Published online Dec 16, 2025. doi: 10.12998/wjcc.v13.i35.115280
Revised: November 5, 2025
Accepted: December 8, 2025
Published online: December 16, 2025
Processing time: 64 Days and 14.2 Hours
Immunoglobulin A (IgA) vasculitis, formerly known as Henoch-Schönlein pur
This case report describes an 11-year-old black girl with acute onset of rash, joint pain, and abdominal pain, subsequently diagnosed with IgA vasculitis.
The case highlights the importance of early recognition and supportive man
Core Tip: Immunoglobulin A (IgA) vasculitis, rare in black children, can be missed when classic purpura is subtle on dark skin. Early triad recognition—palpable rash, arthralgia, abdominal pain—and prompt supportive care secure rapid recovery. This case describes an 11-year-old Black girl with classic systemic features—palpable purpura, abdominal pain, and arthritis—but with atypical dermatologic presentation due to skin pigmentation. The report underscores the diagnostic challenges of identifying vasculitic rashes in darker skin and highlights the need for inclusive dermatologic education and region-specific research on IgA vasculitis presentation and outcomes in African children.
- Citation: Nurani KM, Natalia G, Kadernani N, Kadernani K. Systemic and dermatological findings of immunoglobulin A vasculitis in a black child: A case report. World J Clin Cases 2025; 13(35): 115280
- URL: https://www.wjgnet.com/2307-8960/full/v13/i35/115280.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i35.115280
Immunoglobulin A (IgA) vasculitisis the most common form of systemic vasculitis in children and is characterized by the deposition of IgA immune complexes in small vessels[1]. It primarily affects the skin, gastrointestinal tract, joints, and kidneys[2]. The exact etiology remains unclear, but infections, particularly viral upper respiratory tract infections, have been implicated as potential triggers[2]. Despite being well-documented in lighter-skinned populations, reports of IgA vasculitis in Black children remain sparse. Additionally, dermatologic findings in individuals with darker skin tones are less well characterized, making diagnosis more difficult. This gap in documentation underscores the importance of this case in contributing to the understanding of IgA vasculitis in Black-skinned individuals.
An 11-year-old Black female presented to the hospital as a referred from a peripheral facility with a one-day history of a non-pruritic rash, abdominal pain, and joint pain.
The rash appeared as patchy, raised, hyperpigmented macules on the dorsal and ventral aspects of her hands and extended to her elbows. Similar lesions were present on her feet and knees. The rash was non-itchy and did not blanch on pressure. Approximately four hours after the onset of the rash, she developed sharp periumbilical abdominal pain, which was aggravated by food intake. The pain was associated with bilious, non-bloody vomiting that occurred postprandially. She also experienced acute-onset pain affecting multiple joints, including the wrists, elbows, ankles, and small joints of the hands and feet. The joint pain was associated with swelling, stiffness, warmth, and a severely limited range of motion, impairing her ability to walk.
She reported a dry cough two weeks before admission, which resolved after taking over-the-counter antihistamines. There was no history of recent infections, travel, or contact with individuals with similar symptoms. Her past medical history was unremarkable apart from a history of rickets in infancy. She had no known allergies and no history of chronic illnesses.
Her personal and family history was unremarkable.
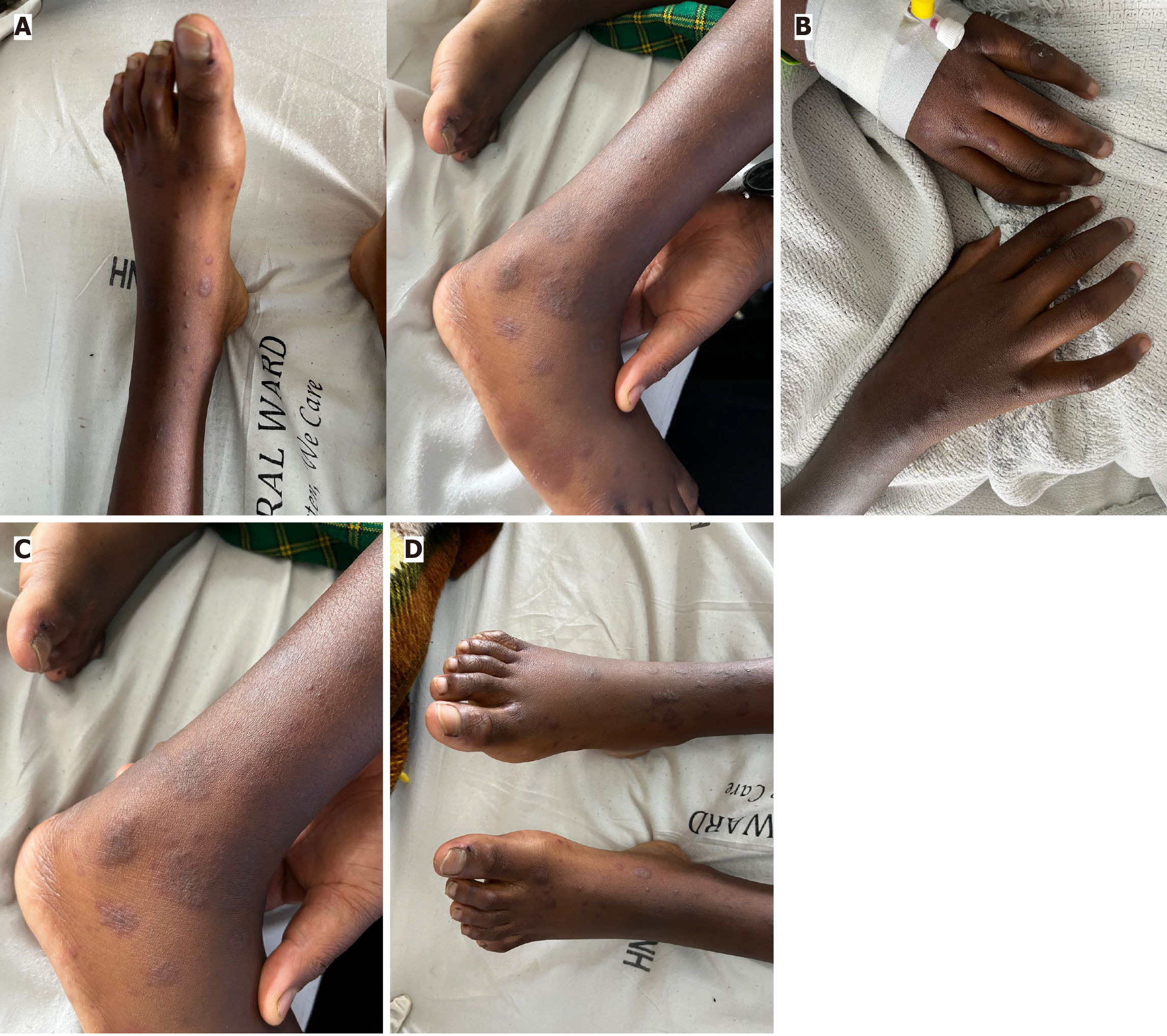
On physical examination, she appeared ill but was hemodynamically stable. Her vital signs were within normal limits, and she was afebrile. A hyperpigmented, non-blanching rash was observed on the extremities, consistent with vasculitis but atypical in its presentation due to her skin tone (Figure 1). Joint examination revealed tenderness and swelling, limiting the assessment of power and reflexes due to pain. Abdominal examination revealed a soft, non-distended abdomen with no organomegaly or masses. There were no signs of peritoneal irritation.
A complete blood count showed leukocytosis with a white blood cell count of 18.52 × 109/L (reference range: 4.00-10.00). Neutrophilia was present, with a neutrophil count of 16.64 × 109/L (reference range: 2.00-7.50), while lymphocytes were reduced at 0.96 × 109/L (reference range: 1.50-3.50). The hemoglobin level was 11.4 g/dL, and platelet count was within normal limits at 263 × 109/L. The erythrocyte sedimentation rate was elevated at 25 mm/hour (reference range: < 20 mm/hour). An abdominal ultrasound revealed normal liver, gallbladder, pancreas, spleen, and kidney findings. There was no evidence of visceral lymphadenopathy, intra-abdominal mass lesions, or peritoneal fluid collection. The bowel loops showed normal peristalsis, and there were no features suggestive of appendicitis.
No special notes.
The clinical presentation of palpable purpura, arthralgia, and abdominal pain, combined with the preceding upper respiratory infection and supportive laboratory findings, was consistent with IgA vasculitis. However, the recognition of the rash as purpuric was challenging due to the patient’s skin tone, highlighting the need for increased awareness of how vasculitic lesions manifest in darker-skinned individuals. Differential diagnoses considered included juvenile idiopathic arthritis, systemic lupus erythematosus (SLE), immune thrombocytopenic purpura, thrombotic thrombocytopenic purpura, Kawasaki disease, and sepsis with disseminated intravascular coagulation. However, the absence of thrombocytopenia, hemolysis, mucocutaneous involvement, or severe systemic inflammation helped rule out these conditions.
The patient was initiated on nonsteroidal anti-inflammatory drugs for pain control, ceftriaxone for empirical antimicrobial coverage, and omeprazole for gastric protection. Supportive care was provided, and serial follow-up assessments were conducted.
Over the course of hospitalization, her abdominal pain gradually resolved, the hyperpigmented rash began to fade, and she regained the ability to walk. However, joint pain persisted, though with improving mobility. The patient fully recovered and was discharged.
IgA vasculitis, previously known as Henoch–Schönlein purpura, represents the most common systemic vasculitis in children, with a peak incidence between ages 4 years and 7 years[3]. It is an immune complex–mediated small vessel vasculitis characterized by the deposition of predominantly IgA1 antibodies in vessel walls, commonly triggered by infections, vaccinations, or medications[2]. The classical clinical tetrad includes palpable purpura without thrombocytopenia, arthralgia or arthritis, abdominal pain, and renal involvement[4].
This case illustrates a classic but diagnostically challenging presentation of IgA vasculitis in an 11-year-old Black female. Although the patient fulfilled the 2006 EULAR/PRINTO/PRES classification criteria for IgA vasculitis[5], her presentation highlights a critical diagnostic challenge in dermatologic assessment: The under-recognition of purpuric lesions in individuals with darker skin tones. In lighter-skinned individuals, IgA vasculitis’s purpura are often described as erythematous or violaceous and readily apparent on inspection[6]. However, in this patient, the cutaneous lesions appeared as hyperpigmented macules, raising concerns for diagnostic delays in populations with richly pigmented skin. The failure of many medical textbooks and image databases to adequately represent dermatologic disease in skin of color contributes to diagnostic inequities and poorer outcomes[7].
Beyond dermatologic presentation, the patient experienced acute-onset polyarticular arthritis, affecting both large and small joints, and causing marked functional impairment. Arthritis is reported in up to 80% of pediatric IgA vasculitis cases, often presenting early and resolving without permanent joint damage[4]. Notably, joint involvement may be more severe and migratory in older children, which aligns with this patient's age and symptom burden.
The abdominal symptoms were particularly prominent, characterized by sharp periumbilical pain and bilious vom
Of note, the patient was afebrile, hemodynamically stable, and had no clinical signs of renal involvement at the time of presentation. These findings, along with normal platelet counts and a reassuring abdominal ultrasound, helped narrow the differential diagnosis. Meningococcemia, often characterized by acute fever, hypotension, and petechial or purpuric rash in a toxic-appearing patient, was considered unlikely given the stable vitals and absence of systemic toxicity[11]. SLE was ruled out due to the lack of constitutional symptoms, cytopenias, or multisystem involvement[12]. Similarly, serum sickness, typically triggered by drug or antigen exposure and associated with fever, urticaria, and arthralgia, was not supported by the history or clinical findings[13]. The absence of thrombocytopenia further supported the diagnosis of IgA vasculitis over thrombocytopenic purpura. While a definitive diagnosis of IgA vasculitis is clinical, adjunctive investigations including urinalysis, renal function tests, and abdominal imaging are essential to assess for complications and guide follow-up[2].
The patient's history of a self-limiting dry cough two weeks prior suggests a viral prodrome, a common trigger in paediatric IgA Vasculitis. Respiratory tract infections precede over 50% of cases, and while streptococcal infections are well-known culprits, other non-specific viral illnesses may also initiate the pathogenic IgA1 immune response[14,15]. Emerging research confirms that IgA vasculitis cases often follow spikes in respiratory pathogens—not climate—supporting an infectious trigger hypothesis. Although temperate regions report seasonal peaks during autumn and winter, these trends align with infection surges, reinforcing that infection, may trigger disease onset[16-18].
Management of IgA vasculitis remains primarily supportive. In this case, analgesics and close monitoring were appropriate given the absence of renal compromise or gastrointestinal complications requiring surgical evaluation. The role of corticosteroids remains debated; however, evidence suggests they may hasten symptom resolution in cases of severe abdominal or joint involvement, though they do not prevent long-term renal damage[19,20]. Importantly, long-term prognosis hinges on the extent of renal involvement, which may emerge later in the disease course. This necessitates regular urinalysis and blood pressure monitoring during follow-up[21].
This case highlights the importance of recognizing IgA vasculitis in children with multisystem symptoms, especially when the characteristic purpura may appear subtle on darker skin tones. Clinicians should be aware that lesions may present as hyperpigmented or violaceous macules rather than the classic bright purpura seen in lighter skin. The case also underscores the need for better dermatologic and pediatric training inclusive of skin of color and for region-specific research on IgA vasculitis presentation and outcomes.
| 1. | Sestan M, Jelusic M. Diagnostic and Management Strategies of IgA Vasculitis Nephritis/Henoch-Schönlein Purpura Nephritis in Pediatric Patients: Current Perspectives. Pediatric Health Med Ther. 2023;14:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 2. | Roache-Robinson P, Killeen RB, Hotwagner DT. IgA Vasculitis (Henoch-Schönlein Purpura). 2023 Sep 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 3. | Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002;360:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 472] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 4. | Saulsbury FT. Clinical update: Henoch-Schönlein purpura. Lancet. 2007;369:976-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, Buoncompagni A, Lazar C, Bilge I, Uziel Y, Rigante D, Cantarini L, Hilario MO, Silva CA, Alegria M, Norambuena X, Belot A, Berkun Y, Estrella AI, Olivieri AN, Alpigiani MG, Rumba I, Sztajnbok F, Tambic-Bukovac L, Breda L, Al-Mayouf S, Mihaylova D, Chasnyk V, Sengler C, Klein-Gitelman M, Djeddi D, Nuno L, Pruunsild C, Brunner J, Kondi A, Pagava K, Pederzoli S, Martini A, Ruperto N; Paediatric Rheumatology International Trials Organisation (PRINTO). EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 888] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 6. | Di Ludovico A, Rinaldi M, Lauriola F, Ciarelli F, La Bella S, Gualdi G, Chiarelli F, Bailey K, Breda L. The Diagnostic Role of Skin Manifestations in Rheumatic Diseases in Children: A Critical Review of Paediatric Vasculitis. Int J Mol Sci. 2024;25:7323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Trapani S, Micheli A, Grisolia F, Resti M, Chiappini E, Falcini F, De Martino M. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005;35:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Chen SY, Kong MS. Gastrointestinal manifestations and complications of Henoch-Schönlein purpura. Chang Gung Med J. 2004;27:175-181. [PubMed] |
| 10. | Ebert EC. Gastrointestinal manifestations of Henoch-Schonlein Purpura. Dig Dis Sci. 2008;53:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Rausch-Phung EA, Siddiqui JA, Gulick PG. Meningococcemia. 2025 Apr 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 12. | Levy DM, Kamphuis S. Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am. 2012;59:345-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 13. | Rixe N, Tavarez MM. Serum Sickness. 2023 Aug 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 14. | Bayındır Y, Başaran Ö, Bilginer Y, Özen S. Vasculitis in Children. Turk Arch Pediatr. 2024;59:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 15. | Weiss PF, Klink AJ, Luan X, Feudtner C. Temporal association of Streptococcus, Staphylococcus, and parainfluenza pediatric hospitalizations and hospitalized cases of Henoch-Schönlein purpura. J Rheumatol. 2010;37:2587-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Felix A, Assad Z, Bidet P, Caseris M, Dumaine C, Faye A, Melki I, Kaguelidou F, Valtuille Z, Ouldali N, Meinzer U. Common Seasonal Pathogens and Epidemiology of Henoch-Schönlein Purpura Among Children. JAMA Netw Open. 2024;7:e245362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Wang B, Xiang W, Zhu Z, Shen H. The influence of respiratory infections on Henoch-Schönlein purpura in children. BMC Infect Dis. 2025;25:118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Xu L, Li Y, Wu X. IgA vasculitis update: Epidemiology, pathogenesis, and biomarkers. Front Immunol. 2022;13:921864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 19. | Ronkainen J, Koskimies O, Ala-Houhala M, Antikainen M, Merenmies J, Rajantie J, Ormälä T, Turtinen J, Nuutinen M. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Weiss PF. Pediatric vasculitis. Pediatr Clin North Am. 2012;59:407-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Davin JC, Coppo R. Henoch-Schönlein purpura nephritis in children. Nat Rev Nephrol. 2014;10:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/