Published online Nov 26, 2025. doi: 10.12998/wjcc.v13.i33.112684
Revised: August 26, 2025
Accepted: November 5, 2025
Published online: November 26, 2025
Processing time: 110 Days and 15 Hours
Hypoganglionosis is a rare gastrointestinal acquired motility disorder that res
A 33-year-old female presented to the emergency room with massive abdominal distension that rapidly progressed to abdominal compartment syndrome. The pa
Abdominal compartment syndrome can develop secondary to excessive colonic distension. This extreme but rare situation must be addressed immediately. Hypo
Core Tip: We present a case of abdominal compartment syndrome, a condition characterized by increased intra-abdominal pressure causing severe physiological disturbance and multiorgan failure. This situation can occur with a rare gastrointestinal acquired motility disorder called hypoganglionosis. We learned that such condition could present in adult life, developing massive colonic distension, fecal impaction and overwhelming abdominal distension causing abdominal compartment syndrome. It should be recognized that emergency life-saving resection may become necessary in such cases.
- Citation: Bergeron E, Gologan A. Abdominal compartment syndrome with colonic hypoganglionosis and massive colonic distension in a young adult: A case report. World J Clin Cases 2025; 13(33): 112684
- URL: https://www.wjgnet.com/2307-8960/full/v13/i33/112684.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i33.112684
Hypoganglionosis, a rare gastrointestinal acquired motility disorder, resembles Hirschsprung’s disease[1,2]. Either of these diseases can manifest in the adult life[1,3-5]. Hypoganglionosis displays, unlike Hirschsprung’s disease, lower presence and sparse distribution of myenteric and submucosal ganglia throughout the large intestine[2,6]. Few cases of abdominal compartment syndrome associated with Hirschsprung’s disease or hypoganglionosis have been reported[7-10]. Abdominal compartment syndrome is a condition characterized by an increase in intra-abdominal pressure that causes severe physiological disturbance leading to multiorgan failure[11,12]. Massive fecal impaction can ultimately lead to overwhelming abdominal distension causing abdominal compartment syndrome[10]. A case of adult hypoganglionosis with massive rectal and colonic distension causing rapidly progressive abdominal compartment syndrome is presented and discussed.
A 33-year-old female presented to the emergency room of a trauma center with a reported history of recent abdominal distension and tenderness, along with rectal pain.
The patient was an uninsured black female from Bahamas, living in Canada for two years. On a first visit at the emergency room, she reported low central abdominal pain associated with rectal pressure. She reported a past history of possible Hirschsprung’s disease. A diagnosis of rectal fecaloma was made, and she was offered a rectal evacuation by the emergency doctor. She refused and quit the emergency room. She returned three days later with increased abdominal distension and pain, and vomiting of fecaloid material. She denied fever. She was unable to evacuate gas and stools. She was able to breath normally. A surgical consultation was obtained. It was not possible to extract the fecaloma at the bedside. The patient was admitted under close observation. She was hydrated, and prescribed analgesics and acetylcysteine orally and rectally. The patient did not tolerate oral acetylcysteine, and the nurses were unable to instill medication into the rectum because of pain. The patient was reassessed in the middle of the night. She was not vomiting. A discussion with the patient was carried out. The rectal evacuation of the fecaloma could be attempted under general anesthesia. However, the possibility to effectively evacuate the fecaloma remained reserved, owing to the hugeness of the colon and rectum. The marked colorectal dilatation rendered the removal of the fecaloma very difficult and risky. The possibility of abdominal surgery was high and presented to the patient. At this time, the patient preferred to think at the situation and discuss with her parents. A reassessment and further discussion were planned in the morning.
Reassessment early in the morning in the presence of the patient’s mother revealed shortness of breath, increased abdominal distention and worsened rectal and abdominal pain. The decision to transfer to the operating room for laparotomy and the possibility of resection and ostomy was discussed and undertaken. The patient was brought to the operating room three hours later.
The patient was reluctant to give past medical history, and thus the history obtained from the patient was fragmentary. She had constipation that was ill-defined. She denied taking medication and had not had medical attention since her arrival in Canada. She recalled a cesarean section eight years before. She said that she does not know how the diagnosis of Hirschsprung’s disease was done. She said that she had an operation; we thus suspect that she could have a rectal biopsy.
The patient had a cesarean section eight years before. Her eight-year-old boy was reported to be in good health. She denied any particular medical related problem in the family.
Upon admission, the patient was awake, well oriented and collaborative. Temperature was normal. She was eupneic, with a blood pressure of 110/75 mmHg and pulse at 90 beats/minute. The abdomen was very distended and sensitive. Even if the abdomen was tense, it remained depressive without rebound tenderness. There was a suprapubic transversal scar from previous cesarean section and no other abdominal scar. Rectal examination was painful, and the rectum demonstrated an impacted huge and hard fecaloma. At night, she was still eupneic with a blood pressure of 145/90 mmHg and pulse at 120 beats/minute. Temperature was normal. The abdomen showed the same distension and tenderness; however, remained depressible. In the morning, the patient was looking less comfortable. Her pulse rate was 90 beats/minute, blood pressure was 105/60 mmHg, and respiratory rate was 26 breaths/minute. Temperature was normal. Her abdomen was more distended, although still depressible. She was still afebrile.
White cell count was 27.0 × 109/L (Normal: 3.8-10.7). Hemoglobin level, coagulation parameters, renal and liver function tests were all within the normal limits.
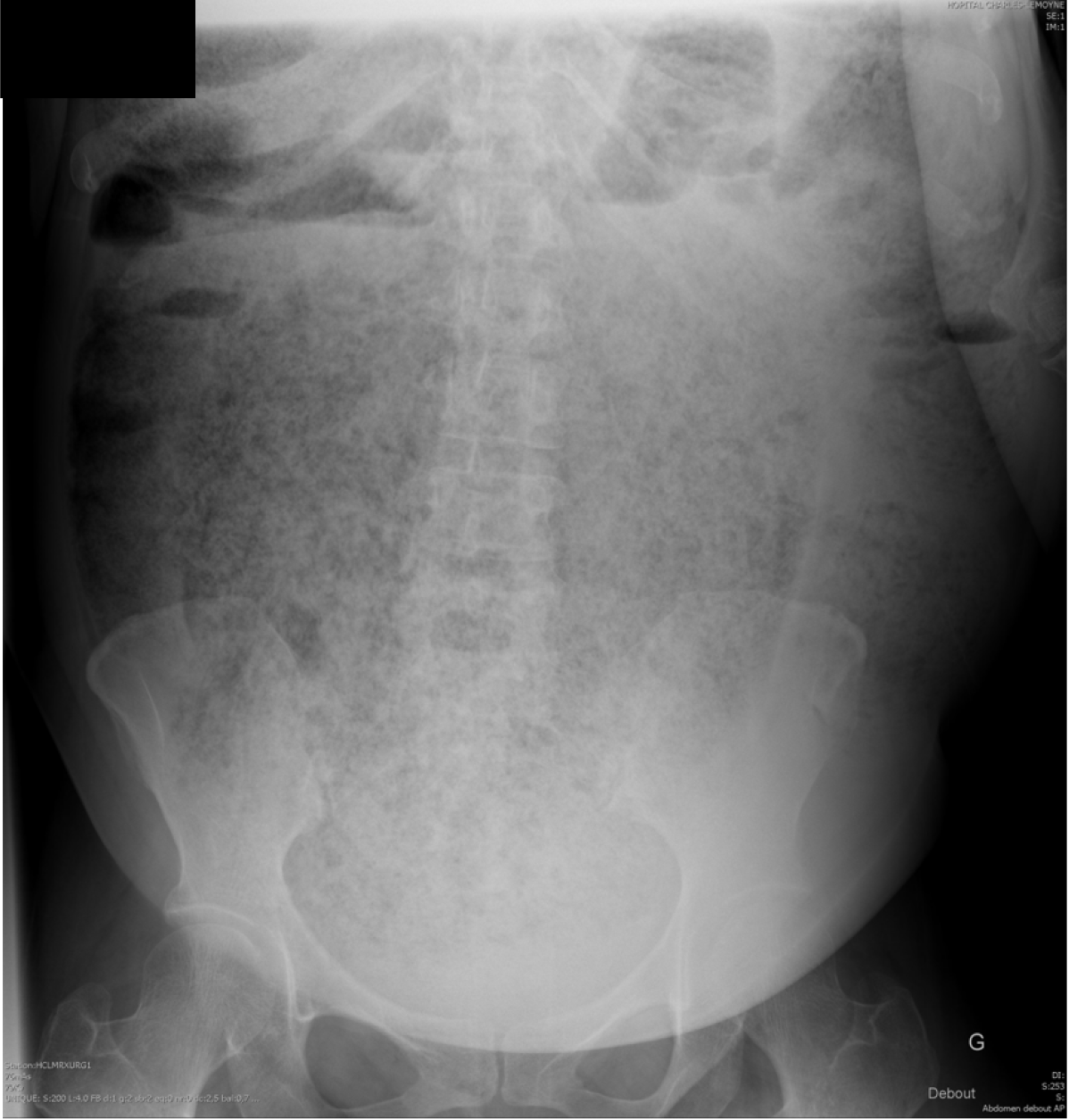
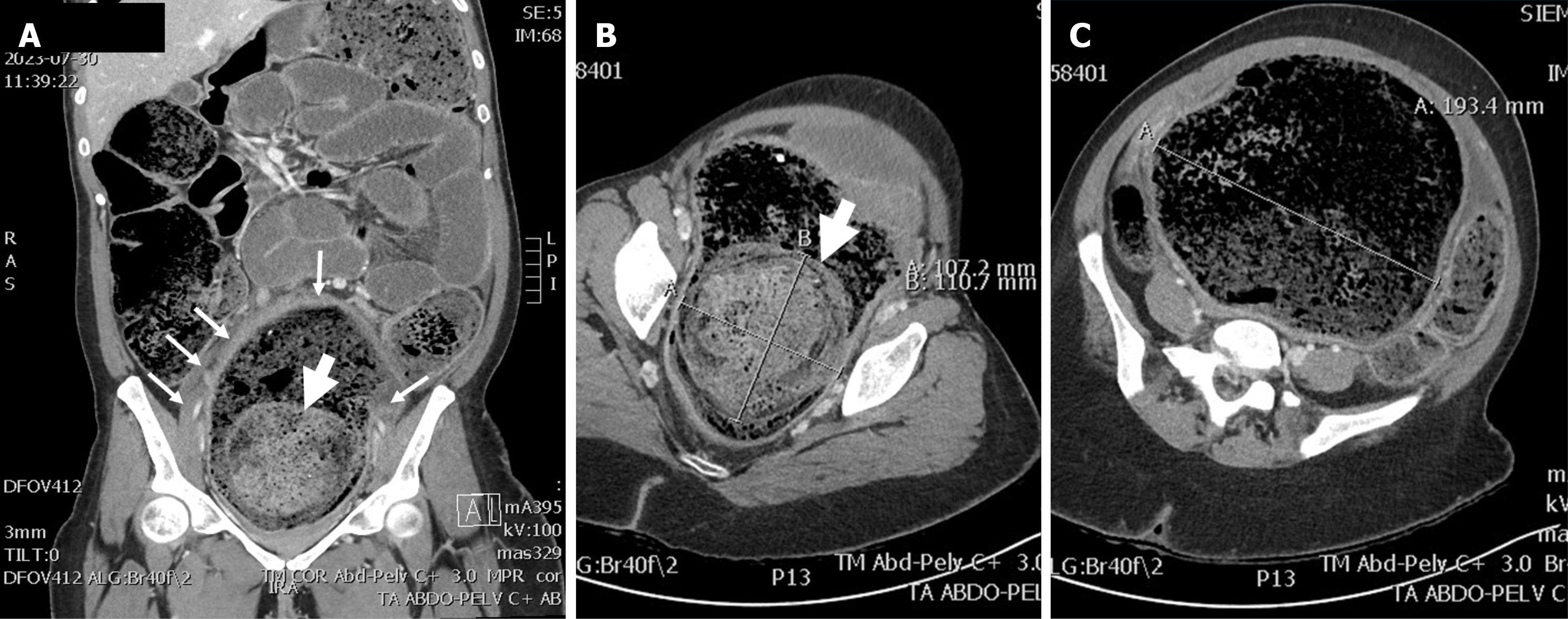
Initial plain film showed a huge and diffuse accumulation of feces (Figure 1). An abdominal computed tomography scan demonstrated accumulation of feces in all parts of the colon and rectum (Figure 2A). The rectum reached 193 mm in diameter (Figure 2C) and a more prominent and dominating mass of stools with a diameter of 110 mm was within the rectum (Figure 2B and C).
Hypoganglionosis, probably acquired, causing megacolon with abdominal compartment syndrome.
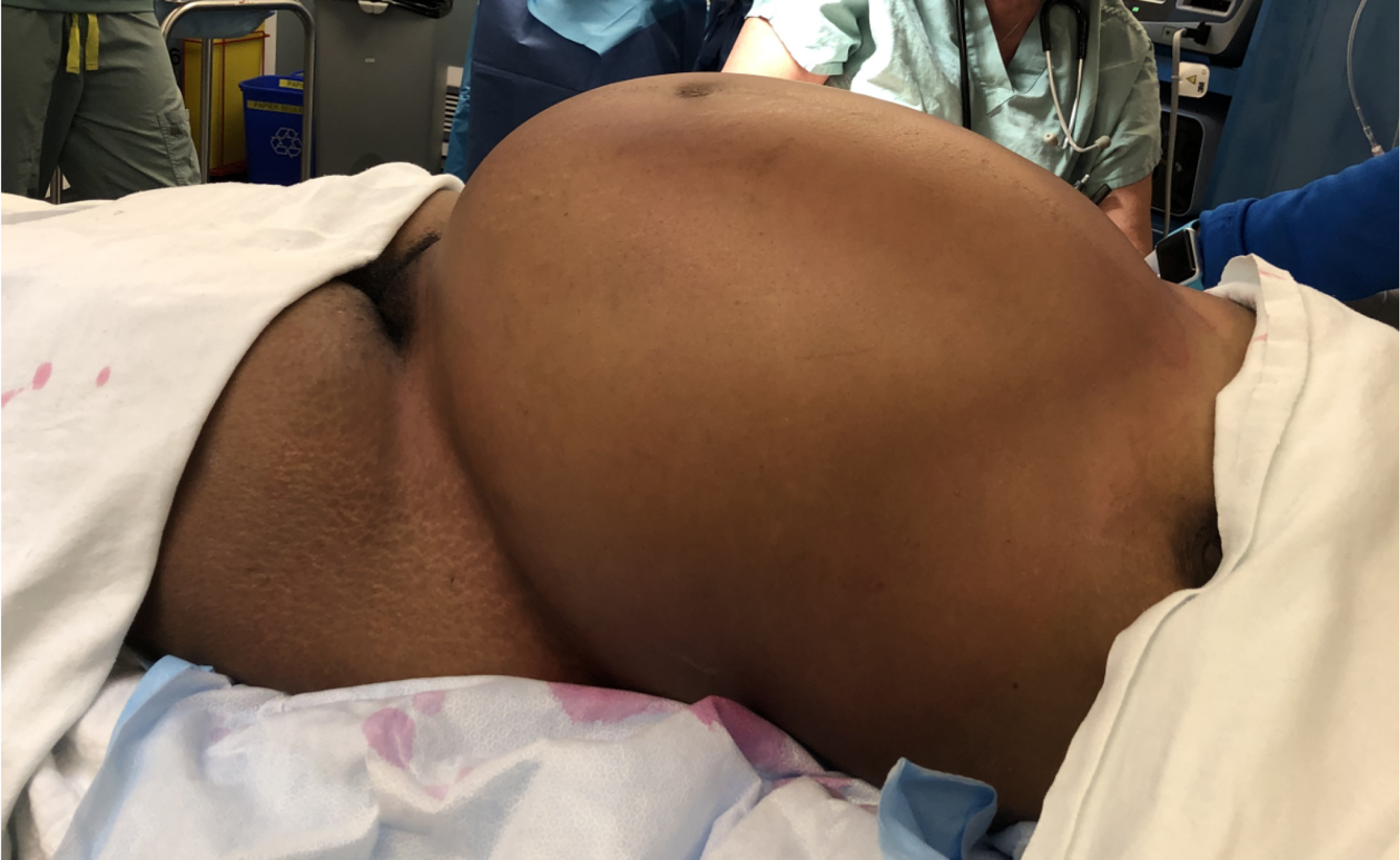
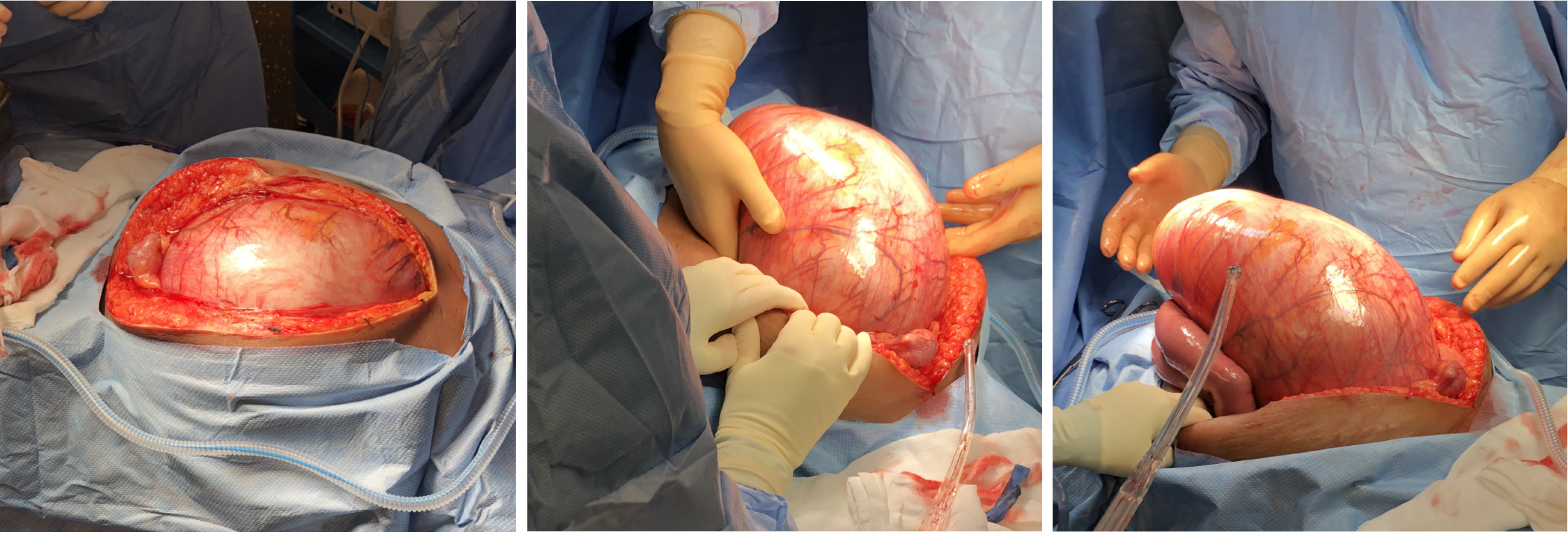
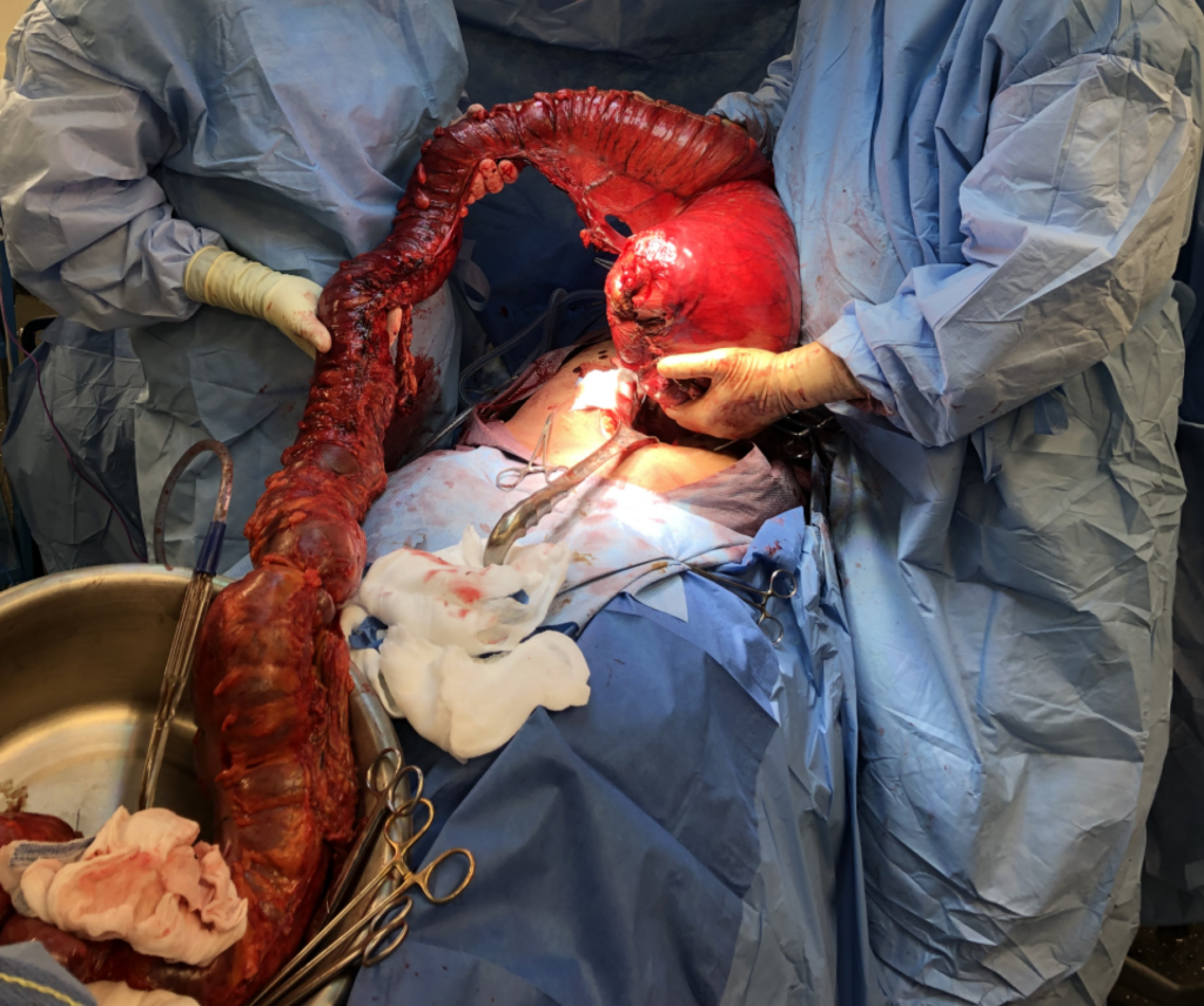
The patient was brought urgently to the operating room (Figure 3). Fast anesthesia setup was carried out. After tracheal intubation, the anesthetist noticed high ventilatory pressures. The systolic blood pressure also dropped to around 70 mmHg with pulse rate increasing to 150 beats/minute. The patient necessitated phenylephrine administration. While installing the bladder catheter, the nurse noticed fecal discharge from the vagina. The vagina was so compressed that a good examination could not be done. No evident lesion was observed at the distal vaginal orifice. A midline xiphopubic incision was rapidly carried out. Upon opening, the enlarged rectum emerged from the laparotomy incision (Figure 4). Just after the incision, the ventilatory pressure decreased and systolic blood pressure could be maintained over 100 mmHg.
As the first step, the rectum was addressed. The dominating mass of stools in the lower rectum was felt hard. It was possible to milk it proximally. Then the rectum was transected at the sacral promontory level. In order to achieve the rectal division without provoking a massive fecal contamination, strong Heaney hysterectomy clamps were used to gradually divide the rectum, using transfixing 0 polypropylene sutures for both proximal and distal stumps. The thickness of the rectal wall was one centimeter. Just this part of the surgical intervention took about one hour to be achieved. The distal stump was reinforced with a 0 polypropylene running suture. The total proctocolectomy was completed (Figure 5) leaving a 10-to-12-centimeter rectal stump, and an end ileostomy was carried out. The intervention lasted 190 minutes. The removed colon was 500 cm long. The predominating rectal fecaloma was measured at 15 cm in diameter. There were many linear lacerations throughout the colon demonstrating impending rupture. The patient received a total of 2600 mL of crystalloids solution and 500 mL of albumin solution. She received no transfusion. She was brought to the intensive care unit intubated but without aminotropic drugs.
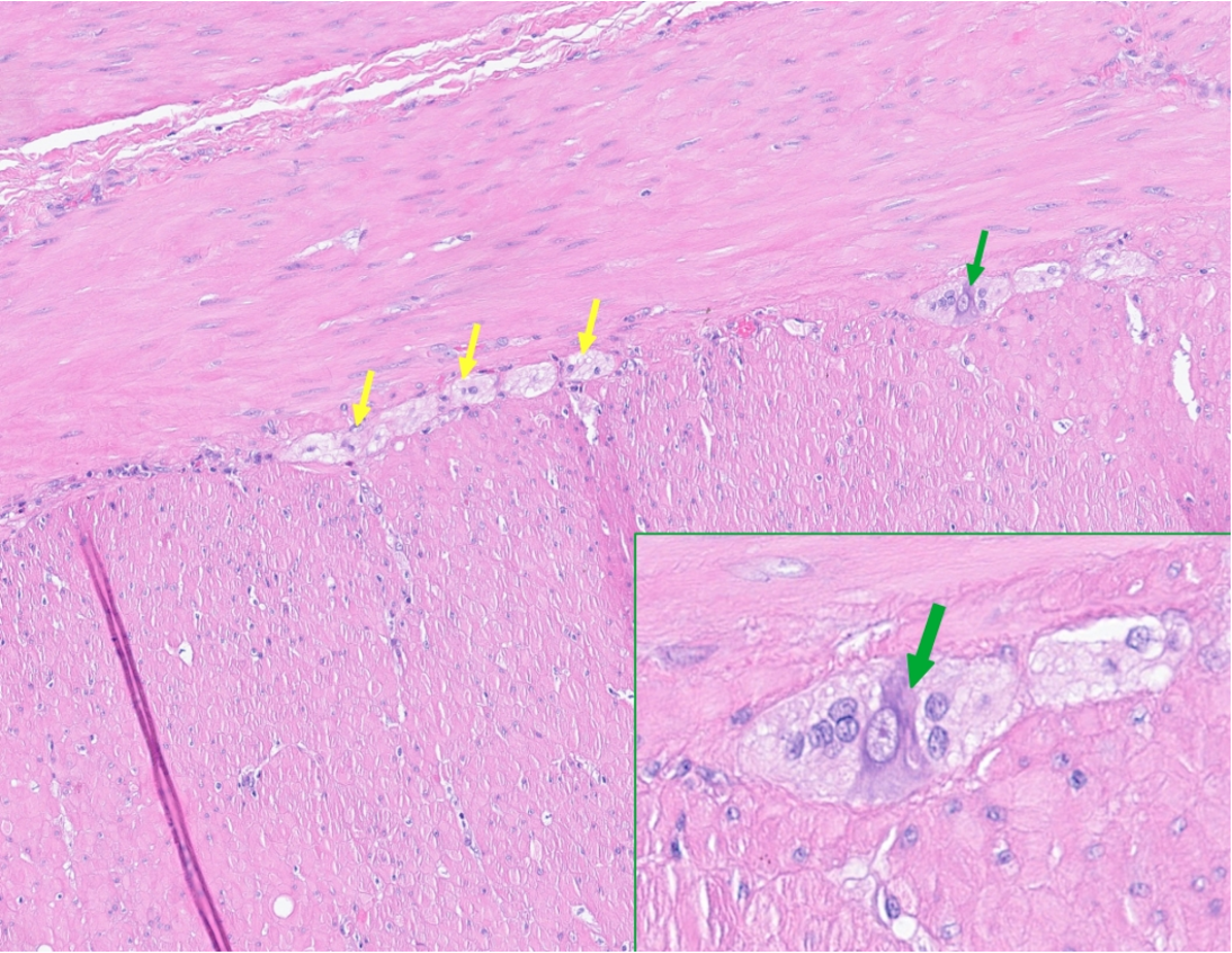
The colon shows markedly reduced ganglion cells, most pronounced distally where they are almost absent (Figure 6). On histologic examination, there is hyperplastic muscularis propria in the distal colon and thinning of the muscularis propria in the proximal colon. There are foci of lympho-plasmocytic infiltration between the muscularis propria layers, where the myenteric plexuses should be. Focally, small infiltrates of lymphocytes and plasma cells are seen between the layers of the muscularis propria, although direct infiltration of the remaining ganglions cannot be documented.
The patient was stabilized in the intensive care unit. She was extubated few hours after transfer to intensive care unit. During the immediate postoperative period, white blood count was 3.6 × 109/L (Normal: 3.8-10.7). Lactates and pH were within normal limits. She was discharged from the intensive care unit after 5 days. She was kept on ceftriaxone and metronidazole for seven days. On the seventh postoperative day, the patient was brought back to the operating room in order to evaluate the rectovaginal fistula. Under general anesthesia, examination of the posterior fornix revealed a small reddish zone of inflammation, which was an evident site of fistula. Stools were evacuated from the rectal stump, which was thoroughly washed with saline and then iodine solution. The rectal stump was satisfactorily closed. The vagina was also washed with saline solution and vaginal douching was prescribed for the postoperative period.
The rest of hospital stay was uneventful. The patient started to eat rapidly, and ileostomy worked normally, with soft stools. The wound healed without infection. She was discharged 10 days after the colectomy without any discharge from the vagina. She presented for follow-up after three weeks. She had no complaints. She had no vaginal discharge. Her abdomen was flat with a very well looking ileostomy. The wound healed without any complication. The patient was planning to return to her original country. A letter was given with a summary of hospitalization for an eventual consultation in surgery. We have no more follow-up thereafter.
Hypoganglionosis is a rare gastrointestinal motility disorder that closely resembles Hirschsprung’s disease[1,2]. It manifests mainly in newborns and infants. However, it can manifest in the adult life[1,3-5]. It causes constipation that can reach catastrophic proportions. Unlike Hirschsprung’s disease characterized by the absence of ganglionic cells of the myenteric and submucosal nervous plexus[7], hypoganglionosis displays the presence, but low number, of myenteric and submucosal ganglia with sparse distribution throughout the large intestine[1,6]. Because of overlap with Hirschsprung’s disease, a definitive diagnosis of myenteric hypoganglionosis requires multiple full thickness biopsies or extensive pathological examination of resected bowel segments[13]. The decline in the number of intestinal ganglion cells reaches 12% to 40% that of a normal innervated colon. Hypoganglionosis represents only 3% to 5% of all congenital intestinal neurological diseases[14]. Hypoganglionosis can also be acquired[4,15-17]. In the acquired form of hypoganglionosis, histologic examination shows gliosis amid a reduced quantity of ganglion cells within the myenteric plexus with preserved plexus size, suggesting that the myenteric plexus initially contained ganglion cells, thereafter destroyed[13,18].
Acquired hypoganglionosis is even rarer[2], and may results from various causes such as Chagas disease, bacterial and viral infections, autoimmune disorders, ischemia[16,19]. The patient reported a past history of Hirschsprung’s disease and operation. The circumstances could not be well clarified except for an imprecise history of chronic constipation. The patient had no abdominal scar except the one from cesarean section. We presumed that she had a rectal biopsy and a false diagnosis of Hirschsprung’s disease because paucity of the ganglion cells could have been seen. The final diagnosis is clearly not Hirschsprung’s disease[10]. Because of the rarity of this specific diagnosis, there is little documentation and no consensus on the specific cause or initiating process of this disease[3,16,20].
However, even if she effectively had a biopsy, the sample might be small and unrepresentative, compared to what we evidently obtained. She was thus probably investigated earlier for constipation. Here again, we further underline the paucity of the history as she did not report chronic abdominal distension, at least not as such as on presentation. The report from the patient was suggestive of a recent and relatively rapid increase in abdominal gait. To a certain point, there was surely an abdominal distension considering the thickness of the colonic wall and the high distension and elongation of the large bowel.
The recently emerging distension in this patient, who was otherwise in good health except for the current situation, might be explained by acquired hypoganglionosis. Patients with acquired hypoganglionosis, unlike the congenital disease, developed symptoms later in life with a long period of chronic constipation or intestinal pseudo-obstruction[16] and the condition is frequently diagnosed after emergency surgery for complications[3]. In these cases of hypoganglionosis, a pathological inflammatory response probably leads to degeneration and destruction of ganglion cells in the lamina propria[2,16,21]. De Giorgio et al[19] well described "enteric ganglionitis" with dense lymphocytic and plasma cell infiltrate that is primarily confined to the myenteric plexus in inflammatory neuropathies. As a consequence, this condition can lead to intestinal pseudo-obstruction and megacolon. However, limited knowledge does not allow conclusions to be drawn about "enteric ganglionitis" as a single etiology for megacolon. No specific cause could have been identified or thought of in the present case. While a recent report after pregnancy[22], the cesarian section eight years ago makes it difficult to establish any relationship between the past pregnancy in this case.
Only few cases of abdominal compartment syndrome associated with Hirschsprung’s disease or hypoganglionosis have been reported[7-10]. Massive fecal impaction leads to megarectum causing abdominal compartment syndrome[10] and also to other abdominal catastrophes such as colorectal obstruction, volvulus, perforation, or necrosis[4,5,8,10,16]. Consensus definitions and clinical practice guidelines have been established for the abdominal compartment syndrome[11,12]. Any increase of intra-abdominal contents is a risk factor for the abdominal compartment syndrome[12], and its diagnosis can be made clinically without the measurement of intra-abdominal pressures using the combination of clinical investigation findings showing huge mass effect from massive fecal impaction[8,9].
In our case, abdominal compartment syndrome became evident owing to parameters observed at the time of anesthesia even though the intra-abdominal pressure was not measured. The situation became an immediate emergency as abdominal compartment syndrome progression was life-threatening. Immediate improvement of the clinical parameters after laparotomy also points towards the correct diagnosis of abdominal compartment syndrome.
Because of the emergency, no alternative, either pharmacological or non-pharmacological[23], was conceivable. The surgery was aimed at solving the life-threatening situation, irrespective of suspected etiology or not. Total colectomy with ileostomy was the only option. The rectal stump usually needs to be evaluated afterwards[1], but diagnosis is already identified in this case. Abdominal resection and pull through procedure is the treatment of choice in adult Hirschsprung’s disease[10,24] and is feasible after a Hartmann’s intervention[10,22]. Low anterior resection gives fair to good results in about 85% of cases, however, leaving a residual hypoganglionic segment in place[10]. Subsequent ileorectal anastomosis could be considered after six to twelve months, however, leaving behind a residual, short, hypoganglionic rectal stump[10].
Acquired hypoganglionosis, a disease of children and young adults[1,3-5], is certainly underreported, particularly in cases with chronic constipation without obvious distension. When chronic constipation remains refractory despite usual differential diagnosis and conservative treatment, particularly when associated with abdominal distention and megacolon, diagnostic tests such as rectal imaging, anorectal manometry and full-thickness rectal biopsy should be considered in order to better define the patient’s condition[25]. In the present case, the lympho-plasmocytic infiltrates suggest a possible auto-immune process. Although the lack of definitive diagnostic criteria and consensus on effective treatment of acquired hypoganglionosis[2,16], in cases of inflammatory neuropathies of the enteric nervous system, steroids alone or combined with other immunosuppressive therapy can be attempted and dramatically improve the clinical picture[19]. Alternatives of pharmacological treatments (transcutaneous electrical nerve stimulation, fecal microbiota transplantation, probiotic, acupuncture) have demonstrated good results in improving chronic constipation in adults and elderly[23,26], but results for children remain inconclusive[27]. Could severe megacolon be prevented or at least detected early? The question is worth asking but is beyond the scope of this paper. However, in severe and refractory and well-established megacolon, surgery remains the definitive treatment option[15,16,20].
In conclusion, we described a potentially catastrophic case of abdominal compartment syndrome secondary to severe colonic hypoganglionosis with excessive distension. This is an extreme but rare situation, and such cases must be addressed immediately. However, it must be recognized that hypoganglionosis is probably more prevalent in cases of severe constipation.
| 1. | Ito T, Kimura T, Yagami T, Maeda N, Komura M, Ohnishi N, Fujita N, Arai K, Tomioka H, Miyatake S, Kobayashi K. Megacolon in an adult case of hypoganglionosis, a pseudo-Hirschsprung's disease: an autopsy study. Intern Med. 2008;47:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Taguchi T, Ieiri S, Miyoshi K, Kohashi K, Oda Y, Kubota A, Watanabe Y, Matsufuji H, Fukuzawa M, Tomomasa T. The incidence and outcome of allied disorders of Hirschsprung's disease in Japan: Results from a nationwide survey. Asian J Surg. 2017;40:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Aldossary MY, Privitera A, Elzamzami O, Alturki N, Sabr K. A Rare Case of Adult-Onset Rectosigmoid Hypoganglionosis. Am J Case Rep. 2018;19:557-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Alsulimani S, Haoues N, Aljuhani AM, Fayoumi N, Al-Sawat A. Sigmoid Volvulus as a Clinical Manifestation of Acquired Colonic Hypoganglionosis: A Case Report. Cureus. 2023;15:e33950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Kahramanca S, Özgehan G, Celep BR, Seker GE, Arıkök AT, Küçükpınar T. Isolated Adult Hypoganglionosis Resulting in Toxic Megacolon: A Case Report. Kafkas J Med Sci. 2014;4:36-39. [DOI] [Full Text] |
| 6. | Kapur RP, Bellizzi AM, Bond S, Chen H, Han JS, LeGallo RD, Midgen C, Poulin AA, Uddin N, Warren M, Velázquez Vega JE, Zuppan CW. Congenital Myenteric Hypoganglionosis. Am J Surg Pathol. 2021;45:1047-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Chuang FJ, Lim AE, Cooper ML, Townend P, Parker DJ. An unusual case of abdominal compartment syndrome from a massive faecaloma. J Surg Case Rep. 2022;2022:rjac348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Usuda D, Takanaga K, Sangen R, Higashikawa T, Kinami S, Saito H, Kasamaki Y. Abdominal compartment syndrome due to extremely elongated sigmoid colon and rectum plus fecal impaction caused by disuse syndrome and diabetic neuropathy: a case report and review of the literature. J Med Case Rep. 2020;14:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Ho S, Krawitz R, Fleming B. Massive faecal impaction leading to abdominal compartment syndrome and acute lower limb ischaemia. BMJ Case Rep. 2018;2018:bcr2018225202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Miyamoto M, Egami K, Maeda S, Ohkawa K, Tanaka N, Uchida E, Tajiri T. Hirschsprung's disease in adults: report of a case and review of the literature. J Nippon Med Sch. 2005;72:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Coccolini F, Roberts D, Ansaloni L, Ivatury R, Gamberini E, Kluger Y, Moore EE, Coimbra R, Kirkpatrick AW, Pereira BM, Montori G, Ceresoli M, Abu-Zidan FM, Sartelli M, Velmahos G, Fraga GP, Leppaniemi A, Tolonen M, Galante J, Razek T, Maier R, Bala M, Sakakushev B, Khokha V, Malbrain M, Agnoletti V, Peitzman A, Demetrashvili Z, Sugrue M, Di Saverio S, Martzi I, Soreide K, Biffl W, Ferrada P, Parry N, Montravers P, Melotti RM, Salvetti F, Valetti TM, Scalea T, Chiara O, Cimbanassi S, Kashuk JL, Larrea M, Hernandez JAM, Lin HF, Chirica M, Arvieux C, Bing C, Horer T, De Simone B, Masiakos P, Reva V, DeAngelis N, Kike K, Balogh ZJ, Fugazzola P, Tomasoni M, Latifi R, Naidoo N, Weber D, Handolin L, Inaba K, Hecker A, Kuo-Ching Y, Ordoñez CA, Rizoli S, Gomes CA, De Moya M, Wani I, Mefire AC, Boffard K, Napolitano L, Catena F. The open abdomen in trauma and non-trauma patients: WSES guidelines. World J Emerg Surg. 2018;13:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 12. | Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, Sugrue M, Cheatham M, Ivatury R, Ball CG, Reintam Blaser A, Regli A, Balogh ZJ, D'Amours S, Debergh D, Kaplan M, Kimball E, Olvera C; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 841] [Cited by in RCA: 891] [Article Influence: 68.5] [Reference Citation Analysis (3)] |
| 13. | Singh N, Petrancosta J, O'Daniel E, Nurko S, Calabro K. A Case Report of a Child with Constipation Diagnosed with Acquired Myenteric Hypoganglionosis. Reports (MDPI). 2025;8:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Dingemann J, Puri P. Isolated hypoganglionosis: systematic review of a rare intestinal innervation defect. Pediatr Surg Int. 2010;26:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Pan ZP, Huang LQ, Cui JH. Acquired segmental sigmoid hypoganglionosis: A case report. Medicine (Baltimore). 2020;99:e18803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Tominaga T, Nagayama S, Takamatsu M, Miyanari S, Nagasaki T, Yamaguchi T, Akiyoshi T, Konishi T, Fujimoto Y, Fukunaga Y, Ueno M. A case of severe megacolon due to acquired isolated hypoganglionosis after low anterior resection for lower rectal cancer. Clin J Gastroenterol. 2020;13:328-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Kwok AM, Still AB, Hart K. Acquired segmental colonic hypoganglionosis in an adult Caucasian male: A case report. World J Gastrointest Surg. 2019;11:101-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Yoshimaru K, Taguchi T, Obata S, Takemoto J, Takahashi Y, Iwanaka T, Yanagi Y, Kuda M, Miyoshi K, Matsuura T, Kinoshita Y, Yoshioka T, Nakazawa A, Oda Y. Immunostaining for Hu C/D and CD56 is useful for a definitive histopathological diagnosis of congenital and acquired isolated hypoganglionosis. Virchows Arch. 2017;470:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | De Giorgio R, Guerrini S, Barbara G, Stanghellini V, De Ponti F, Corinaldesi R, Moses PL, Sharkey KA, Mawe GM. Inflammatory neuropathies of the enteric nervous system. Gastroenterology. 2004;126:1872-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Qadir I, Salick MM, Barakzai A, Zafar H. Isolated adult hypoganglionosis presenting as sigmoid volvulus: a case report. J Med Case Rep. 2011;5:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Holland-Cunz S, Göppl M, Rauch U, Bär C, Klotz M, Schäfer KH. Acquired intestinal aganglionosis after a lytic infection with varicella-zoster virus. J Pediatr Surg. 2006;41:e29-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Sergi W, Serra F, Cucciarrè G, De Ruvo N, Gelmini R. Complication of Hirschsprung's disease immediately after pregnancy: A rare case report. Int J Surg Case Rep. 2021;83:105893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Tan S, Peng C, Lin X, Peng C, Yang Y, Liu S, Huang L, Bian Y, Li Y, Xu C. Clinical efficacy of non-pharmacological treatment of functional constipation: a systematic review and network meta-analysis. Front Cell Infect Microbiol. 2025;15:1565801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Faucheron JL, Verot PL, Sage PY, Girard E, Trilling B. Maladie de Hirschsprung chez l’adulte. In: Techniques chirurgicales appareil digestif. Paris: Elsevier Masson SAS, 2009: 40-602. [DOI] [Full Text] |
| 25. | Cho YS, Lee YJ, Shin JE, Jung HK, Park SY, Kang SJ, Song KH, Kim JW, Lim HC, Park HS, Kim SJ, Cha RR, Bang KB, Bang CS, Yim SK, Ryoo SB, Kye BH, Ji WB, Choi M, Sung IK, Choi SC; Korean Society of Neurogastroenterology and Motility. 2022 Seoul Consensus on Clinical Practice Guidelines for Functional Constipation. J Neurogastroenterol Motil. 2023;29:271-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 26. | Zheng H, Chen Q, Chen M, Wu X, She TW, Li J, Huang DQ, Yue L, Fang JQ. Nonpharmacological conservative treatments for chronic functional constipation: A systematic review and network meta-analysis. Neurogastroenterol Motil. 2019;31:e13441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Wegh CAM, Baaleman DF, Tabbers MM, Smidt H, Benninga MA. Nonpharmacologic Treatment for Children with Functional Constipation: A Systematic Review and Meta-analysis. J Pediatr. 2022;240:136-149.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (3)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/