively characterize cutaneous malignancies, defining lesion depth, margins, and vas
Our case adds to this body of work by illustrating how 48 MHz UHFUS with Color Doppler can be applied to a patient with previously treated Co-M on anti-PD-1 therapy, in whom both a pigmented medial canthal lesion and a lateral periocular nodule raised suspicion of recurrence. Unlike most prior ophthalmic reports where HFUS/UBM was primarily used preoperatively to assess conjunctival tumors and intraocular extension[16], here UHFUS was used in a surveillance setting to systematically scan the entire periocular region. This approach revealed not only the clinically evident lesions but also additional subepidermal nodules with malignant-appearing morphology and vascular patterns, effectively “mapping” a multifocal periocular recurrence that would likely have been underestimated by clinical examination alone. The negative 24 MHz cervical lymph node ultrasound, consistent with the absence of regional metastases, further refined staging in a non-invasive manner.
From a temporal perspective, the sequence of events-primary Co-M treated conservatively, a disease-free interval, then development of new periocular nodules in the same anatomical region during follow-up-supports a strong temporal association between the current lesions and tumor recurrence. This is biologically plausible given the known propensity of Co-M for local recurrence and periocular spread. Morphologically, the hypoechoic subepidermal nodules with ir
| Ultrasound feature (HFUS/UHFUS ± Doppler) | Melanoma/malignant melanocytic lesion (suggestive) | Benign melanocytic lesion (more typical) |
| Overall shape/symmetry | Asymmetric, irregular or multilobulated | Symmetric, round/oval |
| Margins | Irregular, ill-defined, infiltrative appearance | Smooth, well-defined |
| Echogenicity | Markedly hypoechoic relative to surrounding tissue | Mildly hypoechoic to isoechoic |
| Internal echotexture | Heterogeneous; disorganised echoes; possible internal hyperechoic foci | Homogeneous or finely granular; usually no marked heterogeneity |
| Intralesional cystic spaces (especially conjunctival nevi) | Usually absent | Often present (small anechoic cysts), particularly in conjunctival nevi |
| Epithelial/epidermal changes | Thickening and/or architectural disruption; possible ulceration | Epidermis/epithelium usually preserved; no disruption |
| Depth/thickness | Greater thickness; extension into hypodermis/subcutaneous tissue; loss of normal planes | Limited depth; confined to superficial dermis/subepithelial tissue; preserved planes |
| Vascularity (color/power Doppler) | Increased intra- and/or perilesional flow; tortuous vessels; higher flow signals | Absent or minimal flow; if present usually faint/peripheral |
| Perilesional reaction | Perilesional edema or architectural distortion may be present | No significant perilesional reaction |
| Multiplicity/satellites | Possible satellite or in-transit nodules; multifocality raises suspicion | Typically solitary; satellites uncommon |
| Change over time (if serial scans available) | Rapid growth and/or increasing vascularity | Stability over time |
This case also raises the question of how best to integrate UHFUS into the multidisciplinary management of Co-M in the era of immunotherapy. Checkpoint inhibitors such as anti-PD-1 agents are increasingly used in advanced or recurrent ocular melanomas and can achieve durable responses, but patterns such as pseudoprogression and mixed responses are recognized in cutaneous melanoma[25]. In our patient, the detection of multiple periocular nodules during ongoing anti-PD-1 therapy emphasizes that local disease may progress despite systemic treatment and that precise local staging remains crucial for planning potential surgery, radiotherapy, or other focal interventions. UHFUS could be particularly valuable for: (1) Baseline mapping before starting systemic therapy; (2) Early detection of subtle new lesions; and (3) Monitoring response of periocular nodules when organ-preserving strategies are pursued.
Several limitations of this case report must be acknowledged. First, it is a single-patient observation from a tertiary referral setting, which limits generalizability. Second, histopathologic confirmation for all UHFUS-detected nodules is not available, preventing a direct one-to-one correlation between imaging and pathology; this is a common constraint in imaging-based follow-up, especially when aggressive surgery is avoided. Third, UHFUS is highly operator-dependent and not yet widely available in ophthalmology or dermatology units; standardized acquisition protocols and lesion descriptors for conjunctival and periocular melanoma are still lacking. Fourth, long-term clinical and imaging follow-up data are limited in this report, so the impact of UHFUS-guided staging on ultimate outcomes (local control, eye preservation, survival) cannot be fully assessed. These methodological limitations mean that our findings should be interpreted as hypothesis-generating rather than definitive.
Despite these constraints, the case has several strengths that support its validity and clinical relevance: (1) A clear temporal sequence from primary Co-M to periocular recurrence; (2) Concordant clinical and sonographic findings across multiple, spatially related nodules; (3) Integration of regional nodal assessment with cervical ultrasound; and (4) Use of a very high-frequency (48 MHz) probe with Doppler, enabling simultaneous evaluation of micro-morphology and vas
| 1. | Russo A, Marinelli L, Patanè V, Alessandrella M, Pezzella MC, Troiani T, Brancaccio G, Scharf C, Argenziano G, Cappabianca S, Reginelli A. Whole-body magnetic resonance imaging for cutaneous melanoma staging: A scientific review. World J Clin Oncol. 2025;16:109206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Papaoikonomou MA, Pavlidis L, Apalla Z, Papas A. Conjunctival Melanoma: A Narrative Review of Current Knowledge. Pigment Cell Melanoma Res. 2025;38:e70006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 3. | Yohannes GB, Scott NL, Li WJ, Karp CL. Systemic immune checkpoint inhibitors: Successful treatment of conjunctival atypical melanocytic proliferation documented by anterior segment optical coherence tomography. Ocul Surf. 2025;38:184-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Churchill RA, Yu CY, Wang KY, Kim BM, Wagner LH, Bradley EA, Tooley AA, Dalvin LA. Clinical features and outcomes of melanoma involving eyelid and conjunctiva. Can J Ophthalmol. 2025;S0008-4182(25)00321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Nguyen NH, Nguyen MP, Mai HK, Do ST, Pham VH, Vuong DTP, Maturi JR, Le HVT, Cluskey PM, Pham VT. The correlation of clinical and histopathological features of eyelid malignancies: a 5-year retrospective study in Vietnam. Int Ophthalmol. 2025;45:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Okongwu CC, Adewara BA, Olaofe OO, Soremekun AI, Ayodele SO, Abdullahi YO, Ewoye EE, Oladele JO. Malignant melanoma of the conjunctiva metastasizing to the submandibular gland: a case report and review of the literature. BMC Ophthalmol. 2025;25:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Qaddoumi AI, Evans WI, Wilson MW. A case of cutaneous melanoma metastatic to the ciliary body and choroid with complete regression via systemic dual checkpoint inhibitor therapy. BMC Ophthalmol. 2025;25:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Vizvári E, Skribek Á, Polgár N, Vörös A, Sziklai P, Tóth-Molnár E. Conjunctival melanocytic naevus: Diagnostic value of anterior segment optical coherence tomography and ultrasound biomicroscopy. PLoS One. 2018;13:e0192908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Troisi M, Vitiello L, Lixi F, Timofte Zorila MM, Abbinante G, Pellegrino A, Namazbayeva A, Adamo GG, Coco G, Cuccu A, Giannaccare G. Clinical Applications of Optical Coherence Tomography and Optical Coherence Tomography Angiography in Uveal Melanoma: A Narrative Review. Diagnostics (Basel). 2025;15:2421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Janowska A, Oranges T, Granieri G, Romanelli M, Fidanzi C, Iannone M, Dini V. Non-invasive imaging techniques in presurgical margin assessment of basal cell carcinoma: Current evidence. Skin Res Technol. 2023;29:e13271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Tao S, Tian Z, Bai L, Wang W, Xu Y, Kuang C, Liu X. Tri-directional x-ray phase contrast multimodal imaging using one hexagonal mesh modulator. Phys Med Biol. 2023;68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Dobre EG, Surcel M, Constantin C, Ilie MA, Caruntu A, Caruntu C, Neagu M. Skin Cancer Pathobiology at a Glance: A Focus on Imaging Techniques and Their Potential for Improved Diagnosis and Surveillance in Clinical Cohorts. Int J Mol Sci. 2023;24:1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Murtagh P, O'Riordan MM, O'Neill V, Cunningham M, D'Arcy F, Eleuteri A, Greene A, Baily C, Kennedy S, Hussain R, Heimann H, Horgan N. Tumour control, eye retention and visual acuity after radiotherapy for choroidal melanoma. BMJ Open Ophthalmol. 2026;11:e002291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Jain P, Finger PT, Fili M, Damato B, Coupland SE, Heimann H, Kenawy N, J Brouwer N, Marinkovic M, Van Duinen SG, Caujolle JP, Maschi C, Seregard S, Pelayes D, Folgar M, Yousef YA, Krema H, Gallie B, Calle-Vasquez A; American Joint Committee on Cancer Ophthalmic Oncology Task Force. Conjunctival melanoma treatment outcomes in 288 patients: a multicentre international data-sharing study. Br J Ophthalmol. 2021;105:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Butt K, Hussain R, Coupland SE, Krishna Y. Correction: Butt et al. Conjunctival Melanoma: A Clinical Review and Update. Cancers 2024, 16, 3121. Cancers (Basel). 2024;16:3721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Finger PT, Tran HV, Turbin RE, Perry HD, Abramson DH, Chin K, Della Rocca R, Ritch R. High-frequency ultrasonographic evaluation of conjunctival intraepithelial neoplasia and squamous cell carcinoma. Arch Ophthalmol. 2003;121:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Guo YW, Rokohl AC, Kopecky A, Heindl LM. Periocular basal cell carcinoma-current treatment concepts. Ann Eye Sci. 2021;6:18. [DOI] [Full Text] |
| 18. | Caviglia M, Kaleci S, Frascione P, Teoli M, Fargnoli MC, Pellacani G, Mandel VD. A Systematic Review and Meta-Analysis of Ocular and Periocular Basal Cell Carcinoma with First-Time Description of Dermoscopic and Reflectance Confocal Microscopy Features of Caruncle Basal Cell Carcinoma. Diagnostics (Basel). 2025;15:1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Chen G, Luo H, Liu W, Liao X, Meng J, Qiu Z, Leng X. Diagnostic Performance of High-Frequency Ultrasound and Ultra-High-Frequency Ultrasound in Distinguishing Dermatofibrosarcoma Protuberans from Dermatofibroma: A 15-year Period Retrospective Analysis. Clin Cosmet Investig Dermatol. 2025;18:3621-3634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Yu J, Xu X, Gao X, Li X, Zhang T, Chen Y, Zhu Y. [Clinical study on the application of ultra-high-frequency ultrasonography in the diagnosis and treatment of recurrent preauricular sinus]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2026;40:76-79;83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Russo A, Patanè V, Fusco L, Faggioni L, Boschetti CE, Santagata M, Neri E, Cappabianca S, Reginelli A. Reliability of Ultrasonographic Assessment of Depth of Invasion and Tumor Thickness in Intraoral Mucosa Lesions: A Preliminary Experience. J Clin Med. 2024;13:2595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Russo A, Patanè V, Gagliardi F, Urraro F, Ronchi A, Vitiello P, Sica A, Argenziano G, Nardone V, Reginelli A. Preliminary Experience in Ultra-High Frequency Ultrasound Assessment of Cutaneous Primary Lymphomas: An Innovative Classification. Cancers (Basel). 2024;16:2456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Hara H, Mihara M. Beyond Ultra-high Frequency: Clinical Feasibility of 10-12 MHz and Portable Ultrasound in Lymphatic Imaging. Plast Reconstr Surg Glob Open. 2025;13:e7357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Płocka M, Czajkowski R. High-frequency ultrasound in the diagnosis and treatment of skin neoplasms. Postepy Dermatol Alergol. 2023;40:204-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 25. | Finger PT, Pavlick AC. Checkpoint inhibition immunotherapy for advanced local and systemic conjunctival melanoma: a clinical case series. J Immunother Cancer. 2019;7:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/