Published online Nov 16, 2025. doi: 10.12998/wjcc.v13.i32.111134
Revised: July 7, 2025
Accepted: October 11, 2025
Published online: November 16, 2025
Processing time: 141 Days and 22.1 Hours
Optic neuritis (ON) is a focal inflammatory demyelinating disorder of the optic nerve. Although classically regarded as a sentinel event for multiple sclerosis (MS), ON also occurs in antibody-mediated entities such as aquaporin-4-IgG-positive neuromyelitis optica spectrum disorder (AQP4-NMOSD) and myelin-oligodendrocyte-glycoprotein-antibody disease. In all these settings biological sex is a pivotal determinant of susceptibility, clinical expression, treatment response and long-term outcome. Data synthesized from an extensive literature analysis utilizing PubMed, Scopus, and Web of Science in this review shows that women experience ON far more frequently – with female-to-male ratios ranging from 3:1 in MS to almost 9:1 in AQP4-NMOSD – yet men, when affected, tend to accumulate irreversible neuro-axonal loss more rapidly. Sex-specific patterns arise at every biological stratum: X-linked gene dosage, epigenetic regulation, hormonal cycles from puberty through menopause, metabolic co-modifiers such as obesity and vitamin-D status, and psychosocial forces that influence healthcare utilization. By weaving these elements into an expanded narrative, the present review provides a detailed resource for clinicians and investigators aiming at gender-tailored management of ON.
Core Tip: Optic neuritis (ON) is affected by sex at all levels, from molecular biology to clinical outcomes. Women are considerably more predisposed to developing ON, particularly in aquaporin-4-IgG-positive neuromyelitis optica spectrum disorder. Men frequently experience more severe long-term neuroaxonal damage. The interaction of genetic, hormonal, metabolic, and behavioral variables highlights the necessity for sex-specific strategies in the diagnosis, treatment, and research of ON. Comprehending these distinctions is crucial for enhancing individualized treatment in ON across various demyelinating conditions.
- Citation: Zeppieri M, Nicolosi SG, D’Esposito F, Musa M, Avitabile A, Gagliano C, Battista M, Barboni P, Capobianco M. Beyond the optic disc: Investigating gender-based differences in optic neuritis. World J Clin Cases 2025; 13(32): 111134
- URL: https://www.wjgnet.com/2307-8960/full/v13/i32/111134.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i32.111134
Optic neuritis (ON) presents with sub-acute monocular visual loss, impaired color vision and peri-ocular pain exacerbated by eye movement. The classic dichotomy distinguishes a typical ON – strongly predictive of MS – from a spectrum of atypical forms that include neuromyelitis optica spectrum disorder (NMOSD), myelin-oligodendrocyte-glycoprotein-antibody disease (MOGAD), parainfectious optic neuritis and optic neuropathies secondary to systemic autoimmune diseases[1-8]. Diagnostic algorithms have evolved beyond simple clinical judgement: The multidimensional classification proposed by Petzold et al[9] integrates clinical presentation with orbital and brain magnetic resonance imaging (MRI), optical-coherence-tomography (OCT) metrics, cerebrospinal-fluid cytology and disease-specific auto-antibodies to label each episode as definite, possible or non-ON.
Sex differences constitute a persistent leitmotif. Women outnumber men in MS-related ON by roughly 3:1, and the imbalance reaches 9:1 in AQP4-NMOSD[4-7]. In MOGAD, by contrast, sex distribution is approximately equal[10]. Paradoxically women tend to recover vision better than men, whereas men – although less frequently affected – are more likely to develop neuro-degenerative sequelae or steroid-refractory relapses[11-13]. Deciphering these patterns requires an integrated appreciation of genetic, hormonal, metabolic, environmental and psychosocial factors. Every thematic
A systematic literature search was performed across three major biological databases—PubMed, Scopus, and Web of Science—to create a thorough and representative synthesis of sex-based variations in ON. The search included all pertinent publications published until April 2025. Keywords and search phrases were chosen to encompass the complete clinical and immunological spectrum of ON and its demyelinating variations, with a specific focus on biological sex as a modifying variable. The strategy incorporated both controlled vocabulary and free-text terminology, amalgamating descriptors for ON and associated disorders—such as NMOSD and MOGAD—with terms denoting sex and gender distinctions, including “female”, “male”, “sex differences”, and “gender”. Boolean operators were employed to narrow the search and improve specificity. Alongside primary database queries, the reference lists of all obtained research were meticulously examined to uncover additional pertinent sources not identified via computerized indexing.
The inclusion criteria were explicitly delineated and uniformly implemented. Only peer-reviewed, full-text studies in English that included sex-disaggregated data on ON or its atypical subtypes were deemed suitable. These comprised original research articles, meta-analyses, and systematic reviews. Studies were rejected if they were abstracts, conference proceedings, non-peer-reviewed, written in languages other than English, or did not include specific stratification by sex. The screening and selection process had methodological transparency and reproducibility; however, a formal meta-analytic synthesis was not conducted due to variability in outcome measures and study designs.
Subsequent to the preliminary screening, pertinent data were separately retrieved by two reviewers and validated by a third to guarantee consistency and precision. The extracted data encompassed demographic variables, including age at onset and sex ratios; clinical outcomes, such as visual acuity scores, relapse frequency, and disability indices like the Expanded Disability Status Scale; imaging parameters obtained from OCT and MRI; and immunological data, including the presence of aquaporin-4 or MOG antibodies. Hormonal impacts, including life stages such as menarche, pregnancy, and menopause, were documented when accessible, with therapeutic response profiles, particularly to corticosteroids and monoclonal antibodies. Where relevant, psychosocial factors and quality-of-life indicators—such as pain perception, sexual dysfunction, and mental health burden—were included to offer a comprehensive knowledge of sex variations in disease impact.
Due to the diversity in research design, outcomes, and methodological rigor among the collected literature, a quantitative meta-analysis proved impracticable. Outcome metrics varied significantly among trials, encompassing high-precision imaging biomarkers and patient-reported outcome measures, hence hindering effective statistical aggregation (Table 1). A qualitative story synthesis was conducted, organized thematically to illustrate the multifaceted influence of sex on ON.
| Condition | Female:male ratio | Ref. |
| Typical MS-related ON | 2-3:1 | Malik et al[5] and Arnett et al[4] |
| Multiple sclerosis (overall) | 2.73:1 (95 %CI: 2.37-3.09) | Arnett et al[4] |
| AQP4-NMOSD (overall) | 8.89:1 | Arnett et al[4] |
| HIV-positive NMOSD | 9-10:1 | Borisow et al[6] |
| HIV-negative NMOSD | ≈ 2:1 | Borisow et al[6] |
| MOGAD | ≈ 1:1 (some series female > male) | Jurynczyk et al[10] and de Mol et al[14] |
In the selection of the papers included in our review paper, we used several conceptual domains of the Newcastle-Ottawa Scale, encompassing sample representativeness, ascertainment of exposure and outcomes, duration and completeness of follow-up, and methodological control for potential confounders, to inform our internal evaluation of study quality. In instances of contradictory findings, precedence has been given to prospective research characterized by higher sample sizes, established outcome criteria, and rigorous statistical adjustments for sex as an independent variable. Redundant findings documented in both basic source materials were consolidated to guarantee coherence and eliminate redundancy. In instances of disparity, priority was assigned to research featuring bigger sample sizes, more precise definitions, and enhanced statistical analysis. The synthesis elucidates the intricate interaction of genetic, hormonal, metabolic, and psychosocial variables that influence the development, progression, and treatment response of optic neuritis in a sex-specific context.
Typical MS-ON – Female:Male 2-3:1[4,5]. Multiple sclerosis (overall) – Pooled Female:Male 2.73:1 (95%CI: 2.37-3.09)[4,5]. AQP4-NMOSD – Female:Male 8.89:1; human immunodeficiency virus (HIV) -positive strata up to 10:1, HIV-negative ≈ 2:1[4]. MOGAD – Typically 1:1; occasional mild female excess[10,14].
Over the past seven decades MS incidence has climbed disproportionately among women. Several lifestyle factors have been hypothesized, including smoking, delayed childbearing and urbanization[15-18]. However, the temporal curve of tobacco use does not mirror the surge in female MS, because smoking rates in women peaked decades later than the incidence shift. Likewise, reduced parity and an older age at first pregnancy may contribute to risk but cannot fully explain the magnitude of the female predominance[19,20].
MS-ON – Women debut in early adulthood; men a few years later and transition more rapidly to progressive forms[8,21,22]. AQP4-NMOSD – Mean onset approximately 40 years; some series show later onset in men[4]. MOGAD – Pediatric onset twice as common as adult onset[10,23,24].
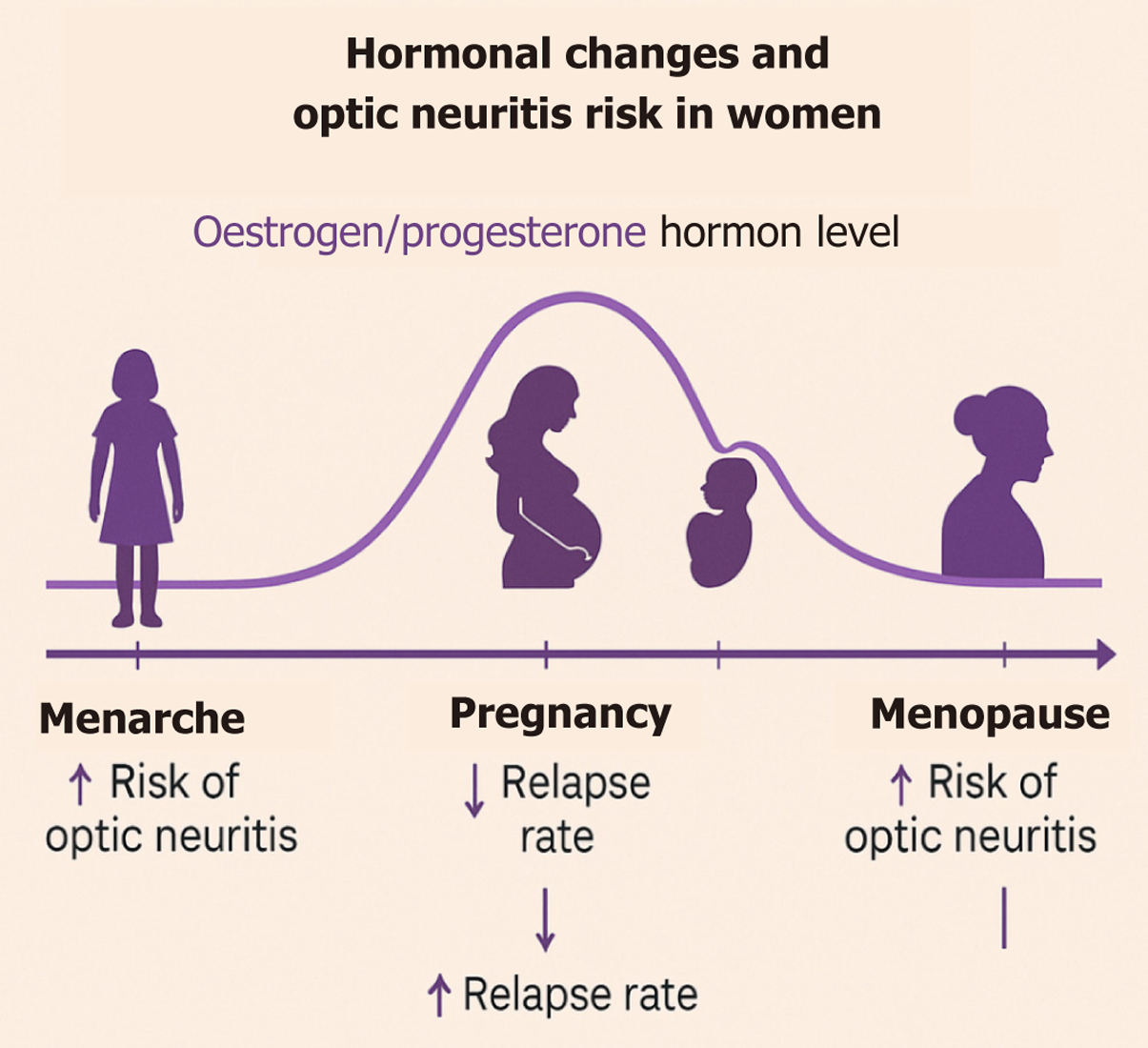
Early menarche (13–15 years of age) is associated with earlier MS onset (approximately 35 years of age)[25,26]. Since obesity accelerates menarche via aromatase-derived estrogen, adolescent body mass index (BMI) indirectly modulates risk[27-29]. Conversely, menopause – characterized by estrogen withdrawal – may aggravate neurodegeneration, although evidence remains sparse[15,30].
High estrogen and progesterone levels (Figure 1) during the third trimester confer a temporary immunological sanctuary: MS relapse rates fall[29-31], OCT demonstrates reduced RNFL loss[5,12,13,32], and NMOSD may remain quiescent in individual cases[27,33,34]. The puerperium is an immunological “snap-back”: Abrupt hormone withdrawal drives a surge in MS and NMOSD relapses[8,35,36]. Exclusive breastfeeding shows conflicting data; some series report protective effects, others note no benefit[8,37,38]. Low parity and delayed first pregnancy, prevalent in modern societies, may partially account for the growing female bias in MS[39-41].
Older ON patients display less optic-disc oedema and more extensive brain plaques, possibly reflecting immunosenescence and lower inflammatory vigor. Seropositivity for AQP4 is more common in the elderly, and age correlates inv
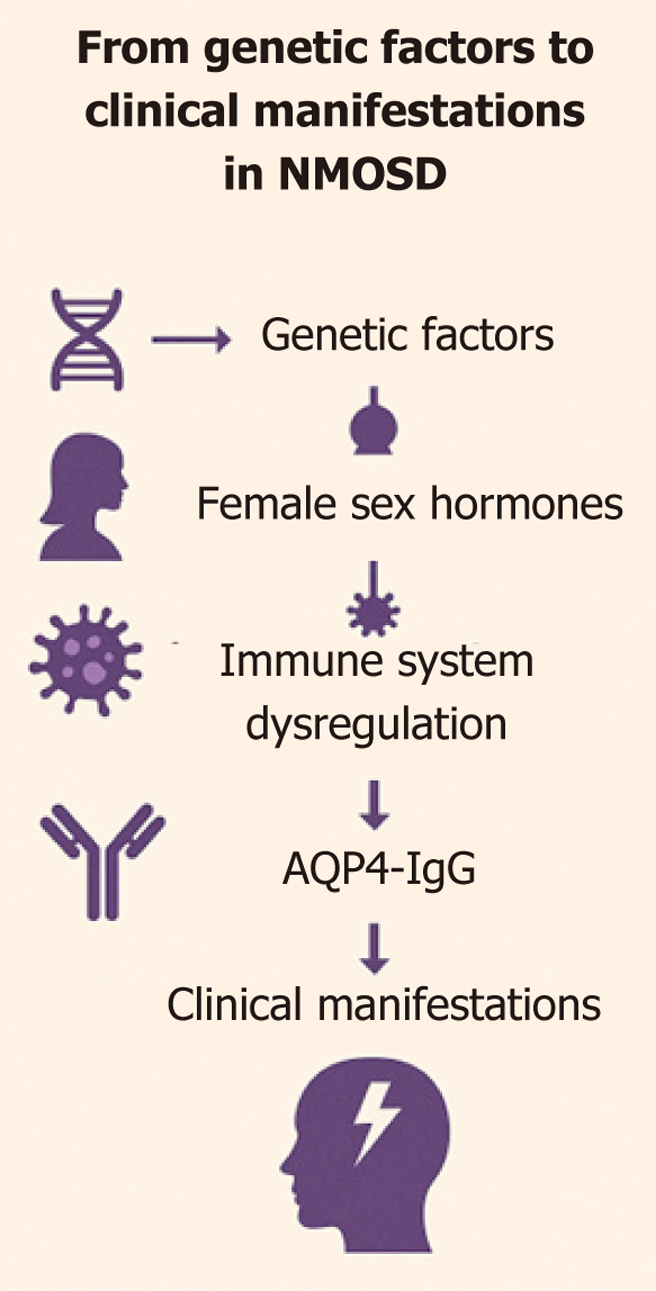
Numerous factors contribute to NMOSD (Figure 2). The X chromosome is enriched in immune-regulatory genes[46-48]. In women, incomplete inactivation allows double-dose expression of TLR-7/8 and other pattern recognition receptors, enhancing viral sensing but increasing autoantibody production. FOXP3 – also X-linked – governs regulatory-T-cell development; sex-biased methylation alters its transcriptional accessibility, influencing susceptibility to experimental autoimmune encephalomyelitis (EAE) and, by extension, MS and NMOSD[8,49-51].
Sex-specific micro-RNAs and Foxp3-locus methylation fine-tune T-cell thresholds. Cellular stress can trigger partial X-reactivation in female lymphocytes, releasing silenced alleles and fueling systemic autoimmunity – a mechanism invoked to explain the higher prevalence of Sjögren’s syndrome and SLE, conditions that share epidemiological patterns with NMOSD[52-55].
There are several hormonal factors to consider (Table 2). Estradiol (E2) and estriol (E3) suppress Th1 cytokines [tumour necrosis factor-α (TNF-α), interferon-γ] and up-regulate interleukin (IL)-10. ER-β activation in microglia dampens iNOS expression, while ER-β signaling in CD11c+ cells reduces nitric-oxide-mediated damage[8,60-62]. In oligodendrocytes ER-β promotes differentiation and remyelination. These mechanisms underpin the clinical observation that high-hormone states (pregnancy, mid-cycle peak) coincide with fewer relapses and milder RNFL thinning[8,13,60,61].
| Hormone (life-phase) | Key immunomodulatory actions | Principal experimental/clinical observations |
| Estradiol/estriol (mid-cycle, pregnancy) | ↓ TNF-α, IFN-γ; ↑ IL-10; ER-β activation quiets microglia and CD11c+ cells; promotes oligodendrocyte maturation and remyelination | Third-trimester pregnancy sharply lowers MS/ON relapse rates; women retain more RNFL after ON in high-estrogen states |
| Progesterone (luteal phase, gestation) | Shifts immunity Th1 → Th2; expands T-regs; suppresses iNOS and Toll-like-receptor signaling; fosters myelin repair | Color-Doppler shows ↑ central-retinal-artery resistance; progesterone analogues ameliorate EAE lesions and enhance remyelination |
| Testosterone/DHT (male dominance, peri-puberty) | Down-regulates Th1/Th17; induces thymic AIRE; inhibits NF-κB & p38-MAPK in microglia; curtails IL-1β, IL-6, TNF-α; modulates Bax/Bcl-2, caspase-3 | Androgen supplementation dampens EAE severity; low testosterone correlates with aggressive MS & greater ON severity in men; DHT shields SH-SY5Y neurons from inflammatory apoptosis |
| Prolactin (lactation, immune-cell secretion) | Dual role – may boost inflammation yet stimulates oligodendrocyte progenitors | Human data inconclusive; experimental models show both aggravation of EAE and promotion of remyelination |
Progesterone shifts immunity toward a Th2 phenotype, augments T-reg numbers, suppresses Toll-like-receptor signaling and promotes myelin repair. Color Doppler studies reveal that progesterone increases central-retinal-artery resistive index, likely by antagonizing estrogen-induced nitric-oxide synthesis[32,33,63-65].
Multiple pathophysiological mechanisms have been suggested to elucidate the relatively poorer visual and neurological outcomes seen in male patients with ON[8,66]. Relative androgen deficit, resulting from either chronic neuroinflammation or intrinsic endocrine dysfunction, may hinder microglial suppression, central immune tolerance, and remy
Testosterone and 5α-dihydrotestosterone (DHT) quell Th1/Th17 differentiation and induce thymic AIRE, broadening central tolerance[8]. In vitro DHT reduces IL-1β, IL-6 and TNF-α release, suppresses COX-2 and NOS, and down-regulates NF-κB and p38-MAPK signaling in microglia, thereby protecting SH-SY5Y neurons from inflammatory damage. DHT also modulates neuronal expression of IL-10, IL-13, Aβ, caspase-3, Bax/Bcl-2 and synaptophysin, collectively curtailing apoptosis and synaptic loss[65-72]. Despite these benefits, epidemiological data show lower serum testosterone in men with aggressive MS, suggesting either consumption by chronic inflammation or an intrinsic endocrine deficit[73].
Prolactin’s role is ambivalent: Experimental data implicate it in disease exacerbation, yet oligodendrocyte-culture studies show enhanced remyelination[8]. Hormone-replacement therapy (HRT) in menopausal women with MS has been insufficiently studied; preliminary surveys hint at subtle immunomodulatory effects but firm recommendations await ran
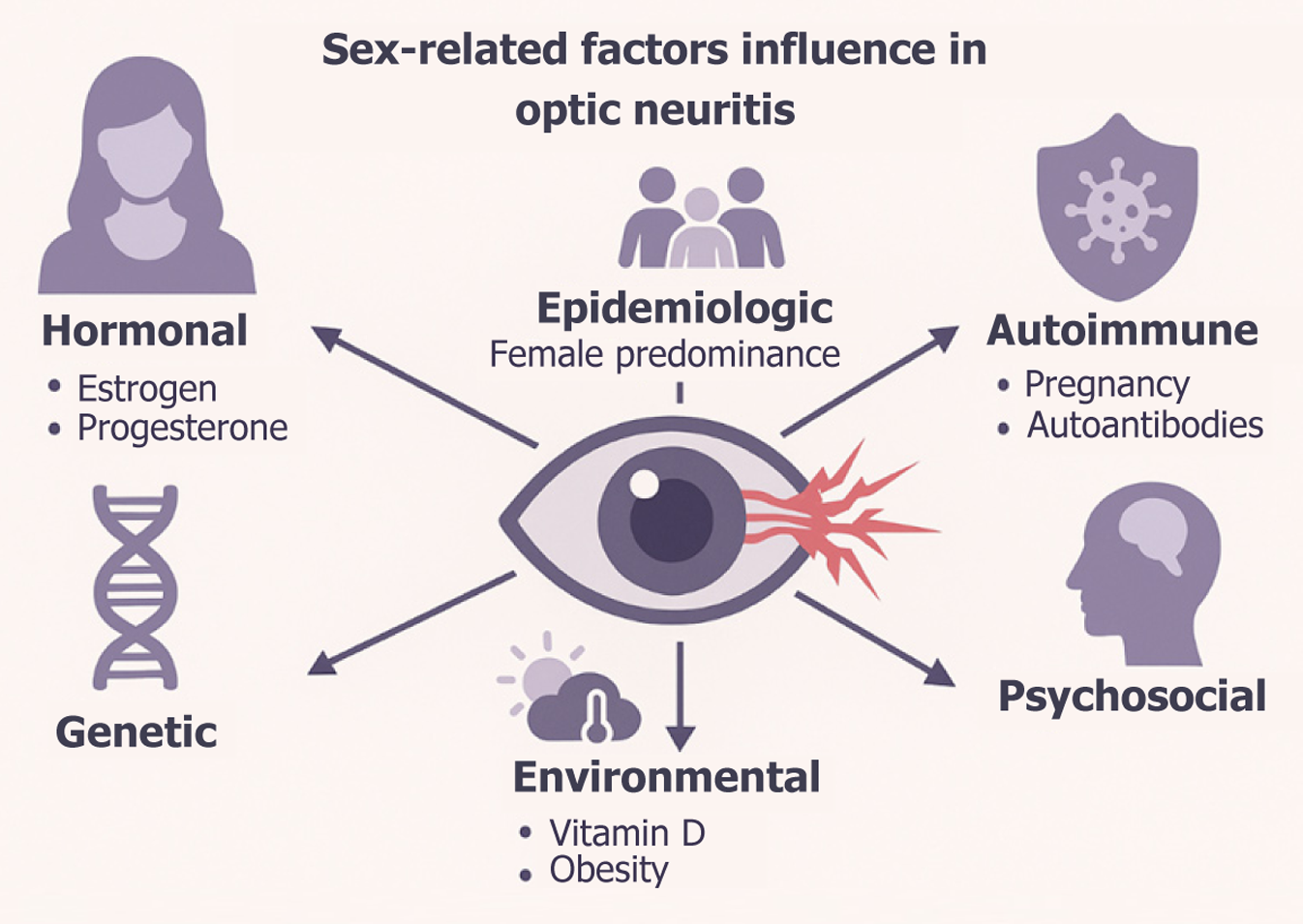
There are several factors involved in the genesis of optic neuritis (Figure 3). Obesity elevates leptin and decreases adiponectin, fostering a chronic inflammatory milieu characterized by high IL-6 and C-reactive-protein (CRP)[74-78]. Mendelian-randomization studies demonstrate a bidirectional causal loop between BMI and CRP. In clinical cohorts higher BMI correlates with more severe ON attacks, greater steroid non-responsiveness and accelerated disability, especially in men[79,80].
A Thai case-control study revealed significantly lower 25(OH)D in all immune-mediated ON subtypes compared with age- and sex-matched controls. Vitamin-D deficiency is more prevalent in women due to estrogen deficits that hamper vitamin-D metabolism and precipitate osteoporosis, thus intersecting with the hormonal axis to modulate demyelinating risk[81,82].
Historical smoking patterns cannot fully explain the female up-swing in MS incidence: Male smoking peaked mid-20th century, whereas female smoking rose later, unaligned with the earlier shift in MS sex ratio. Nonetheless, tobacco remains an adjuvant risk factor, synergizing with HLA-sensitive immune activation[16,17,83].
AQP4-positive NMOSD patients often harbor additional auto-antibodies (ANA, anti-thyroid). Female preponderance mirrors patterns in SLE and Sjögren’s, suggesting systemic immune dysregulation rather than organ-specific autoimmunity[46-48,55,84].
Bidirectional Mendelian-randomization links higher serum progesterone to increased NMOSD susceptibility, inde
AQP4 expression in ovarian and testicular tissue implies a potential for antibody-mediated reproductive disturbances[8]. Female NMOSD patients display lower anti-Müllerian-hormone (AMH) levels, warning of diminished ovarian reserve. In mice, AQP4 knockout reduces fertility, supporting a causal link[87-90].
Men with MS show more pronounced whole-brain and brain-stem atrophy than women. In NMOSD sex-stratified MRI data are scarce; one retrospective review noted higher extra-optic lesion burden in elderly patients, hinting at age-driven hypo-immunity[91-95].
Progesterone elevates central-retinal-artery resistance (Doppler study) by engaging smooth-muscle receptors and sup
Rodent models reveal that neuropathic pain in males hinges on microglial activation and disinhibition of spinal neurons, whereas in females it depends on T-cell-derived mediators and macrophage-sensitization of nociceptors. In ON cohorts women report higher pain intensity, supporting a biological divergence[99-103].
Women with ON and demyelinating disease experience higher rates of depression, fatigue and pain, partly linked to fluctuating sex hormones. Men face elevated suicide risk after diagnosis, may be less inclined to participate in rehabilitation, and often receive less social support[64,104,105]. In NMOSD, both sexes can develop sexual dysfunction, but reduced bioavailable testosterone and elevated SHBG have been specifically correlated with decreased libido, mood disturbance, and diminished quality of life[8,12,66,40,106].
A multicentre survey found that women who had ever used hormonal contraception developed NMOSD at a younger age than non-users, suggesting hormonally modulated disease initiation. Counselling should discuss potential interactions without discouraging effective contraception where clinically necessary[107,108].
Lower AMH in NMOSD signals reduced ovarian reserve. Fertility counselling – encompassing cryopreservation, timing of immunosuppression, and contraceptive planning – is essential. Men with ON and concurrent hypogonadism may require endocrinology referral for testosterone replacement, which could confer neuroprotection and improve mood and sexual function[74,90].
Optimal strategies include achieving disease stability for ≥ 6 months, correcting vitamin-D insufficiency, and avoiding teratogenic agents (teriflunomide, cladribine). High-risk NMOSD patients may benefit from low-dose prednisolone or continuation of compatible monoclonal antibodies through pregnancy. Postpartum relapse surveillance is critical, with early re-initiation of maintenance immunotherapy[36,109-112].
Data are limited, but HRT could theoretically restore estrogen-mediated neuroprotection. Until randomized trials clarify risks and benefits, HRT decisions should be individualized, balancing vasomotor symptom relief against potential immune activation[29,113].
Interferons/Glatiramer Acetate – Equal efficacy, but men more prone to transaminase elevation[114]. S1P Receptor Modulators – Severe lymphopenia predominantly in women; hepatotoxicity in men[115,116]. Teriflunomide/Cladribine – Highly teratogenic; strict contraception required for both sexes[117,118].
Natalizumab – Greater reduction of disability accumulation in women[119]. Anti-CD20 Monoclonal Antibodies – Particularly efficacious in primary-progressive MS (male-biased); necessitate breast-cancer surveillance in women[120,121].
Eculizumab and satralizumab markedly lower relapse risk. Male NMOSD patients exhibit higher rates of high-dose steroid non-response, reinforcing the need for timely biologic escalation[121-124].
Evidence derives from off-label use of MS/NMOSD agents (mycophenolate, azathioprine, rituximab). To date no sex-specific differences in efficacy or safety are apparent, but sample sizes remain small[10].
In light of the identified sexual dimorphisms in pathogenesis and therapeutic efficacy, we advocate for customized monitoring strategies. Male patients with ON should have first endocrinological assessment for hypogonadism, espe
Lymphopenia with S1P modulators warrants closer surveillance in women, while transaminase monitoring is prioritized in men on interferons. Breast-cancer screening is mandatory for women on chronic anti-CD20 therapy. Con
Sex Imbalance – Women dominate NMOSD and MS cohorts, restricting male-specific inferences. Outcome
Gender influences every dimension of optic neuritis – from genetic predisposition and hormonal modulation to metabolic co-risk factors, pain perception, reproductive decisions and therapeutic side-effect profiles. Women are more susceptible yet often recover vision better; men experience fewer attacks but accumulate disability faster and may respond less robustly to corticosteroids. Hormonal transitions (menarche, pregnancy, puerperium, menopause) and environmental factors (vitamin-D insufficiency, obesity, smoking) further modulate risk and outcome. Auto-antibody patterns, ocular hemodynamic, MRI signatures and psychosocial variables all display sex bias. Addressing these nuances demands sex-powered clinical trials, inclusive registries and mechanistic studies that bridge immunology, endocrinology and neuro-ophthalmology. Only through such multidimensional research can clinicians achieve genuinely personalized, gender-responsive care for every individual with optic neuritis.
| 1. | Guier CP, Kaur K, Stokkermans TJ. Optic Neuritis. 2025 Jan 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 2. | Spillers NJ, Luther PM, Talbot NC, Kidder EJ, Doyle CA, Lutfallah SC, Derouen AG, Tirumala S, Ahmadzadeh S, Shekoohi S, Kaye AD, Varrassi G. A Comparative Review of Typical and Atypical Optic Neuritis: Advancements in Treatments, Diagnostics, and Prognosis. Cureus. 2024;16:e56094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Malik A, Ahmed M, Golnik K. Treatment options for atypical optic neuritis. Indian J Ophthalmol. 2014;62:982-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Arnett S, Chew SH, Leitner U, Hor JY, Paul F, Yeaman MR, Levy M, Weinshenker BG, Banwell BL, Fujihara K, Abboud H, Dujmovic Basuroski I, Arrambide G, Neubrand VE, Quan C, Melamed E, Palace J, Sun J, Asgari N, Broadley SA; Guthy Jackson International Clinical Consortium*. Sex ratio and age of onset in AQP4 antibody-associated NMOSD: a review and meta-analysis. J Neurol. 2024;271:4794-4812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Malik MT, Healy BC, Benson LA, Kivisakk P, Musallam A, Weiner HL, Chitnis T. Factors associated with recovery from acute optic neuritis in patients with multiple sclerosis. Neurology. 2014;82:2173-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Borisow N, Kleiter I, Gahlen A, Fischer K, Wernecke KD, Pache F, Ruprecht K, Havla J, Krumbholz M, Kümpfel T, Aktas O, Ringelstein M, Geis C, Kleinschnitz C, Berthele A, Hemmer B, Angstwurm K, Weissert R, Stellmann JP, Schuster S, Stangel M, Lauda F, Tumani H, Mayer C, Zeltner L, Ziemann U, Linker RA, Schwab M, Marziniak M, Then Bergh F, Hofstadt-van Oy U, Neuhaus O, Winkelmann A, Marouf W, Rückriem L, Faiss J, Wildemann B, Paul F, Jarius S, Trebst C, Hellwig K; NEMOS (Neuromyelitis Optica Study Group). Influence of female sex and fertile age on neuromyelitis optica spectrum disorders. Mult Scler. 2017;23:1092-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Jiao Y, Fryer JP, Lennon VA, Jenkins SM, Quek AM, Smith CY, McKeon A, Costanzi C, Iorio R, Weinshenker BG, Wingerchuk DM, Shuster EA, Lucchinetti CF, Pittock SJ. Updated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis optica. Neurology. 2013;81:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Diem L, Hammer H, Hoepner R, Pistor M, Remlinger J, Salmen A. Sex and gender differences in autoimmune demyelinating CNS disorders: Multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD) and myelin-oligodendrocyte-glycoprotein antibody associated disorder (MOGAD). Int Rev Neurobiol. 2022;164:129-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 9. | Petzold A, Fraser CL, Abegg M, Alroughani R, Alshowaeir D, Alvarenga R, Andris C, Asgari N, Barnett Y, Battistella R, Behbehani R, Berger T, Bikbov MM, Biotti D, Biousse V, Boschi A, Brazdil M, Brezhnev A, Calabresi PA, Cordonnier M, Costello F, Cruz FM, Cunha LP, Daoudi S, Deschamps R, de Seze J, Diem R, Etemadifar M, Flores-Rivera J, Fonseca P, Frederiksen J, Frohman E, Frohman T, Tilikete CF, Fujihara K, Gálvez A, Gouider R, Gracia F, Grigoriadis N, Guajardo JM, Habek M, Hawlina M, Martínez-Lapiscina EH, Hooker J, Hor JY, Howlett W, Huang-Link Y, Idrissova Z, Illes Z, Jancic J, Jindahra P, Karussis D, Kerty E, Kim HJ, Lagrèze W, Leocani L, Levin N, Liskova P, Liu Y, Maiga Y, Marignier R, McGuigan C, Meira D, Merle H, Monteiro MLR, Moodley A, Moura F, Muñoz S, Mustafa S, Nakashima I, Noval S, Oehninger C, Ogun O, Omoti A, Pandit L, Paul F, Rebolleda G, Reddel S, Rejdak K, Rejdak R, Rodriguez-Morales AJ, Rougier MB, Sa MJ, Sanchez-Dalmau B, Saylor D, Shatriah I, Siva A, Stiebel-Kalish H, Szatmary G, Ta L, Tenembaum S, Tran H, Trufanov Y, van Pesch V, Wang AG, Wattjes MP, Willoughby E, Zakaria M, Zvornicanin J, Balcer L, Plant GT. Diagnosis and classification of optic neuritis. Lancet Neurol. 2022;21:1120-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 198] [Reference Citation Analysis (0)] |
| 10. | Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, Tackley G, Hamid S, Sheard A, Reynolds G, Chandratre S, Hemingway C, Jacob A, Vincent A, Leite MI, Waters P, Palace J. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128-3138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 571] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 11. | Osborne NR, Davis KD. Sex and gender differences in pain. Int Rev Neurobiol. 2022;164:277-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 12. | Marzoli SB, Criscuoli A. Pain in optic neuropathies. Neurol Sci. 2018;39:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Costello F, Hodge W, Pan YI, Burton JM, Freedman MS, Stys PK, Trufyn J, Kardon R. Sex-specific differences in retinal nerve fiber layer thinning after acute optic neuritis. Neurology. 2012;79:1866-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | de Mol CL, Wong Y, van Pelt ED, Wokke B, Siepman T, Neuteboom RF, Hamann D, Hintzen RQ. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler. 2020;26:806-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Nuzzi R, Scalabrin S, Becco A, Panzica G. Sex Hormones and Optic Nerve Disorders: A Review. Front Neurosci. 2019;13:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2321] [Cited by in RCA: 4420] [Article Influence: 442.0] [Reference Citation Analysis (0)] |
| 17. | Rubtsova K, Marrack P, Rubtsov AV. Sexual dimorphism in autoimmunity. J Clin Invest. 2015;125:2187-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Perkin GD, Bowden P, Rose FC. Smoking and optic neuritis. Postgrad Med J. 1975;51:382-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Thacker EL, O'Reilly EJ, Weisskopf MG, Chen H, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68:764-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Palacios N, Alonso A, Brønnum-Hansen H, Ascherio A. Smoking and increased risk of multiple sclerosis: parallel trends in the sex ratio reinforce the evidence. Ann Epidemiol. 2011;21:536-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca MA. Multiple sclerosis. Nat Rev Dis Primers. 2018;4:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 1044] [Article Influence: 130.5] [Reference Citation Analysis (1)] |
| 22. | Ribbons KA, McElduff P, Boz C, Trojano M, Izquierdo G, Duquette P, Girard M, Grand'Maison F, Hupperts R, Grammond P, Oreja-Guevara C, Petersen T, Bergamaschi R, Giuliani G, Barnett M, van Pesch V, Amato MP, Iuliano G, Fiol M, Slee M, Verheul F, Cristiano E, Fernandez-Bolanos R, Saladino ML, Rio ME, Cabrera-Gomez J, Butzkueven H, van Munster E, Den Braber-Moerland L, La Spitaleri D, Lugaresi A, Shaygannejad V, Gray O, Deri N, Alroughani R, Lechner-Scott J. Male Sex Is Independently Associated with Faster Disability Accumulation in Relapse-Onset MS but Not in Primary Progressive MS. PLoS One. 2015;10:e0122686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, White OB, Broadley S, Lechner-Scott J, Vucic S, Henderson APD, Barnett MH, Reddel SW, Brilot F, Dale RC; Australasian and New Zealand MOG Study Group. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89:127-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 458] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 24. | Cobo-Calvo A, Ruiz A, Rollot F, Arrambide G, Deschamps R, Maillart E, Papeix C, Audoin B, Lépine AF, Maurey H, Zephir H, Biotti D, Ciron J, Durand-Dubief F, Collongues N, Ayrignac X, Labauge P, Meyer P, Thouvenot E, Bourre B, Montcuquet A, Cohen M, Horello P, Tintoré M, De Seze J, Vukusic S, Deiva K, Marignier R; NOMADMUS, KidBioSEP, and OFSEP study groups. Clinical Features and Risk of Relapse in Children and Adults with Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. Ann Neurol. 2021;89:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 25. | Jiang X, Olsson T, Alfredsson L. Age at Menarche and Risk of Multiple Sclerosis: Current Progress From Epidemiological Investigations. Front Immunol. 2018;9:2600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Sloka JS, Pryse-Phillips WE, Stefanelli M. The relation between menarche and the age of first symptoms in a multiple sclerosis cohort. Mult Scler. 2006;12:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Ysrraelit MC, Correale J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology. 2019;156:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 28. | Ramagopalan SV, Valdar W, Criscuoli M, DeLuca GC, Dyment DA, Orton SM, Yee IM, Ebers GC, Sadovnick AD; Canadian Collaborative Study Group. Age of puberty and the risk of multiple sclerosis: a population based study. Eur J Neurol. 2009;16:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Bove R, Okai A, Houtchens M, Elias-Hamp B, Lugaresi A, Hellwig K, Kubala Havrdová E. Effects of Menopause in Women With Multiple Sclerosis: An Evidence-Based Review. Front Neurol. 2021;12:554375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1060] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 31. | Miller DH, Fazekas F, Montalban X, Reingold SC, Trojano M. Pregnancy, sex and hormonal factors in multiple sclerosis. Mult Scler. 2014;20:527-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Madendag Y, Acmaz G, Atas M, Sahin E, Tayyar AT, Madendag IÇ, Özdemir F, Senol V. The Effect of Oral Contraceptive Pills on the Macula, the Retinal Nerve Fiber Layer, and Choroidal Thickness. Med Sci Monit. 2017;23:5657-5661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Hu Y, Zou F, Lu W. Sex hormones and neuromyelitis optica spectrum disorder: a bidirectional Mendelian randomization study. Neurol Sci. 2024;45:4471-4479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 34. | Murgia F, Giagnoni F, Lorefice L, Caria P, Dettori T, D'Alterio MN, Angioni S, Hendren AJ, Caboni P, Pibiri M, Monni G, Cocco E, Atzori L. Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review. Biomedicines. 2022;10:3107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 35. | Tong Y, Liu J, Yang T, Kang Y, Wang J, Zhao T, Cheng C, Fan Y. Influences of pregnancy on neuromyelitis optica spectrum disorders and multiple sclerosis. Mult Scler Relat Disord. 2018;25:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Klawiter EC, Bove R, Elsone L, Alvarez E, Borisow N, Cortez M, Mateen F, Mealy MA, Sorum J, Mutch K, Tobyne SM, Ruprecht K, Buckle G, Levy M, Wingerchuk D, Paul F, Cross AH, Jacobs A, Chitnis T, Weinshenker B. High risk of postpartum relapses in neuromyelitis optica spectrum disorder. Neurology. 2017;89:2238-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Erkkilä H, Raitta C, Iivanainen M, Taskinen E, Unnérus HA, Gummerus M. Optic neuritis during lactation. Graefes Arch Clin Exp Ophthalmol. 1985;222:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Holmqvist P, Hammar M, Landtblom AM, Brynhildsen J. Age at onset of multiple sclerosis is correlated to use of combined oral contraceptives and childbirth before diagnosis. Fertil Steril. 2010;94:2835-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | D'hooghe MB, D'Hooghe T, De Keyser J. Female gender and reproductive factors affecting risk, relapses and progression in multiple sclerosis. Gynecol Obstet Invest. 2013;75:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Nguyen AL, Vodehnalova K, Kalincik T, Signori A, Havrdova EK, Lechner-Scott J, Skibina OG, Eastaugh A, Taylor L, Baker J, McGuinn N, Rath L, Maltby V, Sormani MP, Butzkueven H, Van der Walt A, Horakova D, Jokubaitis VG. Association of Pregnancy With the Onset of Clinically Isolated Syndrome. JAMA Neurol. 2020;77:1496-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Nielsen NM, Jørgensen KT, Stenager E, Jensen A, Pedersen BV, Hjalgrim H, Kjær SK, Frisch M. Reproductive history and risk of multiple sclerosis. Epidemiology. 2011;22:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Luo WJ, Huang QS, He JF, Han M, Liu B, Du Y. Clinical differences between young and older patients with optic neuritis. Ann Eye Sci. 2018;2:67-67. [DOI] [Full Text] |
| 43. | Kunchok A, Malpas C, Nytrova P, Havrdova EK, Alroughani R, Terzi M, Yamout B, Hor JY, Karabudak R, Boz C, Ozakbas S, Olascoaga J, Simo M, Granella F, Patti F, McCombe P, Csepany T, Singhal B, Bergamaschi R, Fragoso Y, Al-Harbi T, Turkoglu R, Lechner-Scott J, Laureys G, Oreja-Guevara C, Pucci E, Sola P, Ferraro D, Altintas A, Soysal A, Vucic S, Grand'Maison F, Izquierdo G, Eichau S, Lugaresi A, Onofrj M, Trojano M, Marriott M, Butzkueven H, Kister I, Kalincik T. Clinical and therapeutic predictors of disease outcomes in AQP4-IgG+ neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2020;38:101868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA. Age and disability accumulation in multiple sclerosis. Neurology. 2011;77:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 45. | Kobayashi T, Shiba T, Kinoshita A, Matsumoto T, Hori Y. The influences of gender and aging on optic nerve head microcirculation in healthy adults. Sci Rep. 2019;9:15636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, Lucchinetti CF, Zéphir H, Moder K, Weinshenker BG. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008;65:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 408] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 47. | Wang X, Yi H, Liu J, Li M, Mao ZF, Xu L, Peng FH. Anti-thyroid antibodies and thyroid function in neuromyelitis optica spectrum disorders. J Neurol Sci. 2016;366:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Pereira WLCJ, Reiche EMV, Kallaur AP, Oliveira SR, Simão ANC, Lozovoy MAB, Schiavão LJV, Rodrigues PRDVP, Alfieri DF, Flauzino T, Kaimen-Maciel DR. Frequency of autoimmune disorders and autoantibodies in patients with neuromyelitis optica. Acta Neuropsychiatr. 2017;29:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Brooks WH. X chromosome inactivation and autoimmunity. Clin Rev Allergy Immunol. 2010;39:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Voskuhl RR, Sawalha AH, Itoh Y. Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression. Mult Scler. 2018;24:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Reddy J, Waldner H, Zhang X, Illes Z, Wucherpfennig KW, Sobel RA, Kuchroo VK. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2005;175:5591-5595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 922] [Cited by in RCA: 1018] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 53. | Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. 2019;13:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 264] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 54. | Brooks WH, Renaudineau Y. Epigenetics and autoimmune diseases: the X chromosome-nucleolus nexus. Front Genet. 2015;6:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Kvarnström M, Ottosson V, Nordmark B, Wahren-Herlenius M. Incident cases of primary Sjögren's syndrome during a 5-year period in Stockholm County: a descriptive study of the patients and their characteristics. Scand J Rheumatol. 2015;44:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Hyun JW, Kim S, Moon J, Park NY, Kang YR, Kim KH, Kim SH, Kim HJ. HLA Association With AQP4-IgG-Positive Neuromyelitis Optica Spectrum Disorder in the Korean Population. Neurol Neuroimmunol Neuroinflamm. 2025;12:e200366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 57. | Grant-Peters M, Passos GRD, Yeung HY, Jacob A, Huda S, Leite MI, Dendrou CA, Palace J. No strong HLA association with MOG antibody disease in the UK population. Ann Clin Transl Neurol. 2021;8:1502-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Li T, Li H, Li Y, Dong SA, Yi M, Zhang QX, Feng B, Yang L, Shi FD, Yang CS. Multi-Level Analyses of Genome-Wide Association Study to Reveal Significant Risk Genes and Pathways in Neuromyelitis Optica Spectrum Disorder. Front Genet. 2021;12:690537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Wang H, Zhong X, Wang K, Qiu W, Li J, Dai Y, Hu X. Interleukin 17 gene polymorphism is associated with anti-aquaporin 4 antibody-positive neuromyelitis optica in the Southern Han Chinese--a case control study. J Neurol Sci. 2012;314:26-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol. 2003;171:6267-6274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 61. | Kim RY, Mangu D, Hoffman AS, Kavosh R, Jung E, Itoh N, Voskuhl R. Oestrogen receptor β ligand acts on CD11c+ cells to mediate protection in experimental autoimmune encephalomyelitis. Brain. 2018;141:132-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 855] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 63. | El-Etr M, Rame M, Boucher C, Ghoumari AM, Kumar N, Liere P, Pianos A, Schumacher M, Sitruk-Ware R. Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex. Glia. 2015;63:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 64. | Vincent K, Tracey I. Hormones and their Interaction with the Pain Experience. Rev Pain. 2008;2:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | He P, Yan S, Zheng J, Gao Y, Zhang S, Liu Z, Liu X, Xiao C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-κB in Male C57BL/6J Mice and BV2 Microglial Cells. J Agric Food Chem. 2018;66:10205-10214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 66. | Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A Reappraisal of Testosterone's Binding in Circulation: Physiological and Clinical Implications. Endocr Rev. 2017;38:302-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 291] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 67. | Liao Z, Vosberg DE, Pausova Z, Paus T. A Shifting Relationship Between Sex Hormone-Binding Globulin and Total Testosterone Across Puberty in Boys. J Clin Endocrinol Metab. 2022;107:e4187-e4196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 68. | Davis SR, Tran J. Testosterone influences libido and well being in women. Trends Endocrinol Metab. 2001;12:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 69. | Huang W, ZhangBao J, Chang X, Wang L, Zhao C, Lu J, Wang M, Ding X, Xu Y, Zhou L, Li D, Behne MK, Behne JM, Yeaman MR, Katz E, Lu C, Quan C; Guthy-Jackson Charitable Foundation International Clinical Consortium (GJCF-ICC). Neuromyelitis optica spectrum disorder in China: Quality of life and medical care experience. Mult Scler Relat Disord. 2020;46:102542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Zhang Y, Zhang Q, Shi Z, Chen H, Wang J, Yan C, Du Q, Qiu Y, Zhao Z, Zhou H. Sexual dysfunction in patients with neuromyelitis optica spectrum disorder. J Neuroimmunol. 2020;338:577093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Yang L, Zhou R, Tong Y, Chen P, Shen Y, Miao S, Liu X. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol Dis. 2020;140:104814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 72. | Chen F, Ghosh A, Wu F, Tang S, Hu M, Sun H, Kong L, Hong H. Preventive effect of genetic knockdown and pharmacological blockade of CysLT(1)R on lipopolysaccharide (LPS)-induced memory deficit and neurotoxicity in vivo. Brain Behav Immun. 2017;60:255-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Wu X, Liu C, Chen L, Du YF, Hu M, Reed MN, Long Y, Suppiramaniam V, Hong H, Tang SS. Protective effects of tauroursodeoxycholic acid on lipopolysaccharide-induced cognitive impairment and neurotoxicity in mice. Int Immunopharmacol. 2019;72:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 74. | Bove R, Musallam A, Healy BC, Raghavan K, Glanz BI, Bakshi R, Weiner H, De Jager PL, Miller KK, Chitnis T. Low testosterone is associated with disability in men with multiple sclerosis. Mult Scler. 2014;20:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 75. | Chitnis T. The role of testosterone in MS risk and course. Mult Scler. 2018;24:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Timpson NJ, Nordestgaard BG, Harbord RM, Zacho J, Frayling TM, Tybjærg-Hansen A, Smith GD. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes (Lond). 2011;35:300-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 77. | van Dielen FM, van't Veer C, Schols AM, Soeters PB, Buurman WA, Greve JW. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int J Obes Relat Metab Disord. 2001;25:1759-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 78. | Procaccini C, Pucino V, Mantzoros CS, Matarese G. Leptin in autoimmune diseases. Metabolism. 2015;64:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 79. | Sbarbati A, Osculati F, Silvagni D, Benati D, Galiè M, Camoglio FS, Rigotti G, Maffeis C. Obesity and inflammation: evidence for an elementary lesion. Pediatrics. 2006;117:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Palavra F, Almeida L, Ambrósio AF, Reis F. Obesity and brain inflammation: a focus on multiple sclerosis. Obes Rev. 2016;17:211-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Chu DT, Rosso M, Gonzalez CT, Saxena S, Healy BC, Weiner HL, Chitnis T. Obesity is associated with the Optic Neuritis severity in Male patients with Multiple Sclerosis. Mult Scler Relat Disord. 2021;51:102910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 82. | Puangsricharoen B, Vanikieti K, Jindahra P, Padungkiatsagul T. Serum Vitamin D Levels and Status in Thai Optic Neuritis Subjects: A Case-Control Study. Clin Ophthalmol. 2022;16:3381-3389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 83. | Agmon-Levin N, Theodor E, Segal RM, Shoenfeld Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin Rev Allergy Immunol. 2013;45:256-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 84. | Sellner J, Kraus J, Awad A, Milo R, Hemmer B, Stüve O. The increasing incidence and prevalence of female multiple sclerosis--a critical analysis of potential environmental factors. Autoimmun Rev. 2011;10:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 85. | Rider V, Abdou NI, Kimler BF, Lu N, Brown S, Fridley BL. Gender Bias in Human Systemic Lupus Erythematosus: A Problem of Steroid Receptor Action? Front Immunol. 2018;9:611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Balogh A, Karpati E, Schneider AE, Hetey S, Szilagyi A, Juhasz K, Laszlo G, Hupuczi P, Zavodszky P, Papp Z, Matko J, Than NG. Sex hormone-binding globulin provides a novel entry pathway for estradiol and influences subsequent signaling in lymphocytes via membrane receptor. Sci Rep. 2019;9:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 87. | Rosner W. The functions of corticosteroid-binding globulin and sex hormone-binding globulin: recent advances. Endocr Rev. 1990;11:80-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 367] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 88. | Saadoun S, Waters P, Leite MI, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica IgG causes placental inflammation and fetal death. J Immunol. 2013;191:2999-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 89. | Jesus TT, Bernardino RL, Martins AD, Sá R, Sousa M, Alves MG, Oliveira PF. Aquaporin-4 as a molecular partner of cystic fibrosis transmembrane conductance regulator in rat Sertoli cells. Biochem Biophys Res Commun. 2014;446:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Thöne J, Lichtenberg S, Stahl A, Pache F, Kleiter I, Ruprecht K, Gold R, Hellwig K. Ovarian Reserve in Women With Neuromyelitis Optica Spectrum Disorder. Front Neurol. 2018;9:446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Sun XL, Zhang J, Fan Y, Ding JH, Sha JH, Hu G. Aquaporin-4 deficiency induces subfertility in female mice. Fertil Steril. 2009;92:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Nytrova P, Dolezal O. Sex bias in multiple sclerosis and neuromyelitis optica spectrum disorders: How it influences clinical course, MRI parameters and prognosis. Front Immunol. 2022;13:933415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 93. | Kalron A, Menascu S, Dolev M, Givon U. The walking speed reserve in low disabled people with multiple sclerosis: Does it provide greater insight in detecting mobility deficits and risk of falling than preferred and fast walking speeds? Mult Scler Relat Disord. 2017;17:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Rojas JI, Patrucco L, Besada C, Funes J, Cristiano E. [Sex-related differences in atrophy and lesion load in multiple sclerosis patients]. Neurologia. 2013;28:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Cornblath DR, Gorson KC, Hughes RA, Merkies IS. Observations on chronic inflammatory demyelinating polyneuropathy: A plea for a rigorous approach to diagnosis and treatment. J Neurol Sci. 2013;330:2-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Souza MA, Souza BM, Geber S. Progesterone increases resistance of ophthalmic and central retinal arteries in climacteric women. Climacteric. 2013;16:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Oishi A, Ohmichi M, Takahashi K, Takahashi T, Mori-Abe A, Kawagoe J, Otsu R, Mochizuki Y, Inaba N, Kurachi H. Medroxyprogesterone acetate attenuates estrogen-induced nitric oxide production in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Sri-udomkajorn S, Pongwatcharaporn K. Clinical features and outcome of childhood optic neuritis at Queen Sirikit National Institute of Child Health. J Med Assoc Thai. 2011;94 Suppl 3:S189-S194. [PubMed] |
| 99. | Keogh E. The gender context of pain. Health Psychol Rev. 2021;15:454-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 100. | Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 2020;21:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 467] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 101. | Mogil JS. Sex-based divergence of mechanisms underlying pain and pain inhibition. Curr Opin Behav Sci. 2018;23:113-117. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH, Huh Y, Furutani K, He Q, Tao X, Ko MC, Bortsov A, Donnelly CR, Chen Y, Nackley A, Berta T, Ji RR. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron. 2021;109:2691-2706.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 103. | Lopes DM, Malek N, Edye M, Jager SB, McMurray S, McMahon SB, Denk F. Sex differences in peripheral not central immune responses to pain-inducing injury. Sci Rep. 2017;7:16460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 104. | Lenert ME, Avona A, Garner KM, Barron LR, Burton MD. Sensory Neurons, Neuroimmunity, and Pain Modulation by Sex Hormones. Endocrinology. 2021;162:bqab109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 105. | Pritschet L, Taylor CM, Santander T, Jacobs EG. Applying dense-sampling methods to reveal dynamic endocrine modulation of the nervous system. Curr Opin Behav Sci. 2021;40:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 106. | Maggio M, Lauretani F, Basaria S, Ceda GP, Bandinelli S, Metter EJ, Bos AJ, Ruggiero C, Ceresini G, Paolisso G, Artoni A, Valenti G, Guralnik JM, Ferrucci L. Sex hormone binding globulin levels across the adult lifespan in women--the role of body mass index and fasting insulin. J Endocrinol Invest. 2008;31:597-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 107. | Bove R, Elsone L, Alvarez E, Borisow N, Cortez MM, Mateen FJ, Mealy MA, Mutch K, Tobyne S, Ruprecht K, Buckle G, Levy M, Wingerchuk DM, Paul F, Cross AH, Weinshenker B, Jacob A, Klawiter EC, Chitnis T. Female hormonal exposures and neuromyelitis optica symptom onset in a multicenter study. Neurol Neuroimmunol Neuroinflamm. 2017;4:e339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 108. | Andersen JB, Wandall-Holm MF, Magyari M. Pregnancy outcomes following maternal or paternal exposure to teriflunomide in the Danish MS population. Mult Scler Relat Disord. 2022;59:103529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 109. | D'Souza R, Wuebbolt D, Andrejevic K, Ashraf R, Nguyen V, Zaffar N, Rotstein D, Wyne A. Pregnancy and Neuromyelitis Optica Spectrum Disorder - Reciprocal Effects and Practical Recommendations: A Systematic Review. Front Neurol. 2020;11:544434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Watanabe S, Misu T, Miyazawa I, Nakashima I, Shiga Y, Fujihara K, Itoyama Y. Low-dose corticosteroids reduce relapses in neuromyelitis optica: a retrospective analysis. Mult Scler. 2007;13:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 111. | Dost-Kovalsky K, Thiel S, Ciplea AI, Gold R, Hellwig K. Cladribine and pregnancy in women with multiple sclerosis: The first cohort study. Mult Scler. 2023;29:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 112. | Shim HK, Cho HL, Lee SH. Spinal tuberculosis at the posterior element of spinal column: case report. Clin Neurol Neurosurg. 2014;124:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Anagnostis P, Bitzer J, Cano A, Ceausu I, Chedraui P, Durmusoglu F, Erkkola R, Goulis DG, Hirschberg AL, Kiesel L, Lopes P, Pines A, van Trotsenburg M, Lambrinoudaki I, Rees M. Menopause symptom management in women with dyslipidemias: An EMAS clinical guide. Maturitas. 2020;135:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 114. | Kitley J, Woodhall M, Leite MI, Palace J, Vincent A, Waters P. Aquaporin-4 antibody isoform binding specificities do not explain clinical variations in NMO. Neurol Neuroimmunol Neuroinflamm. 2015;2:e121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Warnke C, Dehmel T, Ramanujam R, Holmen C, Nordin N, Wolfram K, Leussink VI, Hartung HP, Olsson T, Kieseier BC. Initial lymphocyte count and low BMI may affect fingolimod-induced lymphopenia. Neurology. 2014;83:2153-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 116. | Arber C, Angelova PR, Wiethoff S, Tsuchiya Y, Mazzacuva F, Preza E, Bhatia KP, Mills K, Gout I, Abramov AY, Hardy J, Duce JA, Houlden H, Wray S. iPSC-derived neuronal models of PANK2-associated neurodegeneration reveal mitochondrial dysfunction contributing to early disease. PLoS One. 2017;12:e0184104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 117. | European Medicines Agency. Aubagio (teriflunomide): EPAR product information. 2023 revision. Available from: https://www.ema.europa.eu/en/documents/product-information/aubagio-epar-product-information_en.pdf. |
| 118. | Varytė G, Arlauskienė A, Ramašauskaitė D. Pregnancy and multiple sclerosis: an update. Curr Opin Obstet Gynecol. 2021;33:378-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 119. | Wolters PL, Vranceanu AM, Thompson HL, Martin S, Merker VL, Baldwin A, Barnett C, Koetsier KS, Hingtgen CM, Funes CJ, Tonsgard JH, Schorry EK, Allen T, Smith T, Franklin B, Reeve S; REiNS International Collaboration. Current Recommendations for Patient-Reported Outcome Measures Assessing Domains of Quality of Life in Neurofibromatosis Clinical Trials. Neurology. 2021;97:S50-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 120. | Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS; ORATORIO Clinical Investigators. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med. 2017;376:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1381] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 121. | Geevasinga N, Howells J, Menon P, van den Bos M, Shibuya K, Matamala JM, Park SB, Byth K, Kiernan MC, Vucic S. Amyotrophic lateral sclerosis diagnostic index: Toward a personalized diagnosis of ALS. Neurology. 2019;92:e536-e547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 122. | Pittock SJ, Berthele A, Fujihara K, Kim HJ, Levy M, Palace J, Nakashima I, Terzi M, Totolyan N, Viswanathan S, Wang KC, Pace A, Fujita KP, Armstrong R, Wingerchuk DM. Eculizumab in Aquaporin-4-Positive Neuromyelitis Optica Spectrum Disorder. N Engl J Med. 2019;381:614-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 625] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 123. | Yamamura T, Kleiter I, Fujihara K, Palace J, Greenberg B, Zakrzewska-Pniewska B, Patti F, Tsai CP, Saiz A, Yamazaki H, Kawata Y, Wright P, De Seze J. Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. N Engl J Med. 2019;381:2114-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 124. | Kim HJ, Lee J. Response to letter regarding article 'Comparison of myelin water fraction values in periventricular white matter lesions between multiple sclerosis and neuromyelitis optica spectrum disorder'. Mult Scler. 2017;23:304-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 125. | Humphrey MB, Russell L, Danila MI, Fink HA, Guyatt G, Cannon M, Caplan L, Gore S, Grossman J, Hansen KE, Lane NE, Ma NS, Magrey M, McAlindon T, Robinson AB, Saha S, Womack C, Abdulhadi B, Charles JF, Cheah JTL, Chou S, Goyal I, Haseltine K, Jackson L, Mirza R, Moledina I, Punni E, Rinden T, Turgunbaev M, Wysham K, Turner AS, Uhl S. 2022 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2023;75:2088-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 126. | Mani S. Response to "Letter to the editors" in regard to the article 'Genetic heterogeneity of mitochondrial genome in thiamine deficient Leigh syndrome patients'. J Neurol Sci. 2019;407:116441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Levin KH, Shanafelt TD, Keran CM, Busis NA, Foster LA, Molano JRV, O'Donovan CA, Ratliff JB, Schwarz HB, Sloan JA, Cascino TL. Author response: Burnout, career satisfaction, and well-being among US neurology residents and fellows in 2016. Neurology. 2018;90:250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Noncommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/