Published online Nov 16, 2025. doi: 10.12998/wjcc.v13.i32.111525
Revised: July 25, 2025
Accepted: October 14, 2025
Published online: November 16, 2025
Processing time: 133 Days and 10.2 Hours
To evaluate the diagnostic utility of endobronchial ultrasound (EBUS)-guided mediastinal lymph node fenestration biopsy in atypical sarcoidosis using fine biopsy forceps [i.e., EBUS-transbronchial forceps biopsy (TBFB)].
In this case series, two atypical sarcoidosis cases admitted in 2024 were ret
Both cases demonstrated non-caseating granulomatous inflammation on his
Core Tip: This report highlights the diagnostic value of endobronchial ultrasound-guided transbronchial forceps biopsy (EBUS-TBFB) for atypical sarcoidosis. In two asymptomatic cases with non-specific imaging mimicking malignancy, conventional EBUS-guided fine-needle aspiration yielded inconclusive cytology. By contrast, EBUS-TBFB - using a 1.2-mm forceps via the same puncture site - obtained larger histological samples (≥ 3 mm), confirming non-caseating granulomas. This technique overcomes transbronchial needle aspiration’s limitations for benign diseases, improving diagnostic accuracy and guiding critical management decisions: Observation for localized disease (Case 1) or timely immunosuppression for systemic involvement (Case 2). EBUS-TBFB is a promising tool for radiologically ambiguous sarcoidosis.
- Citation: Yu WX, Zhan FF, Hong PY, Huang MH, Chen YY, Lin YL, Zhang XB. Atypical sarcoidosis diagnosed using endobronchial ultrasound-guided mediastinal lymph node biopsy with fine biopsy forceps: Two case reports. World J Clin Cases 2025; 13(32): 111525
- URL: https://www.wjgnet.com/2307-8960/full/v13/i32/111525.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i32.111525
Sarcoidosis is a multisystem granulomatous disorder characterized by non-caseating granulomas, predominantly affecting the lungs and mediastinal lymph nodes. While bilateral hilar lymphadenopathy is its classic radiographic hallmark, atypical presentations - such as unilateral masses, pleural involvement, or extrapulmonary lesions - often mimic malignancies and complicate diagnosis. Approximately 10%-15% of patients remain asymptomatic, further delaying detection. Conventional endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) frequently yields inadequate cytological samples for diagnosing granulomatous diseases. EBUS-guided transbronchial forceps biopsy (EBUS-TBFB) has emerged to address this limitation by enabling histological sampling through the same puncture tract, yet its utility in radiologically ambiguous sarcoidosis is underreported. We present two asymptomatic cases with positron emission tomography-computed tomography (PET-CT) findings initially suggestive of malignancy, where EBUS-TBFB secured definitive histopathological confirmation of sarcoidosis. These cases illustrate the technique’s critical role in diagnosing atypical presentations and guiding management.
Case 1: A 43-year-old female was admitted to the hospital with the complaint of “incidental lung shadows detected 20 days earlier”.
Case 2: A 52-year-old female was admitted with a chief complaint of “incidental lung mass detected on routine chest CT 20 days prior”.
Case 1: The patient had no symptoms such as cough, sputum, fever, dyspnea, chest tightness, or pain.
Case 2: The patient was asymptomatic and reported no cough, hemoptysis, fever, night sweats, chest pain, or dyspnea.
Case 1: The patient had a 5-year history of chronic sinusitis.
Case 2: The patient denied chronic illnesses or occupational exposures.
Case 1: No smoking or alcohol use, no family history.
Case 2: No smoking or alcohol use, no family history.
Case 1: Physical examination revealed clear lung sounds without crackles or wheezing.
Case 2: Physical examination showed no palpable lymphadenopathy or abnormal breath sounds.
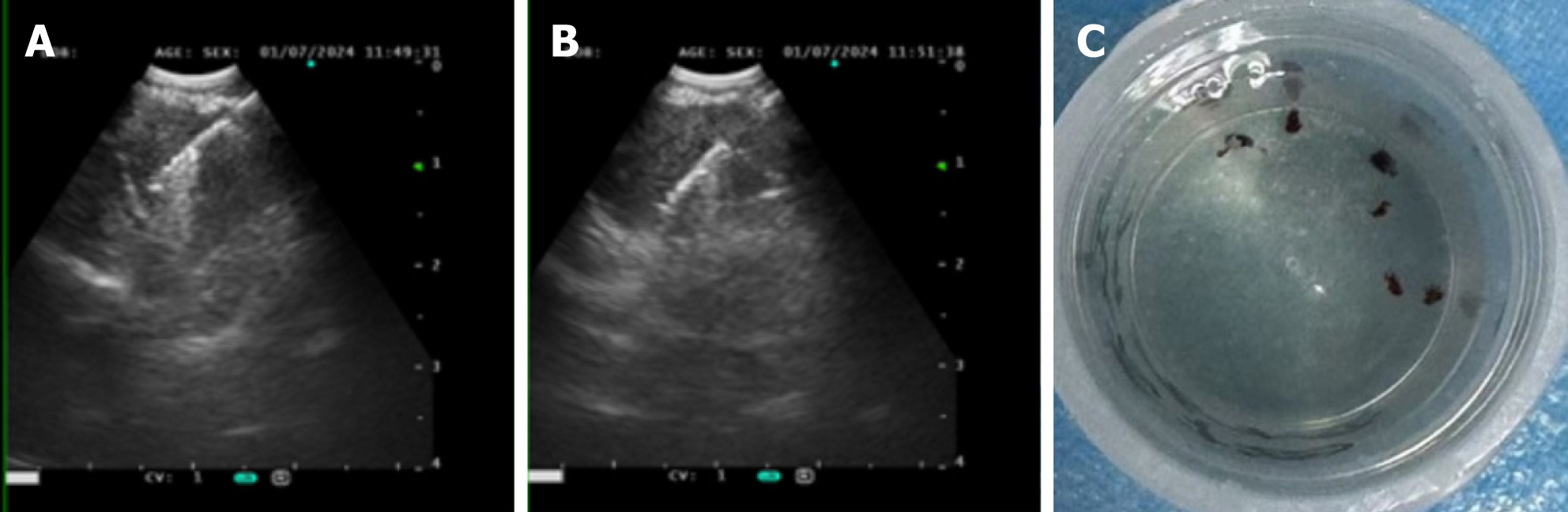
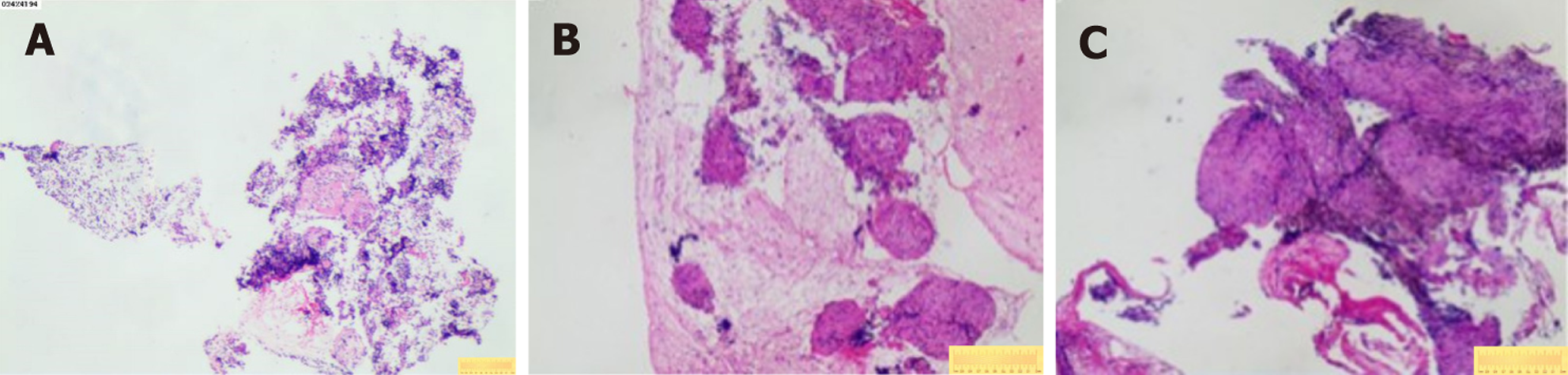
Case 1: Bronchoscopic lavage (June 7, 2024) showed no tumor cells. There were no remarkable results in initial diagnostic evaluations, including routine blood tests, erythrocyte sedimentation rate, biochemistry, coagulation profile, cardiac markers, and serum tumor markers. A repeat procedure was performed on July 1, 2024, under informed consent due to inconclusive findings from the previous bronchoscopy. Bronchoalveolar lavage fluid analysis showed no pathogens but a lymphocyte count of 12002/μL and a CD4/CD8 ratio of 5.81. A biopsy of the right upper lobe apical segment lesion was obtained, followed by EBUS-TBNA using an Olympus 21-G needle to sample station 7 Lymph nodes and posterior upper tracheal soft tissue (Figure 1). Subsequently, EBUS-TBFB was conducted by inserting a 1.2-mm fine biopsy forceps through the pre-established 21-G puncture site under real-time ultrasound guidance. As a result, 4-6 mediastinal biopsies with specimens ≥ 3 mm in diameter were obtained. No post-procedural bleeding was observed. There were scattered epithelial cells, inflammatory infiltrates, and multinucleated giant cells, but no atypia or granulomas in the histopathological analysis of EBUS-TBNA specimens, whereas EBUS-TBFB samples confirmed non-caseating granulomas with multinucleated giant cells (Figure 2A). Immunohistochemistry demonstrated Cytokeratin pan (epithelial+), CD68 (KP-1+), S-100 (−), and Ki-67 (high expression in inflammatory cells), while special stains for acid-fast bacilli, periodic acid-schiff, and Gomori methenamine silver were negative. Serum angiotensin-converting enzyme levels were elevated at 76.3 U/L.
Case 2: Initial diagnostic evaluations revealed thrombocytopenia (55 × 109/L) with normal coagulation parameters, biochemistry, tumor markers, and negative antinuclear antibodies. Pulmonary function tests indicated mild obstructive ventilatory impairment, while a nasopharyngeal biopsy demonstrated lymphoid hyperplasia. There was no remarkable abnormal phenomenon in fundoscopic examination, and a bone marrow biopsy performed on December 17, 2024, showed partial megakaryocyte maturation arrest without atypia or fibrosis. On December 19, 2024, bronchoscopy with bronchoalveolar lavage fluid analysis revealed a CD4/CD8 ratio of 4.68 under general anesthesia. Concurrently, EBUS-TBNA using an Olympus 21-G needle (three passes) and EBUS-TBFB were performed on subcarinal (station 7) lymph nodes via the same puncture site, adhering to the protocol described in Case 1. The EBUS-TBFB yielded specimens with a diameter of at least 3 mm. In accordance with the histopathological examination, non-caseating granulomas without necrosis were identified (Figure 2B and C), and serum angiotensin-converting enzyme levels were elevated at 51.1 U/L.
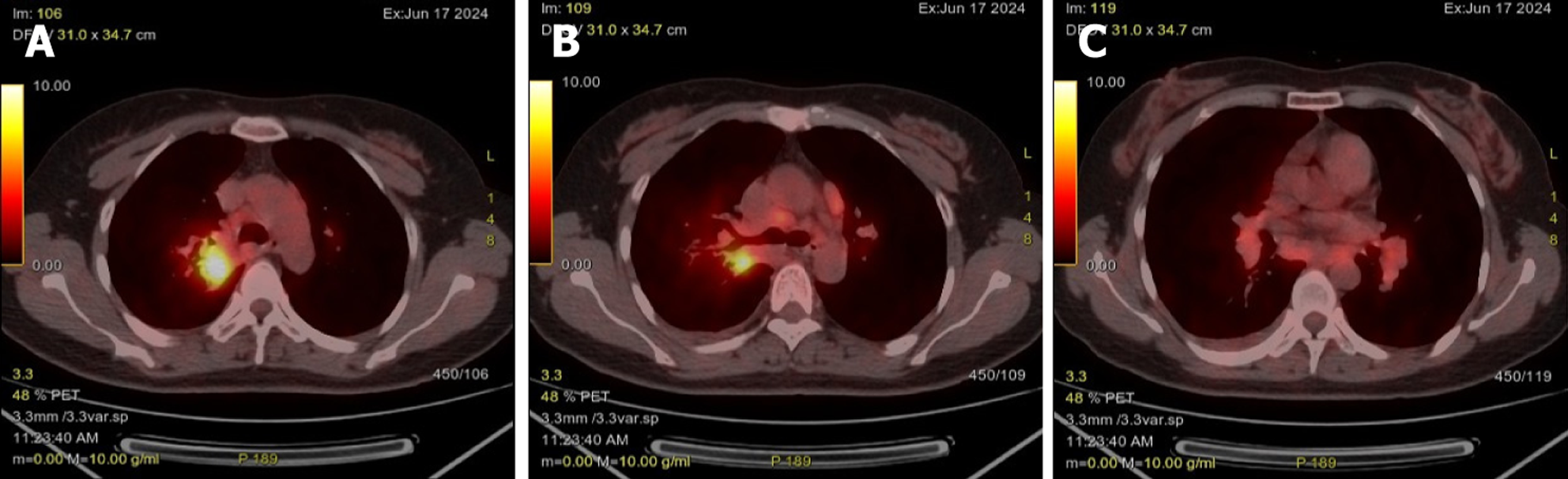
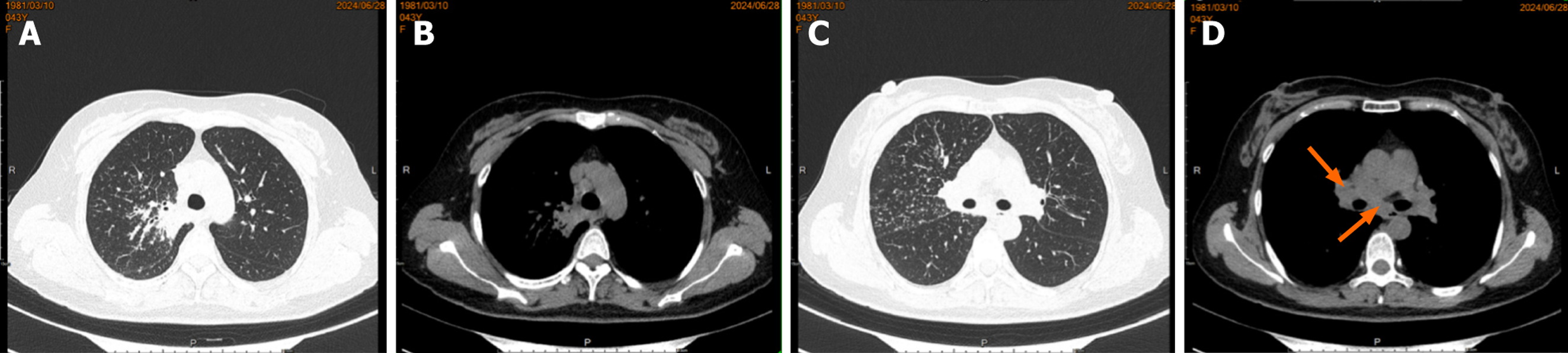
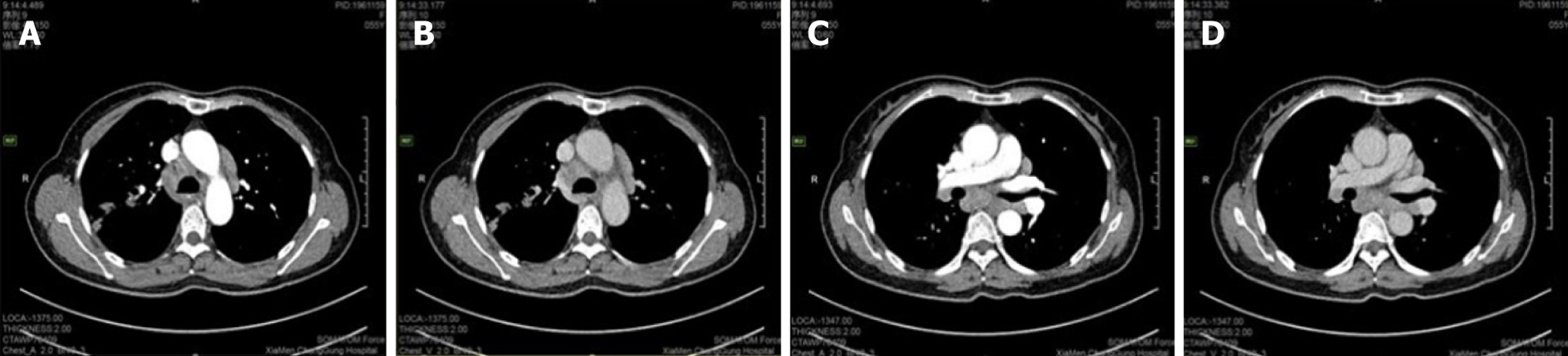
Case 1: A routine chest CT 20 days earlier had revealed a right lung shadow. Subsequent contrast-enhanced chest CT (June 6, 2024) at an external hospital demonstrated a hypervascular right hilar mass with surrounding obstructive pneumonia, scattered enlarged mediastinal lymph nodes, and right pleural effusion. PET-CT suggested malignancy in the right upper lobe with possible pleural involvement, lymphangitic carcinomatosis, and metastases to bilateral hilar/mediastinal lymph nodes (June 17, 2024; Figure 3). In addition, multiple subpleural lung nodules with low metabolic activity were observed. A repeat contrast-enhanced chest CT on June 28, 2024, confirmed a 2.7 cm × 2.0 cm irregular, heterogeneous mass in the right upper lobe, accompanied by obstructive pneumonia and enlarged right hilar and medi
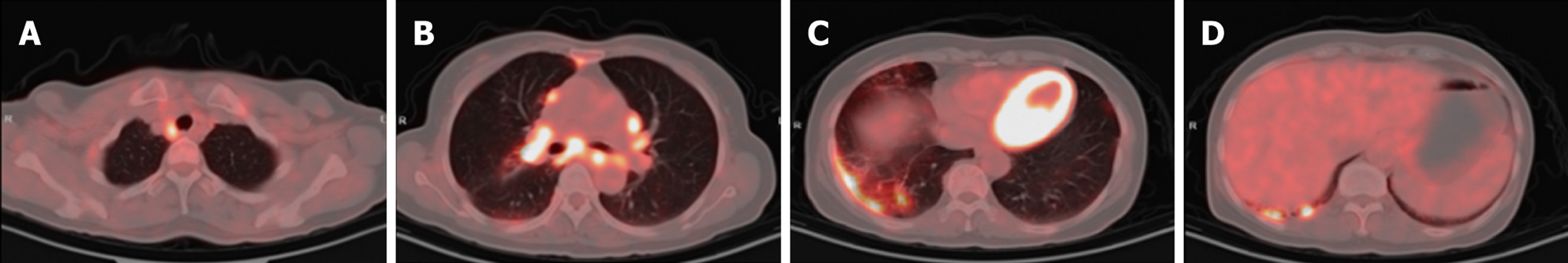
Case 2: Initial contrast-enhanced chest CT at a local hospital revealed multiple enlarged lymph nodes in the right supraclavicular fossa, mediastinum, and bilateral hilar regions (some poorly demarcated from the mid-esophagus), bilateral pulmonary nodules, right bronchial dilation with acute/chronic infection, and localized right pleural thickening (Figure 5). Subsequent PET-CT (December 11, 2024) demonstrated the following symptoms (Figure 6): (1) Widespread hypermetabolic lymphadenopathy (bilateral supraclavicular, mediastinal, hilar, internal mammary, abdominal, and retroperitoneal nodes; SUVmax 8.16; (2) Nasopharyngeal thickening with hypermetabolism; (3) Bilateral hypermetabolic pulmonary consolidations with interstitial changes (prominent in the right upper lobe posterior segment); (4) T11 vertebral focal hypermetabolism (no CT evidence of bone destruction); and (5) Splenomegaly (normal metabolism).
Stage II sarcoidosis.
Sarcoidosis with extrapulmonary involvement.
In alignment with guidelines[1] and patient preference, a conservative management plan involving observation without pharmacotherapy and regular follow-up to monitor disease progression.
Given mild pulmonary dysfunction, thrombocytopenia, and extrapulmonary manifestations, treatment with prednisone (0.5 mg/kg/day) was initiated, along with gastroprotective agents and osteoporosis prophylaxis, and ongoing follow-up was arranged to monitor clinical progression.
Cases 1 and 2: The patient’s condition was stable.
Sarcoidosis is a multisystem inflammatory disorder characterized by non-caseating granulomas and is most commonly found in the lungs and intrathoracic lymph nodes[2]. Although its etiology remains unclear, it is associated with genetic predisposition, environmental triggers, infections, and dysregulated immune responses. The disease typically manifests before age 50, with two incidence peaks: 70% of cases occur between ages 25-40, and a secondary peak is observed in women over 50[2]. As different organs may be involved (e.g., lungs, lymph nodes, skin, eyes, heart), the clinical presentation varies considerably, with 10%-15% of patients remaining asymptomatic[3], as shown by our two cases inci
Classic thoracic imaging features include bilateral hilar/mediastinal lymphadenopathy with pulmonary infiltrates. However, atypical radiological findings - as seen in both our cases - pose diagnostic challenges. Case 1 presented with a right upper lobe mass and paratracheal lymphadenopathy mimicking lung cancer with metastases, while case 2 exhibited multiregional hypermetabolic lymphadenopathy and thrombocytopenia, initially suggestive of lymphoma. These scenarios underscore the necessity of integrating clinical, radiological, and histopathological data for accurate diagnosis. Bronchoscopy with bronchoalveolar lavage lymphocyte subset analysis (increased CD4/CD8 ratio) and mediastinal lymph node sampling are pivotal in such ambiguous cases.
For conventional EBUS-TBNA, 22-G fine-needle aspiration is mainly utilized to obtain cytological specimens, which suffice for malignancy diagnosis but exhibit limited sensitivity for benign diseases such as sarcoidosis. A meta-analysis comparing the diagnostic yields of EBUS-TBNA and EBUS-TBFB revealed that the overall diagnostic rate of EBUS-TBNA for benign diseases was 71.19%, significantly lower than the 86.62% achieved by EBUS-TBFB[4]. To address this limi
It has been reported that the diagnostic rate of conventional EBUS-TBNA for sarcoidosis is only 71.4%[5], while EBUS-TBFB demonstrates significantly higher diagnostic positivity for benign diseases compared to EBUS-TBNA, although no significant difference is observed in malignant cases[6,7]. Notably, increasing the needle size to 19-G in EBUS-TBNA alone did not significantly improve diagnostic sensitivity or specimen adequacy[8]. In contrast, EBUS-TBFB could overcome the limitations of cytological sampling by acquiring larger tissue specimens (≥ 3 mm) from the same puncture site, markedly enhancing the detection rate of benign conditions such as granulomatous inflammation. Furthermore, a significantly higher diagnostic rate compared to TBNA alone can be achieved by combining TBFB with TBNA[9,10]. These findings collectively underscore the promising clinical utility of this approach for benign mediastinal diseases.
Atypical sarcoidosis has posed significant diagnostic dilemmas due to nonspecific imaging and asymptomatic presentations. EBUS-TBFB can improve diagnostic accuracy by overcoming the cytological limitations of TBNA and thus provide robust histological samples critical for distinguishing granulomatous disorders. Our cases highlight its clinical utility in guiding management - avoiding unnecessary treatments in localized disease (case 1) and initiating timely immunosuppression in systemic involvement (case 2). Further validation in larger cohorts is warranted to standardize this approach in routine practice.
| 1. | Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, Abston E, Bernstein RC, Blankstein R, Chen ES, Culver DA, Drake W, Drent M, Gerke AK, Ghobrial M, Govender P, Hamzeh N, James WE, Judson MA, Kellermeyer L, Knight S, Koth LL, Poletti V, Raman SV, Tukey MH, Westney GE, Baughman RP. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201:e26-e51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 699] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 2. | Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1438] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 3. | Iriarte A, Rubio-Rivas M, Villalba N, Corbella X, Mañá J. Clinical features and outcomes of asymptomatic pulmonary sarcoidosis. A comparative cohort study. Respir Med. 2020;169:105998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Yang W, Yang H, Zhang Q, Herth FJF, Zhang X. Comparison between Endobronchial Ultrasound-Guided Transbronchial Node Biopsy and Transbronchial Needle Aspiration: A Meta-Analysis. Respiration. 2024;103:752-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Zhang R, Zhang W, Cheng X, Si D, Liu B, Hu X, Chen X, Su Z. Comparative yield of EBUS-TBNA with EBUS-IFBTLP for diagnosis of mediastinal lymphadenopathy. Ther Adv Respir Dis. 2024;18:17534666241282217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Al Sona M, Esivue O, Benzaquen S. Endobronchial ultrasound (EBUS)-guided transbronchial miniforceps biopsy an urban center experience. J Thorac Dis. 2024;16:183-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Ray AS, Li C, Murphy TE, Cai G, Araujo KLB, Bramley K, DeBiasi EM, Pisani MA, Cortopassi IO, Puchalski JT. Improved Diagnostic Yield and Specimen Quality With Endobronchial Ultrasound-Guided Forceps Biopsies: A Retrospective Analysis. Ann Thorac Surg. 2020;109:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Dhooria S, Sehgal IS, Prasad KT, Muthu V, Dogra P, Saini M, Gupta N, Bal A, Aggarwal AN, Agarwal R. Diagnostic Yield and Safety of the 19-Gauge versus 22-Gauge Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Needle in Subjects with Sarcoidosis (GUESS). Respiration. 2024;103:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Fan Y, Zhang AM, Wu XL, Huang ZS, Kontogianni K, Sun K, Fu WL, Wu N, Kuebler WM, Herth FJF. Transbronchial needle aspiration combined with cryobiopsy in the diagnosis of mediastinal diseases: a multicentre, open-label, randomised trial. Lancet Respir Med. 2023;11:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Rüber F, Wiederkehr G, Steinack C, Höller S, Bode PK, Kölbener F, Franzen DP. Endobronchial Ultrasound-Guided Transbronchial Forceps Biopsy: A Retrospective Bicentric Study Using the Olympus 1.5 mm Mini-Forceps. J Clin Med. 2022;11:4700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/