Published online Nov 26, 2025. doi: 10.12998/wjcc.v13.i33.112607
Revised: August 21, 2025
Accepted: October 28, 2025
Published online: November 26, 2025
Processing time: 112 Days and 18.1 Hours
Elsberg syndrome is a type of postinfectious lumbosacral radiculitis typically tri
A 60-year-old male with diabetes mellitus presented with severe bladder and bowel dysfunction persisting for more than two months, followed by left gluteal and perianal (saddle area) herpes zoster eruption that was accompanied by significant neuropathic pain. Following a suboptimal response to conservative therapy, the patient underwent implantation of a short-term spinal cord stimu
Early implementation of short-term spinal cord stimulation represents a pro
Core Tip: Elsberg syndrome is a type of sacral radiculitis caused by various viruses. We report a rare case of a diabetic patient with refractory Elsberg syndrome secondary to varicella-zoster virus sacral neuropathy. A suboptimal response to conservative therapy was observed, likely compounded by underlying diabetic polyneuropathy, and short-term spinal cord stimulation therapy led to complete neurological recovery and pain resolution, which was sustained at the two-month follow-up.
- Citation: Wang J, Yu XQ, Zhang XG, Song L, Lee J. Short-term spinal cord stimulation for refractory Elsberg syndrome in diabetic neuropathy: A case report. World J Clin Cases 2025; 13(33): 112607
- URL: https://www.wjgnet.com/2307-8960/full/v13/i33/112607.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i33.112607
Elsberg syndrome, also known as lumbosacral radiculomyelitis, is commonly caused by neurotropic viruses such as herpes simplex virus type 2 and varicella-zoster virus (VZV), which affect the sacral nerve roots[1]. Clinical manifestations include bladder/bowel dysfunction, sensory disturbances (including anesthesia, dysesthesia, or hyperesthesia) in the saddle distribution and lower extremities, and flaccid paralysis. Although Elsberg syndrome typically follows a self-limiting course or resolves with antiviral therapy, persistent refractory manifestations documented in clinical ob
A 60-year-old male presented with acute-onset bladder and bowel dysfunction persisting for more than two months, followed by left gluteal and perianal herpes zoster eruption with severe neuropathic pain within two days.
The patient was referred to the pain management clinic for severe dysuria, constipation, and left gluteal/perianal vesicular rash accompanied by debilitating pain (> 2 months). Previously diagnosed with herpes zoster-related neuralgia in the Dermatology Department, he received valacyclovir (1 g ter in die × 7 days), pregabalin (up to 300 mg/day), B-complex vitamins, laxatives, and tamsulosin with a suboptimal response. Despite intermittent urethral catheterization and physical therapy, he required intermittent self-catheterization and digital rectal stimulation, hot compresses, and laxatives to facilitate bladder/bowel evacuation. Persistent perineal pain significantly impaired his quality of life.
His medical history included hypertension diagnosed > 10 years prior and type 2 diabetes mellitus diagnosed > 1 year prior.
There was no significant personal and family history.
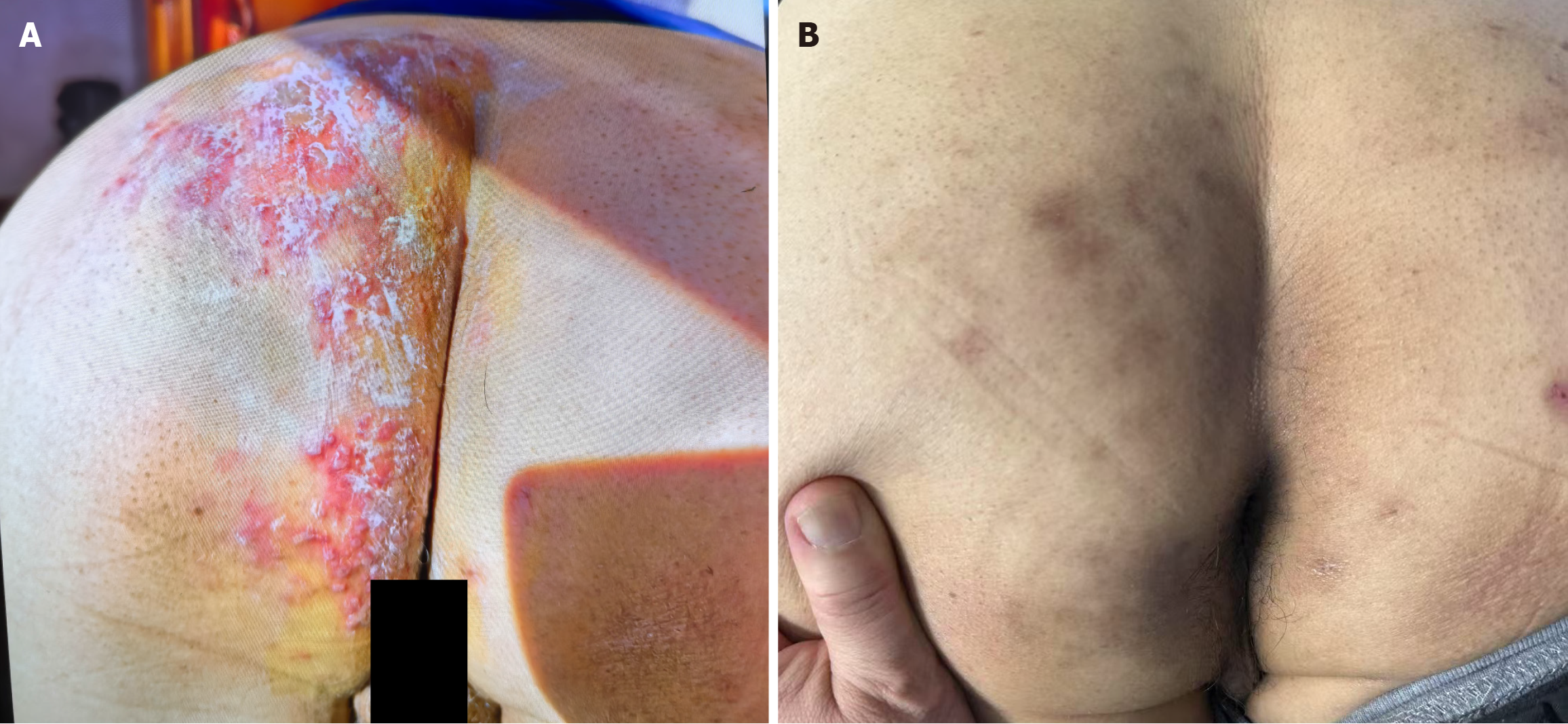
The vital signs on admission were as follows: Temperature, 36.1 °C; heart rate, 69 beats per minute; respiratory rate, 20/minutes; and blood pressure, 145/92 mmHg. The patient exhibited an anxious demeanor with hyperpigmented cutaneous lesions distributed across the left S2-S4 dermatomes, encompassing the gluteal and perianal regions (Figure 1). Neurological assessment revealed cutaneous allodynia to light touch and dermatomal hypoesthesia in affected territories, accompanied by reduced anal sphincter tone (Modified Oxford Scale 2/5). No motor deficits or pathological reflexes were detected. Pain intensity was quantified as 6/11 on the visual analog scale.
The laboratory results revealed glycosuria (4+; reference range: Negative) and a positive fecal occult blood test. Complete blood count, renal/hepatic function tests, electrolyte panel, glycated hemoglobin level, tumor markers (including carcinoembryonic antigen, carbohydrate antigen 19-9, and alpha-fetoprotein levels), coagulation profile, and blood-borne pathogen screening (human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and syphilis) were within normal limits.
Lumbosacral magnetic resonance imaging revealed diffuse degenerative changes accompanied by mild disc bulging spanning from the L2 to S4 vertebral levels. Concurrent urinary tract ultrasonography revealed benign prostatic hyp
The patient was diagnosed with the following conditions: (1) VZV-induced Elsberg syndrome; (2) Herpes zoster-related neuralgia; (3) Stage 3 hypertension (very high-risk group); (4) Type 2 diabetes mellitus; and (5) Benign prostatic hyperplasia.
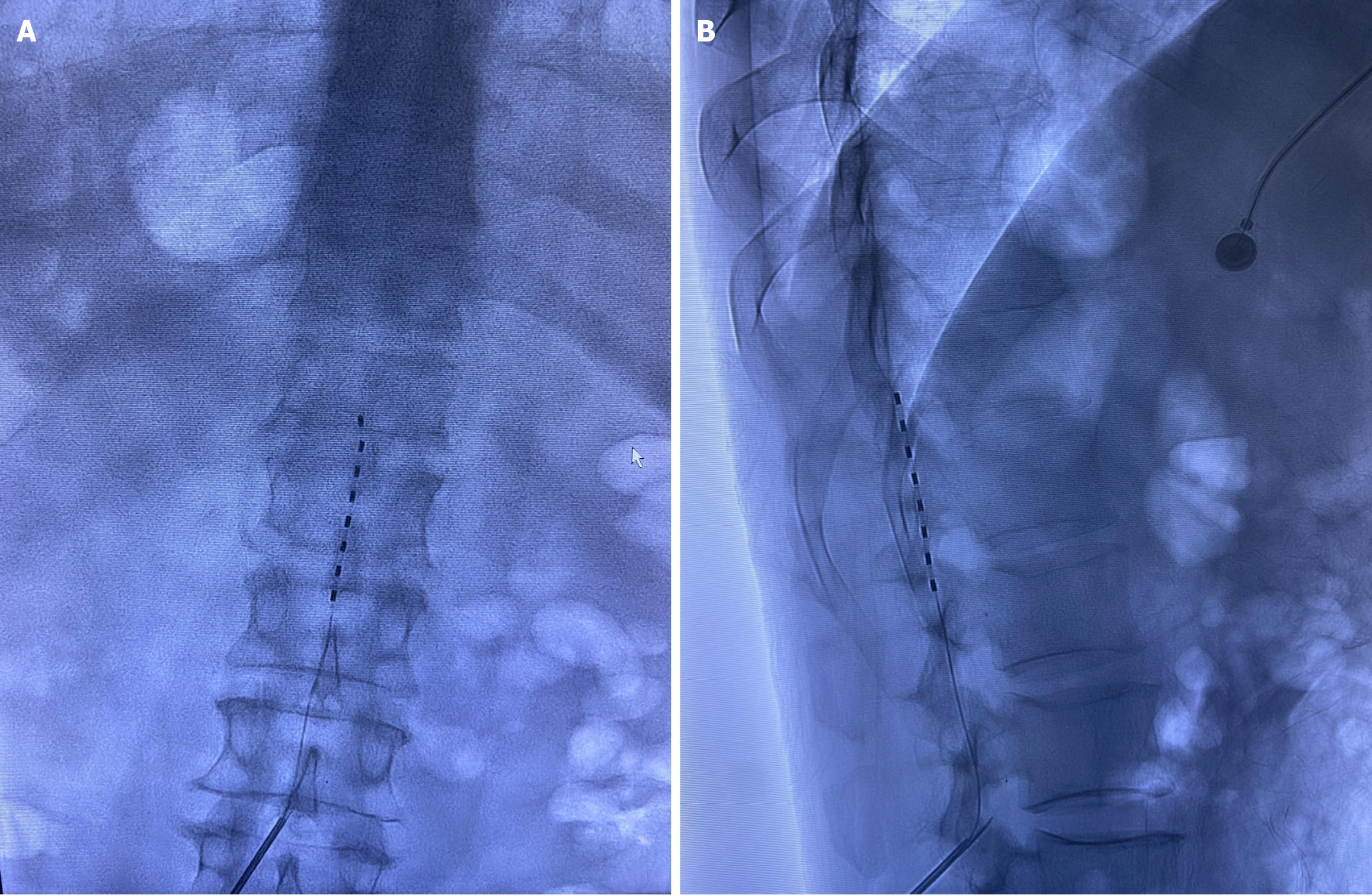
Initial management with pregabalin (150 mg twice a day), mecobalamin (0.5 mg ter in die), tamsulosin (0.2 mg quaque die), and finasteride (5 mg quaque die) provided minimal symptom relief. Ultrasound-guided sacral canal blockade (20 mL of 1% lidocaine) resulted in transient perineal pruritus/pain reduction and immediate restoration of voluntary micturition (approximately 800 mL of urine output), but bowel dysfunction remained. st-SCS was subsequently performed under digital subtraction angiography guidance. A percutaneous electrode lead (PINS Medical TL3213-65, Beijing) was ad
The st-SCS system remained unadjusted for 10 days before explantation. At discharge, normal genitourinary/gastrointestinal function was documented, with residual mild cutaneous hypoesthesia and no pain. At the 2-month postdischarge follow-up conducted via telephone, the patient reported spontaneous voiding and defecation without difficulty, resolution of pain in the herpetic region, and residual mild superficial hypoesthesia localized to the affected dermatomes.
First described by Elsberg in 1913[2], Elsberg syndrome (lumbosacral radiculomyelitis) is characterized by bladder/bowel dysfunction, saddle-distribution sensory abnormalities (anesthesia/hyperesthesia), and flaccid lower extremity paralysis. VZV and herpes simplex virus type 2 are the predominant pathogens[1]. The incidence of herpes zoster in mainland China ranges from 1.9 to 5.8 per 1000 person-years[3], with approximately 4% of cases exhibiting voiding difficulties[4]. A gold-standard diagnostic framework for Elsberg syndrome remains undefined in current clinical practice; in this case, the diagnosis of Elsberg syndrome was established on the basis of the following: (1) Severe bladder/bowel dysfunction; (2) Classic herpes zoster eruption in S2-S4 dermatomes; and (3) Subacute neuropathic pain (> 2 months in duration). Although lumbosacral magnetic resonance imaging revealed no myelitis and cerebrospinal fluid (CSF) virological testing was not performed due to the chronic phase, the constellation of symptoms fulfilled the diagnostic criteria for viral rad
Pathophysiologically, VZV reactivation in the sacral ganglia damages sensory neurons and autonomic fibers in
Herpes zoster-associated neuralgia progresses through three phases: Acute herpetic neuralgia (< 30 days), subacute herpetic neuralgia (30-90 days), and postherpetic neuralgia (> 90 days). First-line therapy for acute herpetic neuralgia includes antivirals, tricyclic antidepressants, vitamin B complex, and physical therapy. A literature review (PubMed/EMBASE: 2000-2023; keywords: Elsberg syndrome, VZV radiculitis) identified 10 reported cases of VZV-associated Elsberg syndrome (Table 1)[6-15]. Six patients achieved complete recovery with acute-phase antiviral therapy, while 3 developed chronic sequelae (including 2 requiring permanent catheterization). Our patient’s refractory symptoms despite > 2 months of conventional treatment may be attributed to diabetic neuropathy[12], which exacerbates neural damage and impairs regeneration.
| Ref. | Region | Sex/age (years) | Disease course | Comorbidities | Clinical manifestations | Treatment | Outcome | Follow-up |
| Nishiyama et al[6], 2023 | Japan | Female/77 | 10 days | None | Urinary retention, genital rash | Acyclovir, acetaminophen, urinary catheter | Persistent postherpetic neuralgia | 6 months |
| Yang et al[7], 2022 | China | Female/74 | 3 weeks | Hypertension and hyperlipidemia | Urinary retention, constipation, sacral numbness | Famciclovir, prednisone, indomethacin, mecobalamin; electroacupuncture | Complete resolution | 1 year |
| Desai et al[8], 2022 | United States | Male/45 | 12 days | Acute ischemic stroke; smoldering myeloma | Urinary retention, fecal incontinence, lumbosacral vesicular rash, right leg paresthesia | Acyclovir, dexamethasone | Required self-catheterization | 1 year |
| Bhagat et al[9], 2021 | United States | Male/56 | 4 days | AIDs; cerebrovascular event | Fever, painful rash, bilateral limb weakness, bowel/bladder dysfunction | Acyclovir; Biktarvy® | Complete resolution | 1 month |
| Santos et al[10], 2021 | Brazil | Male/52 | 2 months | Crohn’s disease; AIDs | Urinary retention and loss of sensation of the genital and left lower limb | Corticosteroid pulse and acyclovir | Incomplete improvement | - |
| Shah et al[11], 2021 | United Kingdom | Male/63 | 2 weeks | - | Low back pain, perineal and genital numbness, together with bilateral lower limb paresthesia and urinary retention | Acyclovir | Death | - |
| Saito et al[12], 2018 | Japan | Female/68 | 10 days | Type 2 diabetes mellitus; hypertension and dyslipidemia | Urinary retention and constipation; rash in the left hip | IV acyclovir, catheterization and methylprednisolone pulse | Complete resolution | 6 months |
| Abe et al[13], 2015 | Japan | Male/57 | 7 days | None | Fever with chills, headache with nausea and vomiting and intention tremors of the bilateral upper limbs, constipation and urinary retention | Acyclovir, bladder catheter | Persistent symptoms | 1 year |
| Fujii et al[14], 2015 | Japan | Male/76 | 8 days | Right testicular hydrocele | Anal pain and voiding difficulty, urinary retention, and incontinence of feces | Acyclovir, methylprednisolone and immunoglobulin | Asymptomatic at discharge | - |
| Matsumoto et al[15], 2012 | Japan | Female/55 | 4 days | Rectal ulcer | Herpes zoster, urinary retention, and constipation | Intravenous acyclovir and oral purgatives urethral catheter | Asymptomatic at discharge | - |
While pulsed radiofrequency ablation targets isolated ganglia, its application to multisegmental sacral radiculitis (S2-S4) is anatomically challenging owing to osseous constraints. Although sacral neuromodulation has demonstrated efficacy in neurogenic bladder/bowel disorders[16,17], its utility in VZV-induced sacral plexopathy remains unestablished. Given the complex presentation of neuropathic pain with autonomic dysfunction in the patient, st-SCS at the conus medullaris level was selected. The proposed mechanisms involved in the treatment method include: (1) The activation of dorsal column promotes neuroplasticity, reestablishing neural continuity and preventing neurofunctional loss[18]; and (2) The classic gate control theory, which states that spinal cord stimulation selectively activates large-diameter Aβ sensory fibers, thereby inhibiting the synaptic transmission of nociceptive signals from small-diameter Aδ and C fibers in the dorsal horn. This “closes the gate” to pain signal transmission to higher central regions[19]. This dual mechanism underpins sustained pain relief and neurological recovery. Given the patient’s subacute presentation, a st-SCS was implanted at the left conus medullaris level (T12-L1 vertebral segments). This cost-effective trial strategy allowed therapeutic efficacy assessment prior to considering permanent implantation. To our knowledge, this represents the first documented case of refractory Elsberg syndrome successfully managed with st-SCS. At the 2-month follow-up, the patient demonstrated complete restoration of bladder and bowel function and resumed baseline activities of daily living. This outcome underscores that early st-SCS intervention during the subacute phase (30-90 days after onset) may interrupt the pathophysiological cascade, preventing irreversible axonal degeneration and the transition to refractory neuropathic pain.
Notwithstanding the constraints inherent in this single-center case report including: (1) Absence of CSF polymerase chain reaction for virological confirmation due to delayed patient presentation; (2) Reliance on subjective symptom reporting without objective urodynamic profiling (e.g., detrusor pressure/flow rates) or quantitative anal sphincter assessment; (3) Lack of neurophysiological validation for sacral autonomic involvement; and (4) Limited initial follow-up period (2 months) precluding long-term therapeutic durability assessment - our findings warrant further investigation. Multicenter collaborations should prioritize: (1) Establishing cohort databases for similar patients to define evidence-based neuromodulation timing criteria, preventing irreversible neural injury; and (2) Implementing standardized objective metrics including etiological confirmation (e.g., CSF polymerase chain reaction), urodynamic studies, qua
This report presents a novel therapeutic approach for refractory VZV-associated Elsberg syndrome, in which st-SCS results in complete symptomatic resolution after failure of conventional therapy. To our knowledge, this represents the first documented case of successful st-SCS management of this condition, suggesting its potential neuroregenerative properties beyond pain modulation.
| 1. | Qadri HM, Pervaiz S, Ijaz M, Fatir CA, Anwar MU, Babar MS, Bashir A. Elsberg syndrome - A systematic review of existing scientific literature from 2000 - 2023. Pak J Med Sci. 2024;40:S103-S113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Savoldi F, Kaufmann TJ, Flanagan EP, Toledano M, Weinshenker BG. Elsberg syndrome: A rarely recognized cause of cauda equina syndrome and lower thoracic myelitis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Yin D, Van Oorschot D, Jiang N, Marijam A, Saha D, Wu Z, Tang H, Diaz-Decaro J, Watson P, Xie X, Ren Y, He Y, Feng Y. A systematic literature review to assess the burden of herpes zoster disease in China. Expert Rev Anti Infect Ther. 2021;19:165-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Chen PH, Hsueh HF, Hong CZ. Herpes zoster-associated voiding dysfunction: a retrospective study and literature review. Arch Phys Med Rehabil. 2002;83:1624-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 361] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 6. | Nishiyama D, Yoshimura S, Shimizuhira C, Ikeda N, Miyamae N, Sumida Y. Elsberg syndrome caused by herpes zoster in the sacral region with preceding urinary retention. Acute Med Surg. 2023;10:e824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Yang LS, Zhang K, Zhou DF, Zheng SZ, Zhang J. Acupuncture for the Elsberg Syndrome Secondary to Varicella-Zoster Virus Infection: a Case Report and Brief Review. J Acupunct Meridian Stud. 2022;15:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Desai R, Welsh CT, Schumann SO 3rd. Elsberg Syndrome, Lumbosacral Radiculopathy, and Myelitis Due to Herpes Zoster in a Patient With Smoldering Myeloma. J Investig Med High Impact Case Rep. 2022;10:23247096211063348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Bhagat YV, Yunasan E, Alzedaneen Y, Muttana S, Michael MB. Treatment of Elsberg Syndrome Causes Fever of Unknown Origin Attributable to Drug Reaction. Cureus. 2021;13:e18510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Santos DH, Carneiro de Oliveira RM, Junior WRF, Olivetti BC. Myelorradiculitis due to Varicella Zoster (Elsberg syndrome) as the first symptom of HIV in a patient with Crohn's disease in use of Infliximab. Mult Scler Relat Disord. 2021;47:102643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Shah R, Jayakumar N, Athar S, Ashwood N. When infection mimics cauda equina syndrome: a cautionary tale. Ann R Coll Surg Engl. 2021;103:e181-e183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Saito H, Ebashi M, Kushimoto M, Ikeda J, Egashira F, Yamaguchi S, Watanabe K, Ogawa K, Suzuki Y, Ishihara H, Fujishiro M. Elsberg syndrome related to varicella zoster virus infection with painless skin lesions in an elderly woman with poorly controlled type 2 diabetes mellitus. Ther Clin Risk Manag. 2018;14:1951-1954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Abe M, Araoka H, Kimura M, Yoneyama A. Varicella Zoster Virus Meningoencephalitis Presenting with Elsberg Syndrome without a Rash in an Immunocompetent Patient. Intern Med. 2015;54:2065-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Fujii K, Moriwaki K, Torii T, Hashimoto K, Shiroyama K, Tajima M, Sanuki M, Kurita S. Urinary retention occurring one week after spinal anesthesia: a case of Elsberg syndrome. Can J Anaesth. 2015;62:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Matsumoto H, Shimizu T, Tokushige S, Mizuno H, Igeta Y, Hashida H. Rectal ulcer in a patient with VZV sacral meningoradiculitis (Elsberg syndrome). Intern Med. 2012;51:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Meng L, Yan Z, Wang X, Zhang Y, Zhu Z, Zhu W, Ling Q, Sun X, Gu Y, Lv J, Li Y. Preliminary analysis of stimulation parameters for sacral neuromodulation in different indications: a multicenter retrospective cohort study from China. Int J Surg. 2024;110:3536-3542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Schwarztuch Gildor O, Neheman A, Vainrib M. Feasibility of Sacral Neuromodulation in Patients With Underlying Neurologic Lower Urinary Tract Dysfunction and Fecal Incontinence. Urology. 2024;188:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Tharu NS, Wong AYL, Zheng YP. Transcutaneous Electrical Spinal Cord Stimulation Increased Target-Specific Muscle Strength and Locomotion in Chronic Spinal Cord Injury. Brain Sci. 2024;14:640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Sdrulla AD, Guan Y, Raja SN. Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms. Pain Pract. 2018;18:1048-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/