Published online Nov 16, 2025. doi: 10.12998/wjcc.v13.i32.111551
Revised: July 20, 2025
Accepted: September 23, 2025
Published online: November 16, 2025
Processing time: 133 Days and 0.5 Hours
Intracranial blister-like microaneurysms are an extremely rare disease. Rupture of intracranial aneurysms can lead to subarachnoid hemorrhage (SAH). Patients with SAH may experience severe neurological symptoms, including severe hea
This article reports the case of 71-year-old female patient with an intracranial aneurysm. The patient experienced a sudden headache with vomiting for 3 hours. Brain computed tomography (CT) scan showed a subarachnoid hemorrhage. She was diagnosed with rupture of an aneurysm and subarachnoid hemorrhage. The aneurysm was located in the choroidal segment of the right internal carotid artery. The size of the aneurysm was 2.00 mm × 1.80 mm × 1.97 mm, and the neck of the aneurysm was less than 0.5 mm wide. We successfully treated this aneu
Endovascular electrocoagulation is an effective and safe method for the treatment of intracranial blister-like microaneurysms.
Core Tip: Microaneurysms with a diameter of less than 2 mm and a neck of less than 0.5 mm are very rare. This type of microaneurysm is characterized by a small lumen and thin wall and is extremely challenging for surgeons. This article introduces the process of endovascular electrocoagulation for the treatment of aneurysms and provides a feasible solution for the treatment of microaneurysms.
- Citation: Zhang ZY, Zhang XY, Lu GZ, Wang SL, Hao JH, Zhang LY. Endovascular electrocoagulation for treating a blister-like microaneurysm with an extremely narrow neck: A case report. World J Clin Cases 2025; 13(32): 111551
- URL: https://www.wjgnet.com/2307-8960/full/v13/i32/111551.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i32.111551
The incidence of intracranial aneurysms is 1%-2%[1]. Microaneurysms with a diameter of less than 3 mm account for 15% of ruptured hemorrhagic aneurysms[2]. Microaneurysms are characterized by a small lumen and thin wall, which leads to limited operational space and difficulty in microcatheter placement, thus increasing the risk associated with endovascular interventional therapy[3]. Schuette et al[4] retrospectively analyzed 347 cases of aneurysms and reported that the risk of intraoperative rupture of aneurysms with a diameter of < 4 mm was 5 times greater than that of larger aneurysms. Brinjikji et al[5] conducted a meta-analysis of endovascular treatment for tiny aneurysms and reported that 95.3% of the 422 aneurysms showed complete or near-complete occlusion after embolization. Rupture of an intracranial aneurysm can cause subarachnoid hemorrhage, which in turn causes severe neurological damage or even death. Subarachnoid hemorrhage from a ruptured intracranial aneurysm is a life-threatening stroke that affects younger patients than those affected by other forms of stroke[6]. The risk of intracranial aneurysms is increased among people with a family history; the risk becomes higher if two or more first-degree relatives have experienced such an event[7]. Factors associated with an increased risk of aneurysm rupture include hypertension, smoking, alcohol abuse, the use of sympathomimetic drugs, and an aneurysm larger than 7 mm[8]. Aneurysmal subarachnoid hemorrhage is more common among women than men, and the incidence increases with age to a peak among people in their 50s[9]. However, very few cases exist in which endovascular electrocoagulation technology has been used to treat blood blister-like microaneurysms. This case demonstrates that endovascular electrocoagulation is an effective and safe approach for treating blood blister-like microaneurysms.
A 71-year-old female experienced a sudden headache with vomiting for 3 hours.
The patient developed a headache 3 hours prior, accompanied by 2 episodes of vomiting of the gastric contents. The patient had not experienced loss of consciousness, limb convulsions, or incontinence of urine or feces. An emergency cranial computed tomography (CT) scan revealed a subarachnoid hemorrhage.
The patient had a previous history of good health and denied a history of hypertension, heart disease, diabetes, or cerebrovascular disease.
The patient had no relevant family history.
The following vital signs were recorded: Respiratory 17 breaths per minute. Pulse 78 beats per minute. Temperature 36.2 °C. Blood pressure 169/78 mmHg. The patient was in a state of shallow coma with a Glasgow Coma Scale score of 12; the eyes were opened in response to verbal stimulation, and irrelevant answers were given. The bilateral pupils were round and equal in size, measuring 2.5 mm in diameter, with a sluggish light reflex. The patient had nuchal rigidity, localized limb pain upon stimulation, and negative bilateral Babinski signs.
Laboratory tests revealed no significant abnormalities.
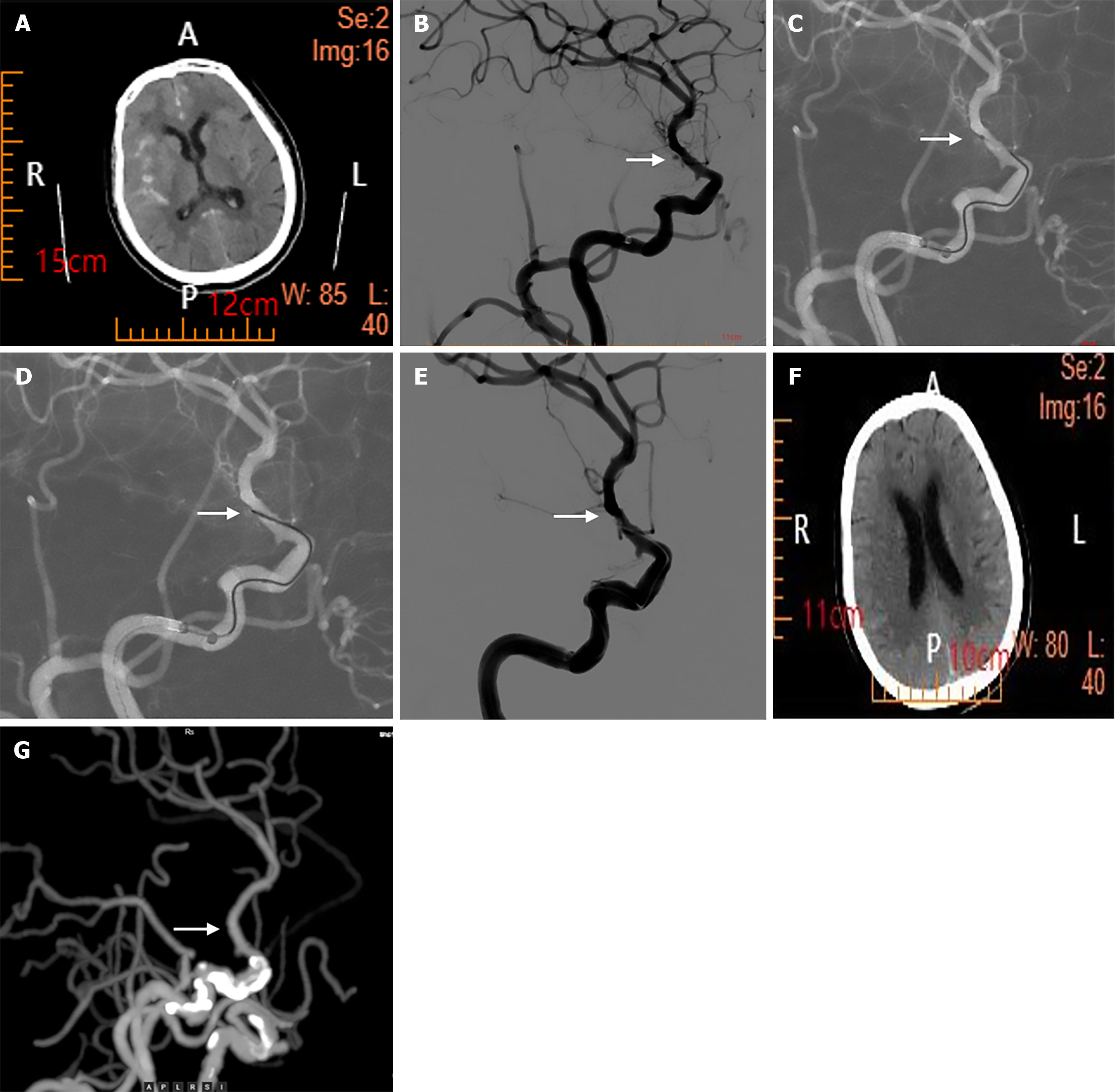
Cranial CT revealed a subarachnoid hemorrhage (Figure 1).
The patient was diagnosed with rupture of an aneurysm in the choroidal segment of the right internal carotid artery and subarachnoid hemorrhage.
The patient was scheduled for cerebral angiography and interventional embolization of the aneurysm. The patient was given 300 mg of aspirin and 300 mg of Plavix before the operation. After general anesthesia was successfully induced, Seldinger puncture was performed on the right femoral artery, and a 6F arterial sheath was inserted. During cerebral angiography, a blister-like microaneurysm was found in the choroidal segment of the internal carotid artery. The size of the aneurysm was 2.00 mm × 1.80 mm × 1.97 mm, and the neck of the aneurysm was less than 0.5 mm wide. The aneurysm neck was so narrow that the Echelon 10 microcatheter could not reach the aneurysm lumen. Thus, we introduced the tip of the ASAHI INTECC 0.014 guidewire into the aneurysm lumen, connected the proximal end to the Solitaire stent detachment system, and coagulated the lesion with a current of 4.8 V and 1.0 mA for 1 minute. After coagulation, repeat angiography was performed, which revealed delayed visualization of the aneurysm. The procedure was repeated for 5 minutes, with further delayed visualization of the aneurysm. Finally, after the third continuous coagulation for 10 minutes, angiography revealed that no contrast agent flowed into the aneurysm lumen. After the site was observed for half an hour, the aneurysm was not revisualized, and the parent artery remained patent.
Three days after the procedure, a follow-up CT revealed no obvious subarachnoid hemorrhage. Three months later, a follow-up cranial computed tomography angiography revealed that the original lesion did not recur and had a good prognosis (Table 1).
| Before surgery | During surgery | 3 days after surgery | 3 months after surgery | |
| Imaging examination | Computed tomography scan showed a subarachnoid hemorrhage | Cerebral angiography revealed an aneurysm in the choroidal segment of the right internal carotid artery; after endovascular electrocoagulation, this site was free of contrast agent extravasation | CT shows no obvious subarachnoid hemorrhage | CT angiography shows no contrast agent extravasation in the original lesion, with a good prognosis |
The wall of the aneurysm is thin and fragile, so the method of clipping the aneurysm carries a high risk. Therefore, they are more amenable to endovascular interventional therapy[10], such as flow diversion, coil therapy, and balloon-assisted or stent-assisted techniques. Flow diversion is a promising treatment option. The principle of this method is that the use of pipeline embolization device can prevent blood from flowing into the aneurysm[11]. However, in this case, the aneurysm was located close to the anterior choroidal artery and posterior communicating artery, flow diversion may affect the blood flow in these branch vessels[12]. Considering that the anterior choroidal artery is involved in supplying blood to important functional areas, a flow diverter device was not used in this patient. Moreover, the high cost of flow diversion is also a factor that cannot be ignored.
Microaneurysms often occur in low-flow arteries and tend to undergo spontaneous embolization, with the potential to transform into benign lesions. Therefore, the risk of such aneurysms is often underestimated. The neck of the aneurysm was less than 0.5 mm wide; thus, the aneurysm neck was too narrow to allow the microcatheter to enter the aneurysm lumen. Therefore, the surgical strategy was adjusted intraoperatively: Microguidewire coagulation was used to occlude the ruptured and bleeding aneurysm. The selection and adjustment of microguidewires are crucial for the success of coagulation procedures. Microguidewires should not only have strong deliverability but also excellent electrical conductivity. Theoretically, greater power and longer coagulation times can promote faster thrombus formation. However, regarding safety considerations, the coagulation power should be reduced to a safe range to avoid damaging the wall of the parent artery. The aneurysm can gradually be occluded by increasing the coagulation time in a stepwise manner.
The mechanism underlying endovascular electrocoagulation for intracranial microaneurysms remains unclear. A possible mechanism is that a certain range of constant direct currents can attract negatively charged factors in the blood, such as platelets, red blood cells, white blood cells, fibrin, and clotting factors, to induce thrombus formation. Ad
In addition, there are several emerging therapies for the treatment of subarachnoid hemorrhage[15]. The use of nanoparticles (NPs) has emerged as a promising new method for targeted drug delivery[16]. Microcatheters can be used to deliver NPs loaded with drugs that promote endothelialization or anti-inflammation at the target site, which significantly increases the local drug concentration, accelerates the healing of aneurysms, and reduces the recurrence rate. We speculate that the combined use of endovascular interventional technology and NPs will lead to better treatment of subarachnoid hemorrhage.
The microguidewire coagulation technique is an effective and simple method for treating ruptured microaneurysms with good efficacy and no need for antiplatelet therapy. Short-term follow-up results have demonstrated its safety and effectiveness, but large samples and long-term follow-up data are still needed for verification.
| 1. | Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014;13:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 2. | van Rooij WJ, Keeren GJ, Peluso JP, Sluzewski M. Clinical and angiographic results of coiling of 196 very small (< or = 3 mm) intracranial aneurysms. AJNR Am J Neuroradiol. 2009;30:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Nguyen TN, Raymond J, Guilbert F, Roy D, Bérubé MD, Mahmoud M, Weill A. Association of endovascular therapy of very small ruptured aneurysms with higher rates of procedure-related rupture. J Neurosurg. 2008;108:1088-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Schuette AJ, Hui FK, Spiotta AM, Obuchowski NA, Gupta R, Moskowitz SI, Tong FC, Dion JE, Cawley CM. Endovascular therapy of very small aneurysms of the anterior communicating artery: five-fold increased incidence of rupture. Neurosurgery. 2011;68:731-7; discussion 737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Brinjikji W, Lanzino G, Cloft HJ, Rabinstein A, Kallmes DF. Endovascular treatment of very small (3 mm or smaller) intracranial aneurysms: report of a consecutive series and a meta-analysis. Stroke. 2010;41:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50:1413-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 461] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 7. | Bor AS, Koffijberg H, Wermer MJ, Rinkel GJ. Optimal screening strategy for familial intracranial aneurysms: a cost-effectiveness analysis. Neurology. 2010;74:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Broderick JP, Brown RD Jr, Sauerbeck L, Hornung R, Huston J 3rd, Woo D, Anderson C, Rouleau G, Kleindorfer D, Flaherty ML, Meissner I, Foroud T, Moomaw EC, Connolly ES; FIA Study Investigators. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. 2009;40:1952-1957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Shea AM, Reed SD, Curtis LH, Alexander MJ, Villani JJ, Schulman KA. Characteristics of nontraumatic subarachnoid hemorrhage in the United States in 2003. Neurosurgery. 2007;61:1131-1137; discussion 1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Sanchez-Mejia RO, Lawton MT. Distal aneurysms of basilar perforating and circumferential arteries. Report of three cases. J Neurosurg. 2007;107:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Zhu D, Fang Y, Yang P, Zhang P, Chen L, Xu Y, Hong B, Huang Q, Liu JM. Overlapped Stenting Combined with Coiling for Blood Blister-Like Aneurysms: Comparison of Low-Profile Visualized Intraluminal Support (LVIS) Stent and Non-LVIS Stent. World Neurosurg. 2017;104:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Kim BM, Shin YS, Baik MW, Lee DH, Jeon P, Baik SK, Lee TH, Kang DH, Suh SI, Byun JS, Jung JY, Kwon K, Kim DJ, Park KY, Kim BS, Park JC, Kim SR, Kim YW, Kim H, Jo K, Yoon CH, Kim YS. Pipeline Embolization Device for Large/Giant or Fusiform Aneurysms: An Initial Multi-Center Experience in Korea. Neurointervention. 2016;11:10-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Kassell NF, Torner JC. Aneurysmal rebleeding: a preliminary report from the Cooperative Aneurysm Study. Neurosurgery. 1983;13:479-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 249] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR; CARAT Investigators. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke. 2008;39:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 391] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 15. | Findlay MC, Kundu M, Nelson JR, Cole KL, Winterton C, Tenhoeve S, Lucke-Wold B. Emerging Treatments for Subarachnoid Hemorrhage. CNS Neurol Disord Drug Targets. 2024;23:1345-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Hey G, Mehkri I, Mehkri Y, Maqbool H, Tahirkheli M, Woodford S, Lucke-Wold B. Nanoparticle-Based Therapies for Management of Subarachnoid Hemorrhage, Neurotrauma, and Stroke. Biomedicines. 2024;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/