Published online Jan 26, 2026. doi: 10.12998/wjcc.v14.i3.114691
Revised: November 18, 2025
Accepted: January 8, 2026
Published online: January 26, 2026
Processing time: 118 Days and 21.9 Hours
Anxiety disorders are highly prevalent in patients with bipolar disorder (BD) and are associated with a more severe illness course and poorer outcomes. A sig
To investigate whether early intervention has a more positive outcome for anxiety disorders in patients who present with clinical high risk factors for BD.
A total of 66 patients were enrolled in this study from January 2021 and December 2022 in Huzhou Third Mu
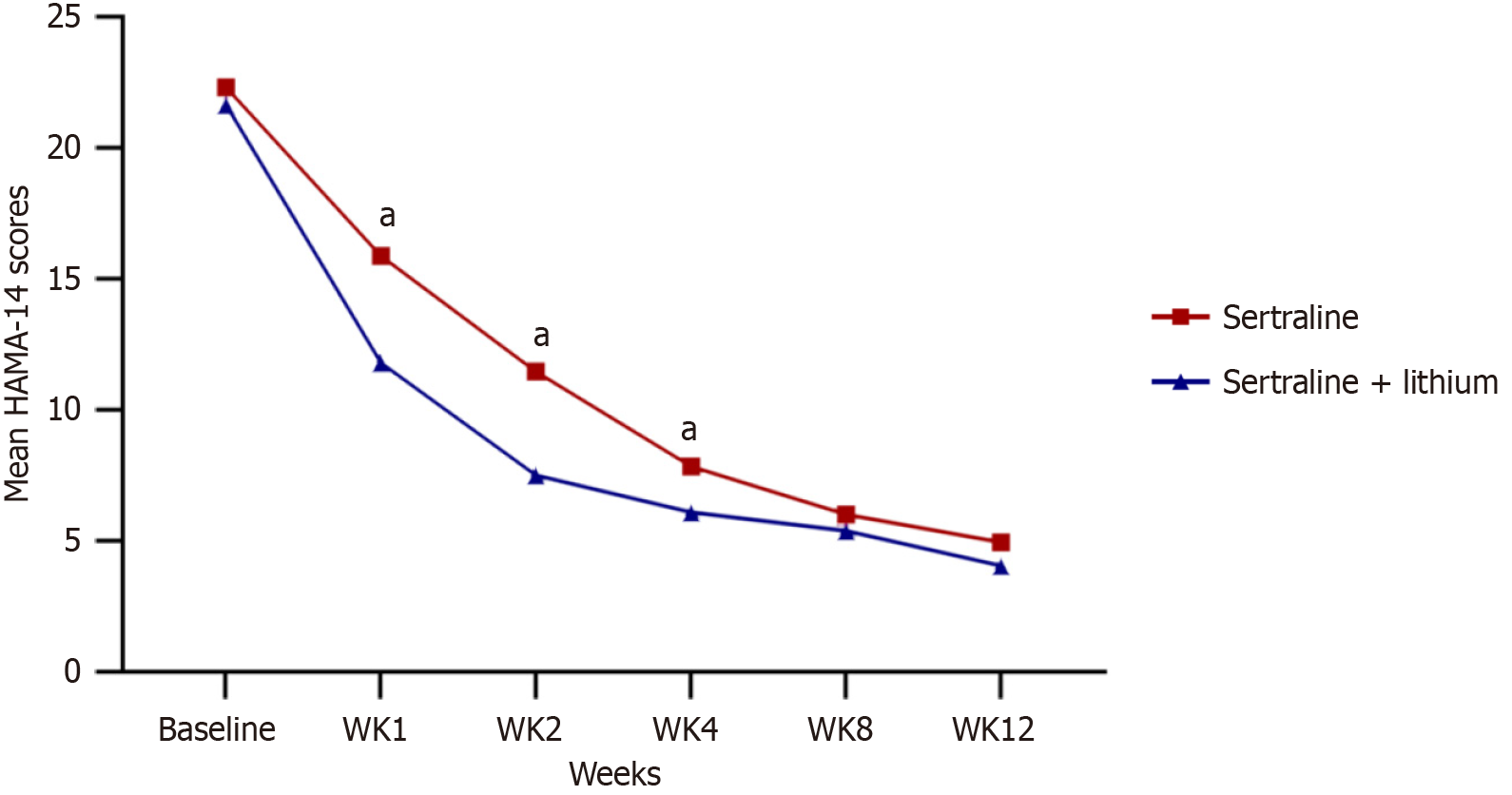
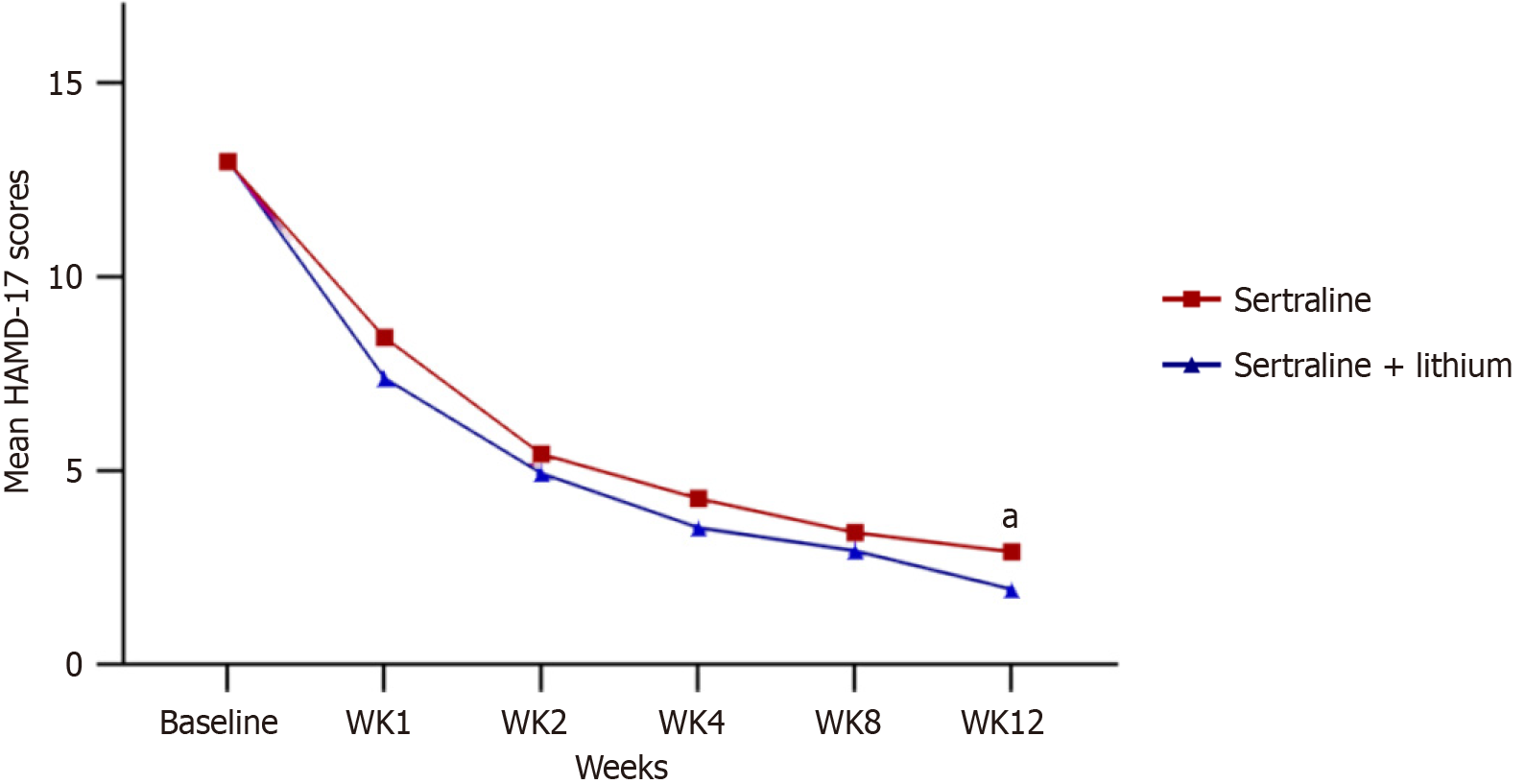
Significant differences in the change of Hamilton Anxiety Rating Scale scores were observed between the two groups at week 1, week 2, and week 4 (P < 0.05). However, after 8 weeks and 12 weeks of treatment, there were no significant different (P = 0.485 and P = 0.206). There was no significant difference in the change over time in Hamilton Depression Rating Scale scores between the treatment groups (P = 0.2), except at week 12 (P = 0.034). No significant differences were observed in the adverse effects reported between patients treated with sertraline alone (18%) and those treated with the combination therapy (21%).
This current double-blind, case-controlled study assessed the effectiveness and tolerability of combined therapy vs monotherapy for anxiety disorder in patients with clinical high-risk factors for BD. In light of the constraints associated with this initial study, the results imply that the combination of sertraline and lithium may provide a more favorable prognosis.
Core Tip: This randomized controlled trial investigated early intervention in 66 antidepressant-naive adults with anxiety disorders who also presented clinical high-risk factors for bipolar disorder. The combination of sertraline and lithium demonstrated a significantly faster reduction in anxiety symptoms within the first four weeks compared to sertraline monotherapy, with comparable safety profiles. These preliminary findings suggest that early adjunctive mood stabilization may accelerate response and potentially mitigate the risk of manic switch in this clinically complex and high-risk population, warranting further investigation in larger trials.
- Citation: Wang H, Wang SL, Ge CJ, Lei LL, Zeng L, Qian MC. Early intervention in anxiety disorder patients with clinical high-risk factors for bipolar disorder: A randomized controlled trial. World J Clin Cases 2026; 14(3): 114691
- URL: https://www.wjgnet.com/2307-8960/full/v14/i3/114691.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i3.114691
Bipolar disorder (BD) is well known for its high comorbidity and misdiagnosis rate, as well as the long delay in diagnosis[1,2]. Recent studies have shown that the occurrence rate of comorbidity in BD, specifically anxiety disorders, throughout a person’s lifetime is 40.5%, while the current prevalence is 38.2%[3]. Several factors have been discovered to impact the coexistence of anxiety disorders in individuals with BD, including the age when symptoms begin, substance use disorders, and the occurrence of manic episodes or psychotic features. Anxiety is highly prevalent among patients with BD and has been associated with significantly lower quality of life and adverse outcomes from the disease[4]. For instance, comorbid generalized anxiety disorder has been correlated with a more severe course of BD, a greater oc
One common phenomenon in clinical practice is the elevated prevalence of misdiagnosis or prolonged delays in diagnosing BD. This is often attributed to the reliance on diagnosis methods such as the International Classification of Diseases or the Diagnostic and Statistical Manual of Mental Disorders criteria, as well as clinical guidelines based on doctors' subjective opinions[2]. The absence of accurate biomarkers further complicates the selection of appropriate therapeutic regimens.
Identifying BD and its co-occurring condition, such as anxiety disorder, at the right time is crucial for various clinical reasons. Such identification allows for a better understanding of the symptoms, progression, treatment, and overall outcome of the disorder. Furthermore, there has been significant research interest in detecting prodromal symptoms of BD, as early intervention could potentially enhance therapeutic efficacy and prognosis[7,8]. It is worth noting that the presence of anxiety disorders during childhood and adolescence can heighten the possibility of developing BD in later stages of life. Therefore, targeting anxiety disorders for early intervention may be a practical approach for individuals with a heightened susceptibility to BD[9].
Previous studies have identified several clinical high-risk factors for BD. These include young onset age (before 18 years old), a family history of BD, noticeable negative thoughts and behaviors, and symptoms of subthreshold mania[10,11]. Limited research and clinical guidelines exist regarding the treatment of anxiety disorders in patients with clinical high-risk factors for BD. Lithium salt, a well-established medication for BD, can be used in various phases of the disorder, including acute and consolidation phases, as well as for the prevention of suicidal risk[12,13]. First-line treatments for anxiety disorder typically involve a combination of pharmacotherapy and psychotherapy. Selective serotonin reuptake inhibitors such as sertraline and serotonin-norepinephrine reuptake inhibitors like venlafaxine extended release are recommended as first-line pharmacotherapy for generalized anxiety disorder, social anxiety disorder, and panic disorder. Sertraline, in particular, is known to be better tolerated compared to other antidepressants[14-16].
The purpose of this study was to investigate the effectiveness of antidepressants and mood stabilizers in treating anxiety disorders that are associated with clinical high-risk factors for BD, in order to gather evidence that can support the early identification and treatment of patients who have potential BD but are initially diagnosed with anxiety disorder.
Inclusion criteria: Participants aged between 18 and 60, diagnosed with an anxiety disorder according to the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013), were included in this research study. The clinical high-risk factors were confirmed using a questionnaire. Participants needed to meet at least one of the following high-risk factors: Age of onset below 18, positive family history of BD, significant negative beliefs and/or behaviors, and subthreshold symptoms of mania, defined as a score between 1 and 5 on the Bech-Rafaelsen Mania Rating Scale (BRAMS; see Supplementary material for details). This assessment was carried out by an experienced clinical psychiatrist (holding an intermediate professional title or above). Enrollment occurred between January 2021 and December 2022 in Huzhou Third Municipal Hospital. The study adhered to the principles of the Declaration of Helsinki and relevant clinical practice guidelines. All participants were required to be psychiatric medication-naïve or to have discontinued any psychotropic medication for at least 2 weeks prior to enrollment. The study protocol was approved by the Huzhou Third Municipal Hospital Ethics Committee. All participants, together with their legal guardians where applicable, provided written informed consent prior to the commencement of the study. The original study, registered at Trials.gov (Identifier: 2020GZ42), was overseen by the Hospital Data and Safety Monitoring Board.
Excluded criteria: The exclusion criteria comprised: (1) Subjects with a past record of drug abuse, alcohol abuse, or other psychoactive substance abuse; (2) individuals with a history of significant medical or neurological ailments, any substantial period of unconsciousness lasting over 10 minutes, or a positive pregnancy test at the beginning of the study; (3) those with clinically significant laboratory or imaging abnormalities; (4) those who do not abide by the examination or cannot complete the examination due to impairments of consciousness, vision, hearing, understanding, and language expression; (5) patients with serious physical diseases (including severe cardiopulmonary, liver, and kidney dysfunction, shock, malignancies, blood diseases, and autoimmune diseases); and (6) those who received immunomodulatory therapy or regular antipyretic/analgesic medication within the six months prior to enrollment.
Using Stata software, participants were randomized via permuted blocked randomization, with varying block sizes, into two distinct groups: Group A (sertraline plus placebo) and group B (sertraline plus lithium carbonate). To preserve blinding, the treatment assignment was concealed from the participants, principal investigator, clinical assessors, and data collectors. The pills were encapsulated excessively for this purpose.
Participants in group A initiated treatment with a daily dose of 50 mg of sertraline and placebo (with a maximum sertraline starting dose of 150 mg taken once daily), while group B received a daily dose of 50 mg of sertraline and 300 mg of lithium carbonate (with a maximum lithium carbonate starting dose of 300 mg taken twice daily). To ensure to
In order to be eligible for participation, all participants were required to have a Hamilton Anxiety Rating Scale (HAMA) score of at least 14 and a Hamilton Depression Rating Scale score of no more than 17[17,18]. Participants were also required to have a baseline BRMAS score under 6[15]. At baseline, socio-demographic characteristics were recorded through self-report questionnaires at each site, with research assistants obtaining supplementary information through further assessments.
Statistical analyses were performed under blinded conditions using SPSS (version 25). We applied a significance level of P < 0.05 throughout the analysis. To compare the baseline variables between the two groups receiving distinct treatments, we employed either the analysis of variance (ANOVA) or the Pearson’s χ2 test. The primary objective was to determine the change in HAMA scores, specifically the 14-item version, over a period of time. To assess the differences in this change, we employed a piece-wise linear mixed-effects model from the baseline to week 12[19]. The treatment condition, time, and the interaction term “visit week × treatment group” were included in the model. Additionally, in the model, we considered the baseline HAMA score and included it as a covariate. To examine the intention-to-treat differences in HAMA-14 scores across various treatment and time conditions, a thorough combined Wald test was conducted. This test assessed the significance of the interaction terms between time and treatment group in the model. Furthermore, in order to evaluate the differences in improvement over time between the treatment conditions, we carried out a joint Wald test. This test involved examining the significance of the treatment × time interaction term.
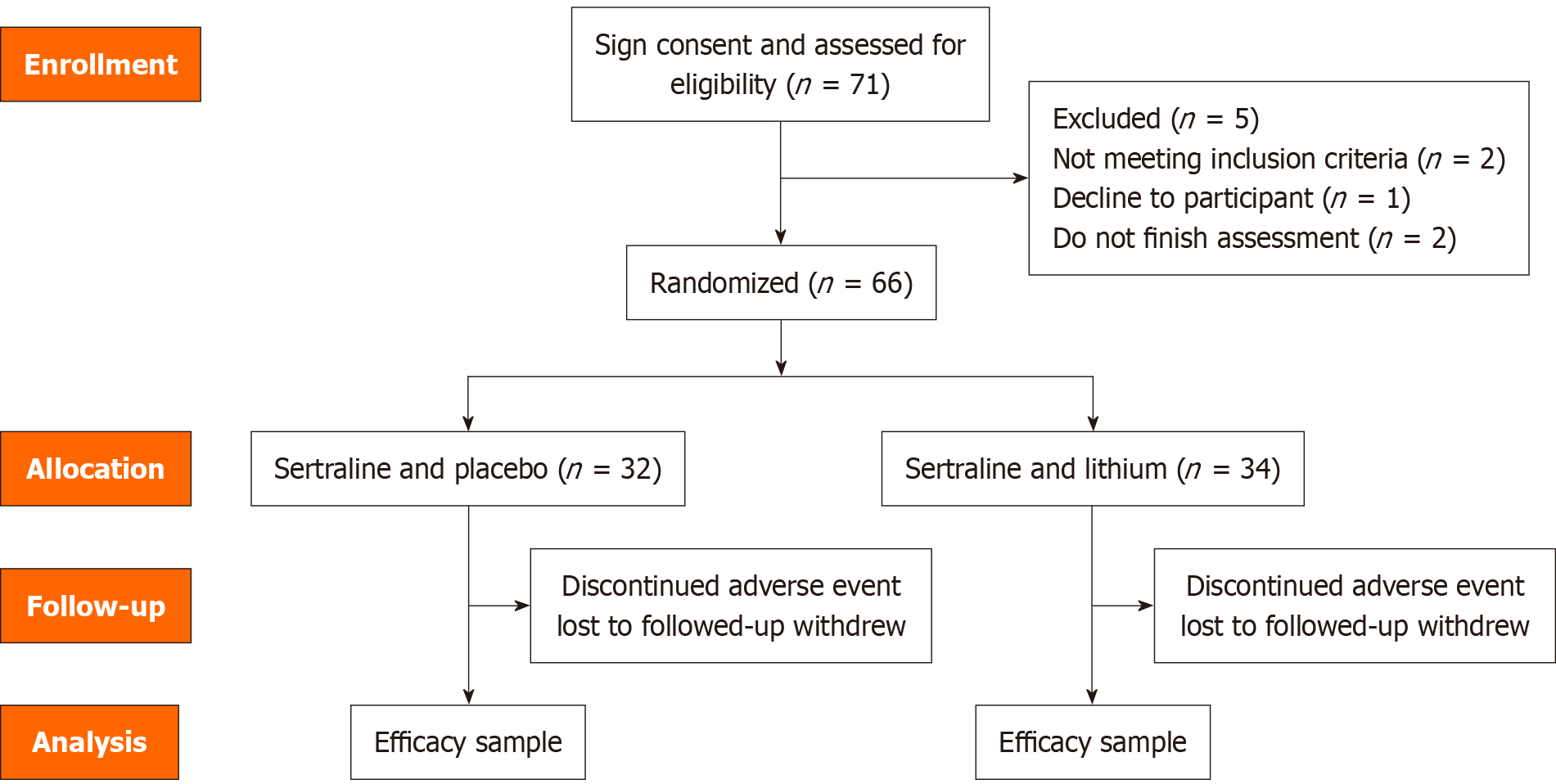
Table 1 and Figure 1 show the demographic comparison by group at study entry. Sixty-six patients were randomized into two groups: Group A (sertraline, n = 32) and group B (sertraline and lithium, n = 34). The study included a total of 41 female participants and 25 male participants. The mean age of group A was 44.31 ± 11.7 years, while group B had a mean age of 43.88 ± 13.25 years (P = 0.89). These indicate that there were no significant differences in baseline characteristics between the two groups.
| Group A (n = 32) | Group B (n = 34) | t or F | df | P value | |
| Age (years) | 44.31 ± 11.7 | 43.88 ± 13.25 | 0.139 | 64 | 0.89 |
| Gender | 0.199 | 1 | 0.655 | ||
| Male | 13 (40.6) | 12 (35.3) | |||
| Female | 19 (59.4) | 22 (64.7) | |||
| Marital status | |||||
| Presently married | 28 (87.5) | 27 (79.4) | 1.244 | 2 | 0.537 |
| Widowed/divorced/separated | 2 (6.3) | 5 (14.7) | |||
| Never married | 2 (6.3) | 2 (5.9) | |||
| Patient’s education | 10.03 ± 3.542 | 9.5 ± 3.711 | 0.594 | 64 | 0.555 |
| Total duration | 64.03 ± 62.92 | 40.59 ± 60.497 | 1.543 | 64 | 0.128 |
| Baseline HAMA-14 | 22.31 ± 2.494 | 21.65 ± 3.004 | 0.976 | 64 | 0.333 |
| Baseline HAMD-17 | 12.97 ± 2.495 | 13 ± 2.425 | 0.052 | 64 | 0.959 |
At week 8, two patients, accounting for 3.3% of the total, requested termination of their treatment based on their personal judgment. One patient was in group A, while the other patient was in group B. And another two patients (3.3%) had their diagnosis revised to BD due to the emergence of manic symptoms (BRMAS scores > 6). Upon unblinding, both were found to have been in the sertraline group and subsequently received mood stabilizers.
Significant differences were observed in the alterations of HAMA-14 scores between the two groups at week 1, week 2, and week 4 (P < 0.05; Figure 2). Specifically, the decrease in HAMA-14 scores after two weeks of treatment was slightly larger in the sertraline and lithium group (-4.08, 95% confidence interval: -5.78 to -2.38) when compared to the sertraline group (4.08, 95% confidence interval: 2.37-5.78). However, no significant differences were observed in the HAMA-14 scores between the two groups at week 8 and week 12 (P = 0.485 and P = 0.206, respectively; Figure 2). Likewise, the alteration in HAMD-17 scores over time exhibited no substantial deviation between the treatment groups (P = 0.2), with the exception of week 12 (P = 0.034; Figure 3). Notably, sensitivity analyses using both completers-only and last-observation-carried-forward approaches consistently replicated the findings of the primary analysis.
There were no serious adverse events reported in this study as a result of the treatment. The groups showed no significant differences in the alterations of systolic and diastolic blood pressure, pulse rate, or weight. Moreover, no significant differences were noted in any laboratory measurements between the treatment groups, except for a singular patient displaying a marginal increase in their free thyroxine level to 1.75 nmol/L. Nevertheless, this elevation lacks any clinical significance.
This study presents a randomized, double-blind study that examined the efficacy of sertraline plus lithium vs sertraline monotherapy for anxiety disorder in individuals with clinical high-risk factors for BD. The original objective of the study was to investigate the effect of early intervention on this particular type of anxiety disorder by analyzing the changes in symptoms throughout the treatment period. To minimize the placebo effect, a blind design was employed, considering the noticeable differences in the appearance of the drugs sertraline and lithium.
Previous studies have utilized early intervention strategies, such as family-focused therapy or psychoeducation, to address unspecified BD or depression[20]. The research included individuals who displayed present mood indications and possessed a family member, either a first or second-degree relative, with a background of BD type I or type II. Over a span of 4 years, the results indicate that therapy that prioritizes the family unit is superior to enhanced care in the ability to diminish the intensity of mood symptoms and lower the likelihood of experiencing an initial episode of mania. Managing anxiety disorder with a high risk for BD in clinical intervention presents a complex challenge. The use of antidepressants for the maintenance treatment of BD has long been a topic of debate[21]. In the current study, we aimed to look for a more reasonable solution by replacing valproate with the emotional stabilizer lithium. This decision was based on previous evidence indicating no significant differences in efficacy between valproate and lithium in terms of time to any mood event[22]. Additionally, we evaluated the effectiveness and safety of the treatment throughout the entire duration.
The results indicate that both groups experienced a reduction in anxiety and depression. Significant differences in HAMA scores were observed between the two groups at week 1, week 2, and week 4, but no difference was found at week 8 and week 12. This suggests that the combination of sertraline and lithium is more effective in rapidly improving anxiety symptoms. One possible explanation for this is that the lithium treatment addresses the overall symptomatology by targeting anxiety disorders with hybrid features for BD. It is worth noting that previous studies did not provide evidence of the anxiolytic effect of lithium.
Except for week 12, when comparing sertraline monotherapy and the combination therapy, the Hamilton Depression Rating Scale scores exhibited no significant between the two groups. However, it is worth noting that the combination therapy showcased a more advantageous effect at this particular time point. In the monotherapy group, two patients exhibited manic symptoms and were subsequently prescribed mood stabilizers. These observations imply that early intervention could potentially result in improved outcomes. However, it is crucial to acknowledge that due to the limited sample size, further cases are required to validate these findings.
Identifying a safe and effective early intervention for anxiety disorder in individuals with clinical high-risk factors for BD is crucial for public health. This intervention would address the needs of individuals who are susceptible to developing BD and simultaneously dealing with anxiety. Such a finding would have significant implications for improving the overall well-being and quality of life for this population. Many individuals seek to recover quickly or prevent the development of BD. This current double-blind, case-controlled study aimed to evaluate the efficacy and tolerability of the combination therapy vs monotherapy for anxiety disorders in this population. In light of the constraints associated with this initial study, the results imply that the combination of sertraline and lithium may offer a more favorable prognosis.
We would like to express our gratitude to Xiu-Juan Hong, from the Department of Pharmacy of our hospital, for her valuable support and assistance in ensuring that the pills were over-encapsulated to maintain blinding. Additionally, we want to express our gratitude to all the participants of this study for their valuable time and significant contribution to the clinical research.
| 1. | Goes FS. The importance of anxiety states in bipolar disorder. Curr Psychiatry Rep. 2015;17:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Hu X, Yu C, Dong T, Yang Z, Fang Y, Jiang Z. Biomarkers and detection methods of bipolar disorder. Biosens Bioelectron. 2023;220:114842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 3. | Yapıcı Eser H, Taşkıran AS, Ertınmaz B, Mutluer T, Kılıç Ö, Özcan Morey A, Necef I, Yalçınay İnan M, Öngür D. Anxiety disorders comorbidity in pediatric bipolar disorder: a meta-analysis and meta-regression study. Acta Psychiatr Scand. 2020;141:327-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Gamage N, Senanayake S, Kumbukage M, Mendis J, Jayasekara A. The prevalence of anxiety and its association with the quality of life and illness severity among bipolar affective disorder patients in a developing country. Asian J Psychiatr. 2020;52:102044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Preti A, Vrublevska J, Veroniki AA, Huedo-Medina TB, Fountoulakis KN. Prevalence, impact and treatment of generalised anxiety disorder in bipolar disorder: a systematic review and meta-analysis. Evid Based Ment Health. 2016;19:73-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Jann MW. Diagnosis and treatment of bipolar disorders in adults: a review of the evidence on pharmacologic treatments. Am Health Drug Benefits. 2014;7:489-499. [PubMed] |
| 7. | Luby JL, Navsaria N. Pediatric bipolar disorder: evidence for prodromal states and early markers. J Child Psychol Psychiatry. 2010;51:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Shao Y, Cheng Y, Gottipati S, Zeng-Treitler Q. Phenotype fingerprinting of bipolar disorder prodrome. Int J Bipolar Disord. 2023;11:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Buckley V, Young AH, Smith P. Child and adolescent anxiety as a risk factor for bipolar disorder: A systematic review of longitudinal studies. Bipolar Disord. 2023;25:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Nery FG, Monkul ES, Lafer B. Gray matter abnormalities as brain structural vulnerability factors for bipolar disorder: A review of neuroimaging studies of individuals at high genetic risk for bipolar disorder. Aust N Z J Psychiatry. 2013;47:1124-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Bundgaard AF, Hemager N, Gantriis DL, Steffensen NL, Burton BK, Ellersgaard D, Christiani CJ, Spang KS, Carlsen AH, Bliksted V, Plessen KJ, Jepsen JRM, Nordentoft M, Mors O, Thorup AAE, Greve AN. Association Between Early Risk Factors and Level of Functioning at Age Seven in Children at Familial Risk for Schizophrenia Or Bipolar Disorder - the Danish High Risk and Resilience Study VIA 7. Scand J Child Adolesc Psychiatr Psychol. 2022;10:12-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, Sharma V, Goldstein BI, Rej S, Beaulieu S, Alda M, MacQueen G, Milev RV, Ravindran A, O'Donovan C, McIntosh D, Lam RW, Vazquez G, Kapczinski F, McIntyre RS, Kozicky J, Kanba S, Lafer B, Suppes T, Calabrese JR, Vieta E, Malhi G, Post RM, Berk M. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1142] [Cited by in RCA: 1212] [Article Influence: 151.5] [Reference Citation Analysis (6)] |
| 13. | Tondo L, Alda M, Bauer M, Bergink V, Grof P, Hajek T, Lewitka U, Licht RW, Manchia M, Müller-Oerlinghausen B, Nielsen RE, Selo M, Simhandl C, Baldessarini RJ; International Group for Studies of Lithium (IGSLi). Clinical use of lithium salts: guide for users and prescribers. Int J Bipolar Disord. 2019;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 14. | Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 15. | Shansis FM, Reche M, Capp E. Evaluating response to mood stabilizers in patients with mixed depression: A study of agreement between three different mania rating scales and a depression rating scale. J Affect Disord. 2016;197:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Szuhany KL, Simon NM. Anxiety Disorders: A Review. JAMA. 2022;328:2431-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 233] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 17. | Thompson E. Hamilton Rating Scale for Anxiety (HAM-A). Occup Med (Lond). 2015;65:601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 331] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 18. | Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): U. S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976. |
| 19. | Laird NM, Ware JH. Random-Effects Models for Longitudinal Data. Biometrics. 1982;38:963-974. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5737] [Cited by in RCA: 5090] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 20. | Salinger JM, O'Brien MP, Miklowitz DJ, Marvin SE, Cannon TD. Family communication with teens at clinical high-risk for psychosis or bipolar disorder. J Fam Psychol. 2018;32:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Yatham LN, Arumugham SS, Kesavan M, Ramachandran K, Murthy NS, Saraf G, Ouyang Y, Bond DJ, Schaffer A, Ravindran A, Ravindran N, Frey BN, Daigneault A, Beaulieu S, Lam RW, Kondapuram N, Reddy MS, Bhandary RP, Ashok MV, Ha K, Ahn YM, Milev R, Wong H, Reddy YCJ; BEAM-BD Trial Group. Duration of Adjunctive Antidepressant Maintenance in Bipolar I Depression. N Engl J Med. 2023;389:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Kang MG, Qian H, Keramatian K, Chakrabarty T, Saraf G, Lam RW, Wong H, Yatham LN. Lithium vs valproate in the maintenance treatment of bipolar I disorder: A post- hoc analysis of a randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2020;54:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |