Published online Nov 16, 2025. doi: 10.12998/wjcc.v13.i32.110897
Revised: July 29, 2025
Accepted: September 17, 2025
Published online: November 16, 2025
Processing time: 134 Days and 18.5 Hours
Malignant pleural mesothelioma (MPM), a rare aggressive malignancy, is pri
A 21-year-old female college student presented with fever (peak 38.4 °C), cough, and white mucoid sputum after cold exposure. Initial evaluation revealed ele
MPM should still be considered an important differential diagnosis in young patients presenting with solitary pleural masses and no history of typical asbestos exposure. F-18 FDG PET/CT, while serving as an essential com
Core Tip: In China, malignant pleural mesothelioma (MPM) is characterized by low definitive diagnosis rates and high misdiagnosis frequencies due to nonspecific clinical manifestations, epidemiological rarity and under-recognition in systemic diagnosis. We present a paradigmatic case of MPM in a young female patient without documented asbestos exposure, detected by F-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT), but failed to demonstrate disseminated pleural micrometastases. This case challenges the conventional risk stratification paradigm (elderly/asbestos-exposed populations), reveals the technical limitations of F-18 FDG PET/CT in detecting localized lesions, and provides evidence-based impetus for enhancing diagnostic vigilance among clinicians and nuclear medicine specialists.
- Citation: Aisikaer A, Sun MM, Shen J. Positron emission tomography/computed tomography in risk-factor-negative young female with malignant pleural mesothelioma: A case report and review of literature. World J Clin Cases 2025; 13(32): 110897
- URL: https://www.wjgnet.com/2307-8960/full/v13/i32/110897.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i32.110897
Malignant pleural mesothelioma (MPM) is an aggressive malignancy originating from mesothelial cells, primarily affecting the pleura[1]. While most cases are linked to asbestos exposure, 5%-10% occur in individuals without such history, often associated with BAP1 gene mutations[2]. World Health Organization (WHO) classifies MPM into three subtypes: Epithelioid (most common), biphasic, and sarcomatoid (most aggressive)[3]. Clinical presentation is nonspecific, typically including dyspnea, chest pain, and weight loss, often leading to delayed diagnosis and poor prognosis[4]. Characteristic computed tomography (CT) findings include irregular pleural thickening (> 1 cm) and effusion. Positron emission tomography (PET)/CT shows increased F-18 fluorodeoxyglucose (FDG) uptake, aiding diagnosis[5]. We report a young female with two FDG-avid (SUVmax = 14.0 and 13.7) pleural masses on PET/CT, confirmed as epithelioid MPM by biopsy.
Two months prior to admission, the patient developed fever (peak temperature 38.4 °C) with cough and white mucoid sputum following cold exposure.
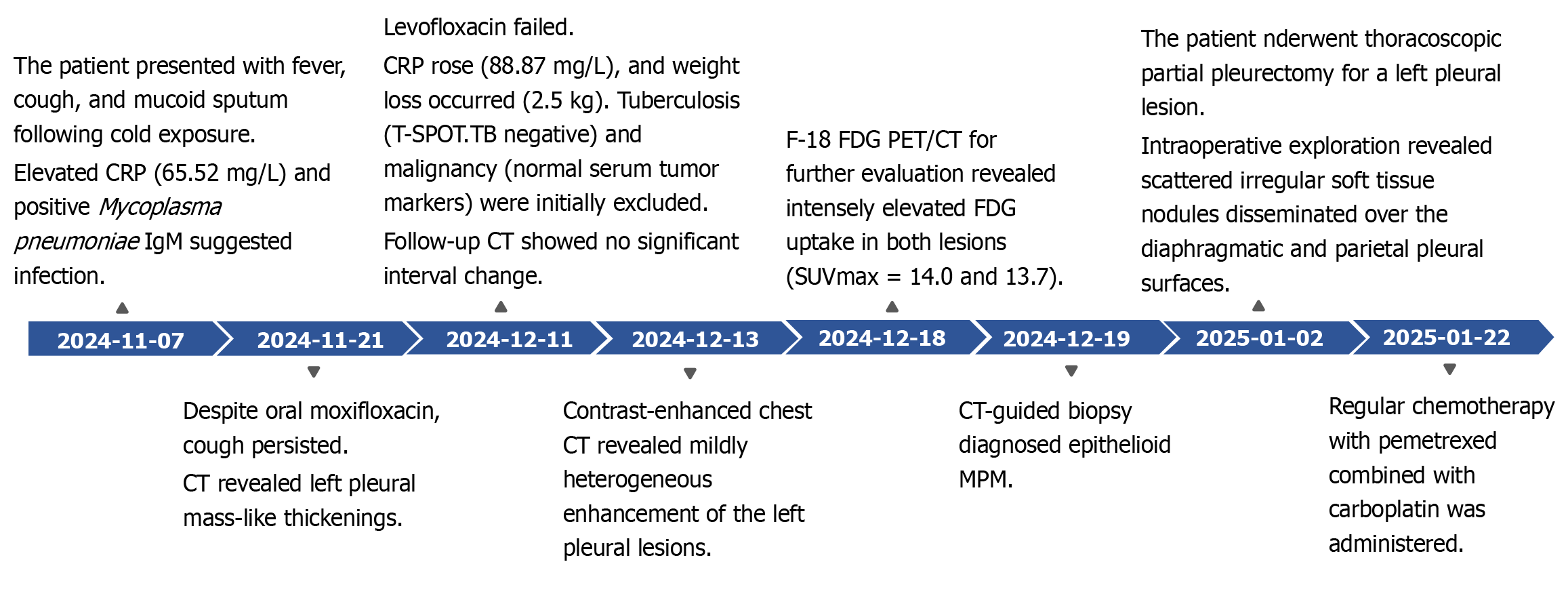
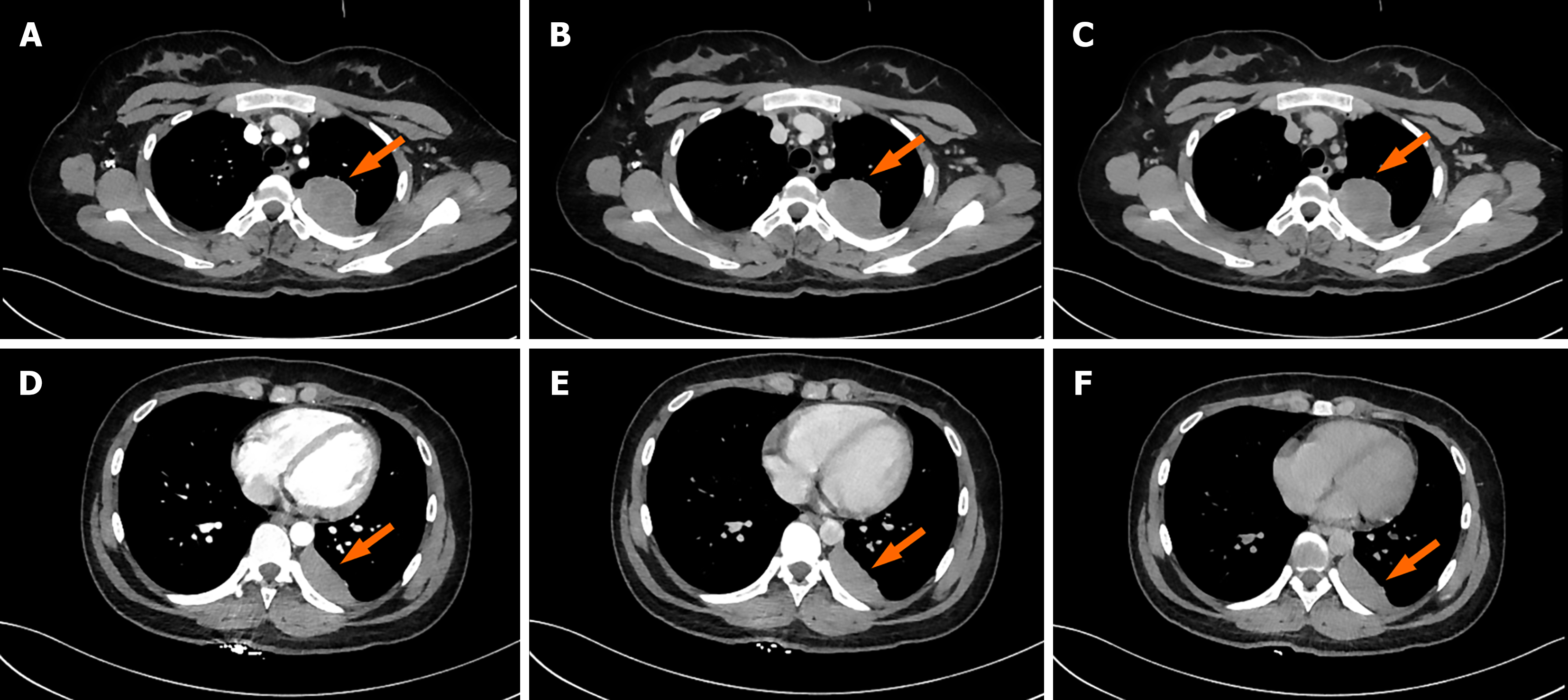
Laboratory investigations revealed markedly elevated C-reactive protein (CRP) (65.52 mg/L; reference range 0–6 mg/L) and Mycoplasma pneumoniae IgM, prompting a 14-day course of intravenous moxifloxacin followed by oral therapy. Although fever resolved after treatment, intermittent cough with white sputum persisted. Chest CT demonstrated multiple mass-like thickenings of the left pleura, leading to antibiotic escalation to levofloxacin. After 2 weeks of targeted therapy without symptomatic improvement – accompanied by 2.5-kg weight loss over 1 month – re-evaluation showed rising CRP (88.87 mg/L) and stable pleural lesions on CT. Subsequent workup returned negative T-SPOT.TB and normal tumor markers [carcinoembryonic antigen (CEA), cytokeratin 19 fragment (CYFRA21-1), neuron-specific enolase (NSE), pro-gastrin-releasing peptide (ProGRP) and squamous cell carcinoma antigen (SCC)]; contrast-enhanced CT revealed heterogeneously enhancing left pleural masses; 18F-FDG PET/CT subsequently identified two broad-based pleural lesions with intensely increased FDG uptake (Figure 1).
She was healthy without a history of personal or family tumors.
She was healthy without a history of personal or family tumors.
On admission, the patient had a body temperature of 38.2 °C, heart rate of 82 bpm, respiratory rate of 20 breaths/minute, and blood pressure of 128/77 mmHg. Respiratory examination revealed clear lung fields with no wheezes, crackles, or pleural friction rub. Percussion elicited resonant notes, and tactile fremitus was normal.
Laboratory examinations revealed elevated CRP level of 65.52 mg/L (reference range: 0–6 mg/L), along with an IgM antibody for M. pneumoniae. Despite 14 days of standardized antibiotic therapy, the CRP level increased further to 88.87 mg/L upon re-evaluation. The T-cell spot test for tuberculosis (T-SPOT.TB) assay yielded negative results. Additionally, tumor marker profiles, including CEA, CYFRA21-1, NSE, ProGRP and SCC, were all within normal ranges.
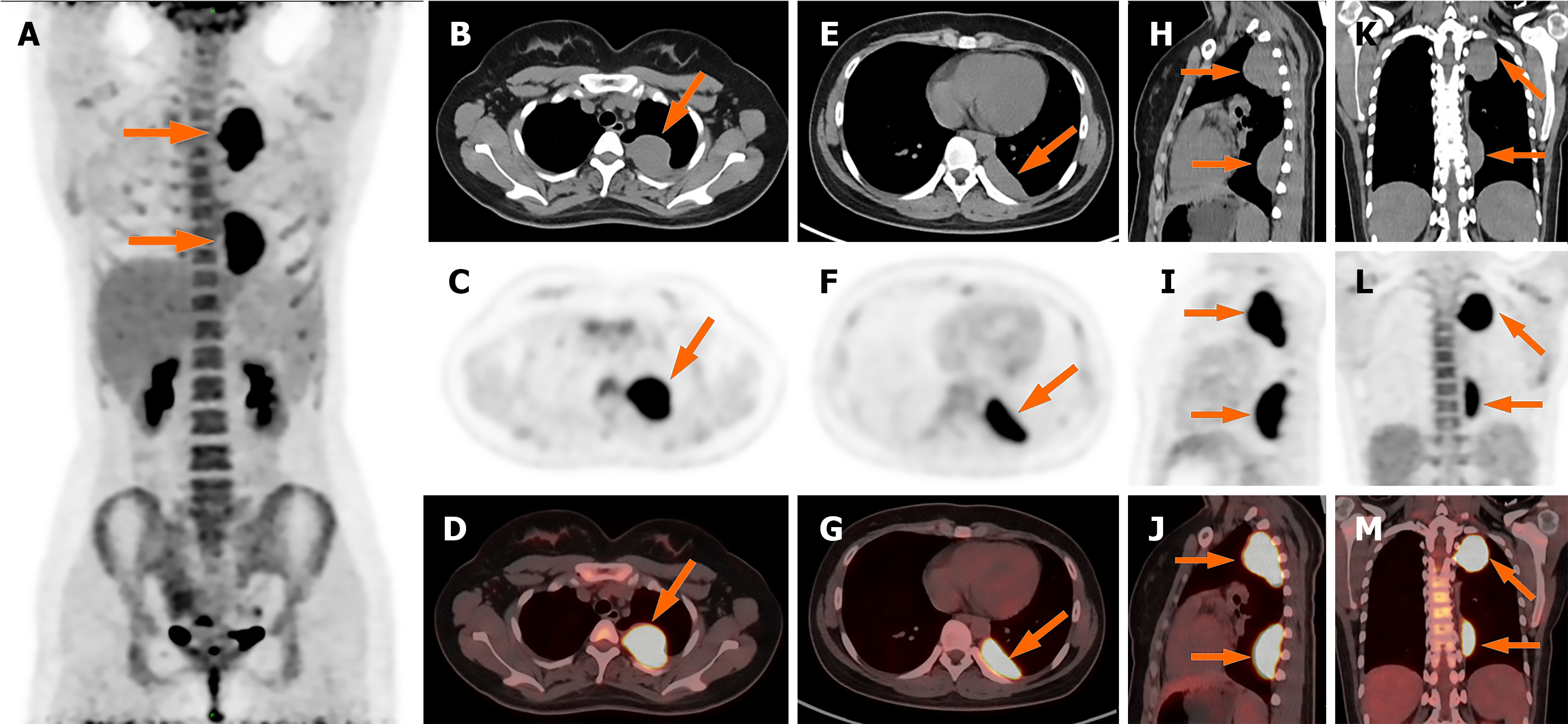
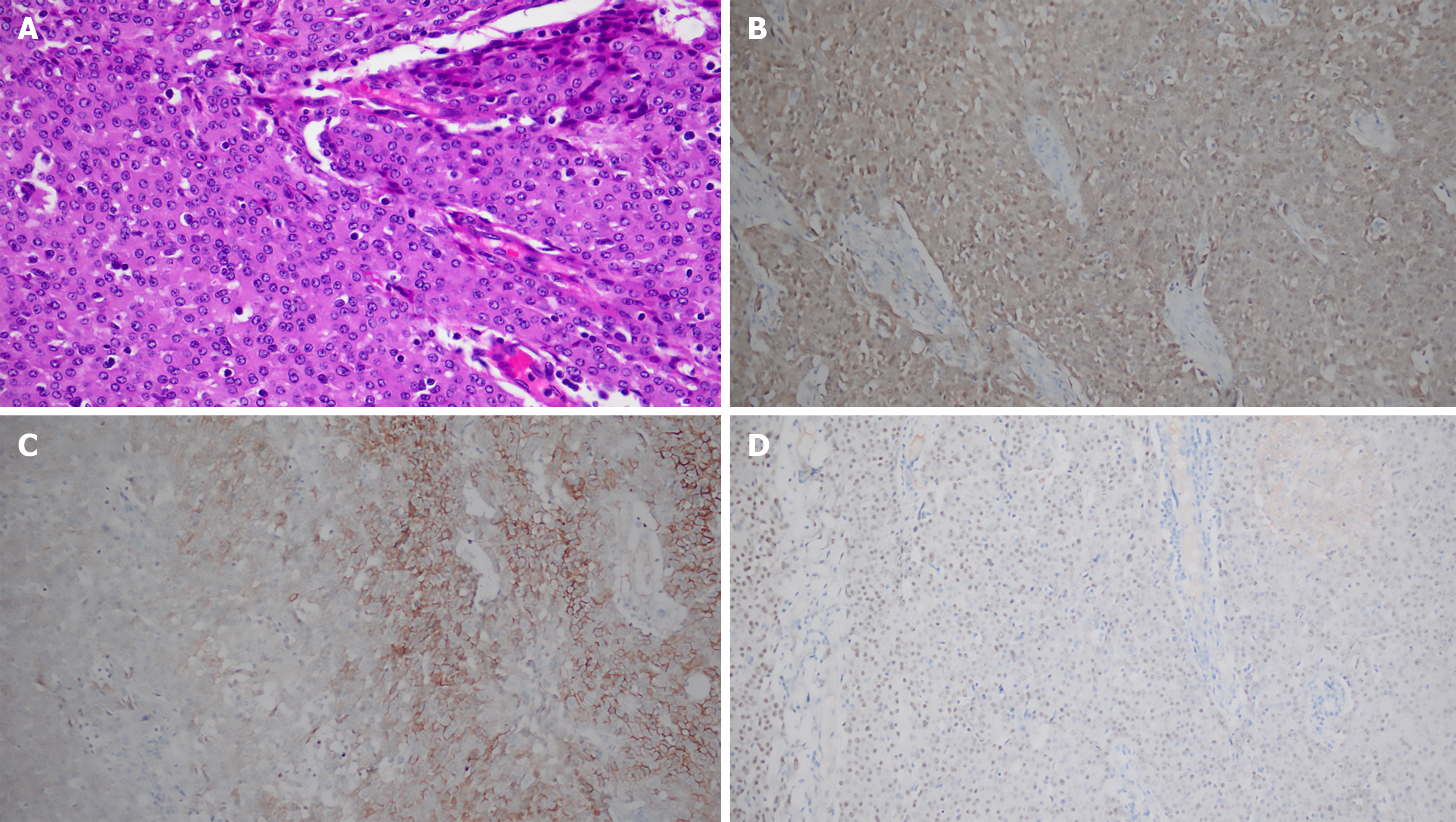
Initial chest CT revealed two pleura-based masses in the left hemithorax with smooth margins, well-defined borders, and broad pleural attachment, measuring 4.6 cm × 3.6 cm × 6.5 cm (46 HU) and 1.8 cm × 4.7 cm × 6.1 cm (38 HU). Following standardized antibiotic therapy, CRP levels increased. Follow-up CT showed no significant interval change. Contrast-enhanced CT demonstrated mild heterogeneous enhancement (Figure 2). F-18 FDG PET/CT for further evaluation revealed intensely elevated FDG uptake in both lesions (SUVmax = 14.0 and 13.7) (Figure 3). To establish a definitive diagnosis, CT-guided percutaneous pleural biopsy was performed in the outpatient setting, confirming epithelioid malignant pleural mesothelioma (Figure 4).
The patient was admitted to our department of thoracic surgery for definitive management and underwent video-assisted thoracoscopic surgery for left pleural lesion resection. Intraoperative exploration revealed scattered irregular soft tissue nodules disseminated over the diaphragmatic and parietal pleural surfaces. The definitive diagnosis of epithelioid malignant pleural mesothelioma with extensive intrapleural seeding metastasis was established based on histopathological correlation.
Intraoperatively, the tumors were friable with hemorrhagic tendency and diffuse pleural cavity implantation metastases, prompting partial pleurectomy. Discharge occurred on postoperative day 7.
Standard chemotherapy with pemetrexed plus carboplatin was initiated. Grade 2 chemotherapy-induced nausea/vomiting (CTCAE v5.0[6]) was managed with ondansetron. Following the KEYNOTE-483 phase 3 trial[7] (NCT02784171) demonstrating 21% mortality reduction with platinum/pemetrexed/pembrolizumab vs chemotherapy alone (hazard ratio 0.79, 95%CI: 0.64–0.98), pembrolizumab was added from cycle 2. Post-cycle 3, CT revealed stable disease (RECIST 1.1[8]) without pneumothorax/pleural effusion, but grade 3 anemia emerged (hemoglobin drop: 118→77 g/L). This chemotherapy-induced myelosuppression required erythropoietin support prior to completing the remaining three cycles.
MPM is a rare and aggressive malignancy originating from mesothelial cells, predominantly in the pleura (accounting for approximately 81% of cases), but also in other sites such as the peritoneum, pericardium and tunica vaginalis. According to the latest global cancer burden data (2020) from the WHO International Agency for Research on Cancer, 30870 new cases were recorded worldwide, representing 0.2% of all new malignancies, with 26278 deaths (0.3% of global cancer mortality)[9]. In China, the crude incidence rates of malignant mesothelioma were reported to be 2.2, 1.9 and 1.6 per million population in 2005, 2010 and 2015, respectively; both national and world age-standardized incidence rates remained relatively stable[10]. MPM is characterized by low definitive diagnosis rates and high rates of misdiagnosis/missed diagnosis in China[1], due to its nonspecific clinical manifestations (e.g., dyspnea, chest discomfort, cough, weight loss, anorexia, fatigue and clubbing) and rarity[4]. The development of MPM is strongly associated with asbestos exposure. Asbestos, a mineral still used in construction, industry and textiles in China, where its use has not been completely banned, necessitates close monitoring of high-risk populations and regions[11]. Occupations with elevated risk include insulation work, asbestos production and manufacturing, heating industries, shipyard work, and brake lining manufacture and repair. A subset of MPM patients without documented asbestos exposure may be associated with BAP1 germline mutations; a phenomenon predominantly observed in young female patients[2]. MPM is frequently diagnosed at advanced stages. Management is challenging, with poor prognosis; the median overall survival for patients with unresectable advanced disease is approximately 12 months[12].
Cross-sectional imaging modalities, including CT, magnetic resonance imaging and F-18 FDG PET/CT, play critical roles in the diagnosis, staging and management of MPM. On CT, MPM is typically characterized by diffuse nodular or mass-like pleural thickening (> 1 cm) with circumferential extension along adjacent pleural surfaces[13]. Involvement of interlobar fissures and associated pleural effusions are commonly observed. Unilateral pleural effusion (74%) and nodular pleural thickening (92%) represent the most frequent CT findings in MPM[14]. Malignant pleural effusion constitutes the initial presentation in most cases, with > 95% of MPM patients developing pleural effusion during their disease course[15]. The mass-like thickening pattern is attributed to the tumor's origin from the parietal pleura, with subsequent spread along pleural surfaces including fissures. Visceral pleural involvement may manifest as a "rind-like" encasement of the lung extending to the mediastinum[16]. CT features suggestive of malignant (versus benign) pleural disease include: (1) Circumferential pleural thickening; (2) Nodular pleural thickening; (3) Pleural thickness > 10 mm; and (4) Mediastinal pleural involvement[17,18]. Although these findings are frequently associated with MPM, they are not pathognomonic. Additionally, pleural plaques are commonly detected, with calcified pleural plaques observed in approximately 20% of cases[17]. While these plaques are generally considered benign indicators of asbestos exposure rather than direct malignant features, they serve as important radiological markers of prior asbestos contact[19,20].
According to the 2018 American Society of Clinical Oncology guidelines, F-18 FDG PET/CT is recommended for initial staging of MPM[12]. By quantifying metabolic activity through 18F-FDG uptake and integrating anatomical data from CT, PET/CT enhances diagnostic accuracy for pleural biopsy and plays a critical role in MPM staging[21]. SUV measurements demonstrate significantly higher FDG avidity in MPM than in benign pleural disease[11]. Bianco et al[22] established an SUVmax cutoff of 2.0 for reliable malignancy detection (sensitivity 88%-100%; specificity 88%-92%). MPM histological subtypes, classified by WHO as epithelioid (55%-65%), biphasic (20%-35%) and sarcomatoid (10%-15%), correlate with SUVmax and prognosis. Epithelioid subtypes exhibit better treatment responses, while sarcomatoid tumors confer poor outcomes[23]. Lim et al[24] reported lower median SUVmax in epithelioid MPM (5.5) vs sarcomatoid/biphasic subtypes (11.7). Similarly, Terada et al[25] observed significantly higher SUVmax in sarcomatoid (10.2 ± 5.4) than epithelioid (4.6 ± 3.9) MPM, supporting SUVmax as a prognostic biomarker for tumor aggressiveness and survival. Nonepithelioid histology consistently predicts reduced survival. An exception was observed in our case: An epithelioid MPM patient exhibited higher FDG uptake than typical sarcomatoid cases, potentially attributable to concurrent M. pneumoniae IgM-positive inflammation. The limitations of PET/CT include false-negative results due to small lesion size (e.g., intraoperatively detected disseminated lesions may remain undetected preoperatively because of their minute size), stemming from its limited spatial resolution—lesions smaller than 8-10 mm are only detectable on PET if they exhibit very high metabolic activity[26]. Additionally, limitations include false-positive or nonspecific results arising from overlapping FDG avidity mechanisms between infection/inflammatory processes and neoplasms.
In this illustrative case, a young female university student was incidentally found to have two left pleural masses > 1 cm thick on CT, demonstrating smooth margins and intense FDG avidity (maximum SUVmax 14.0), raising suspicion for malignancy. Metastatic pleural disease (MPD), the most common malignant pleural condition, must be primarily differentiated from MPM[27]. Lung and breast cancers constitute the predominant etiologies of MPD; consequently, primary tumors in these sites should be excluded when malignant pleural lesions are identified. Comprehensive PET/CT evaluation in this patient revealed no extrathoracic FDG-avid foci beyond the left costal pleura. Furthermore, significant mediastinal and hilar lymphadenopathy is more frequently observed in MPD, likely reflecting the high prevalence of lung cancer metastases within this group. As noted by Rahman et al[28], hilar nodal involvement typically results from parenchymal infiltration rather than direct pleural dissemination, whereas extrapleural and cardiophrenic nodal involvement is characteristic of MPM. This case exhibited no abnormal lymph node enlargement or FDG uptake. In summary, nodular pleural thickening accompanied by hilar/mediastinal lymphadenopathy or hematogenous pulmonary metastases strongly suggests MPD. Conversely, circumferential pleural thickening, interlobar fissure involvement, pericardial invasion, calcified pleural plaques, and absence of hematogenous metastases should heighten suspicion for MPM. Additionally, given this young female university student's immunologically immature status, tuberculosis (TB)-induced pleural pathology must be excluded. TB, a chronic granulomatous disease, commonly manifests as tuberculous pleuritis – a frequent extrapulmonary presentation. Tuberculous pleuritis may demonstrate hypermetabolic pleural nodules on PET/CT[29]. Özmen et al[30] reported no significant difference in SUVmax between TB (mean 6.8 ± 3.5) and MPM cohorts (mean 8.8 ± 5.6). However, extrapulmonary lymph node involvement and concurrent parenchymal lung lesions were identified as key discriminators. In the present case, characteristic TB imaging findings (e.g., tuberculomas and cavitation) were conspicuously absent. Furthermore, a negative T-SPOT.TB effectively ruled out active tuberculous pleuritis. Differential diagnosis must include solitary fibrous tumor of the pleura (SFTP); a rare pleural neoplasm typically presenting as a benign, broad-based extrapulmonary mass that may compress adjacent structures when large. On F-18 FDG PET/CT, SFTP demonstrates smooth margins, occasional calcification, and homogeneous mild FDG uptake (mean SUVmax 2.34, range 1.73-2.81)[31]. While CT features resembled SFTP in this case, intense hypermetabolism on PET (SUVmax > 13) excluded this diagnosis, suggesting that SUVmax can serve as a critical discriminator between MPM and SFTP. Future studies should explore metabolic parameters for differential diagnosis. Intratumoral gas density favors SFTP according to Zhao et al[32].
The integration of metabolic and morphological features by F-18 FDG PET/CT significantly enhances diagnostic accuracy, thereby substantially impacting the management, initial staging and therapeutic decision-making for MPM patients. When combined with a documented asbestos exposure history, mediastinal and diaphragmatic pleural thickenings are recognized as favorable diagnostic indicators of MPM. In young female patients with MPM and without documented asbestos exposure, genetic testing, especially for germline BAP1 mutations, holds significant clinical implications for diagnosis. Furthermore, the absence of abnormal lymph node or distant metastasis detected on PET/CT may identify candidates suitable for surgical intervention or alternative curative approaches. This study aimed to advance the understanding of MPM, particularly in preoperative evaluation, to optimize treatment planning for these challenging tumors.
MPM should still be considered an important differential diagnosis in young patients presenting with solitary pleural masses and no history of typical asbestos exposure. F-18 FDG PET/CT, while serving as an essential component of initial staging for MPM, has some inherent limitations.
| 1. | Wang Q, Xu C, Wang W, Zhang Y, Li Z, Song Z, Wang J, Yu J, Liu J, Zhang S, Cai X, Li W, Zhan P, Liu H, Lv T, Miao L, Min L, Li J, Liu B, Yuan J, Jiang Z, Lin G, Chen X, Pu X, Rao C, Lv D, Yu Z, Li X, Tang C, Zhou C, Zhang J, Guo H, Chu Q, Meng R, Liu X, Wu J, Hu X, Zhou J, Zhu Z, Chen X, Pan W, Pang F, Zhang W, Jian Q, Wang K, Wang L, Zhu Y, Yang G, Lin X, Cai J, Feng H, Wang L, Du Y, Yao W, Shi X, Niu X, Yuan D, Yao Y, Huang J, Wang X, Zhang Y, Sun P, Wang H, Ye M, Wang D, Wang Z, Hao Y, Wang Z, Wan B, Lv D, Yu J, Kang J, Zhang J, Zhang C, Wu L, Shi L, Ye L, Wang G, Wang Y, Gao F, Huang J, Wang G, Wei J, Huang L, Li B, Zhang Z, Li Z, Liu Y, Li Y, Liu Z, Yang N, Wu L, Wang Q, Huang W, Hong Z, Wang G, Qu F, Fang M, Fang Y, Zhu X, Du K, Ji J, Shen Y, Chen J, Zhang Y, Ma S, Lu Y, Song Y, Liu A, Zhong W, Fang W. Chinese expert consensus on the diagnosis and treatment of malignant pleural mesothelioma. Thorac Cancer. 2023;14:2715-2731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Hajj GNM, Cavarson CH, Pinto CAL, Venturi G, Navarro JR, Lima VCC. Malignant pleural mesothelioma: an update. J Bras Pneumol. 2021;47:e20210129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Nicholson AG, Sauter JL, Nowak AK, Kindler HL, Gill RR, Remy-Jardin M, Armato SG 3rd, Fernandez-Cuesta L, Bueno R, Alcala N, Foll M, Pass H, Attanoos R, Baas P, Beasley MB, Brcic L, Butnor KJ, Chirieac LR, Churg A, Courtiol P, Dacic S, De Perrot M, Frauenfelder T, Gibbs A, Hirsch FR, Hiroshima K, Husain A, Klebe S, Lantuejoul S, Moreira A, Opitz I, Perol M, Roden A, Roggli V, Scherpereel A, Tirode F, Tazelaar H, Travis WD, Tsao MS, van Schil P, Vignaud JM, Weynand B, Lang-Lazdunski L, Cree I, Rusch VW, Girard N, Galateau-Salle F. EURACAN/IASLC Proposals for Updating the Histologic Classification of Pleural Mesothelioma: Towards a More Multidisciplinary Approach. J Thorac Oncol. 2020;15:29-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 4. | Sinha S, Swift AJ, Kamil MA, Matthews S, Bull MJ, Fisher P, De Fonseka D, Saha S, Edwards JG, Johns CS. The role of imaging in malignant pleural mesothelioma: an update after the 2018 BTS guidelines. Clin Radiol. 2020;75:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Kandathil A, Subramaniam RM. FDG PET/CT for Primary Staging of Lung Cancer and Mesothelioma. Semin Nucl Med. 2022;52:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 558] [Article Influence: 93.0] [Reference Citation Analysis (2)] |
| 7. | Chu Q, Perrone F, Greillier L, Tu W, Piccirillo MC, Grosso F, Lo Russo G, Florescu M, Mencoboni M, Morabito A, Cecere FL, Ceresoli GL, Dawe DE, Zucali PA, Pagano M, Goffin JR, Sanchez ML, Gridelli C, Zalcman G, Quantin X, Westeel V, Gargiulo P, Delfanti S, Tu D, Lee CW, Leighl N, Sederias J, Brown-Walker P, Luo Y, Lantuejoul S, Tsao MS, Scherpereel A, Bradbury P, Laurie SA, Seymour L. Pembrolizumab plus chemotherapy versus chemotherapy in untreated advanced pleural mesothelioma in Canada, Italy, and France: a phase 3, open-label, randomised controlled trial. Lancet. 2023;402:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22733] [Article Influence: 1337.2] [Reference Citation Analysis (1)] |
| 9. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68393] [Article Influence: 13678.6] [Reference Citation Analysis (201)] |
| 10. | Zhai Y, Hui Z, Chen W, Ying J, Li J, Gao S. The epidemic of malignant mesothelioma in China: a prediction of incidence during 2016-2030. Transl Lung Cancer Res. 2022;11:2403-2411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 11. | Yildirim H, Metintas M, Entok E, Ak G, Ak I, Dundar E, Erginel S. Clinical value of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiation of malignant mesothelioma from asbestos-related benign pleural disease: an observational pilot study. J Thorac Oncol. 2009;4:1480-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Kindler HL, Ismaila N, Armato SG 3rd, Bueno R, Hesdorffer M, Jahan T, Jones CM, Miettinen M, Pass H, Rimner A, Rusch V, Sterman D, Thomas A, Hassan R. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1343-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 13. | Bonde A, Singh R, Prasad SR, Kamireddy D, Aggarwal A, Ramani N, Saboo S, Shanbhogue K, Dasyam AK, Katabathina VS. Mesotheliomas and Benign Mesothelial Tumors: Update on Pathologic and Imaging Findings. Radiographics. 2023;43:e220128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Kawashima A, Libshitz HI. Malignant pleural mesothelioma: CT manifestations in 50 cases. AJR Am J Roentgenol. 1990;155:965-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 83] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Muruganandan S, Duong V. Malignant Pleural Effusion in Malignant Pleural Mesothelioma: An Innocent Bystander? Chest. 2021;160:1602-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 505] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 17. | Nickell LT Jr, Lichtenberger JP 3rd, Khorashadi L, Abbott GF, Carter BW. Multimodality imaging for characterization, classification, and staging of malignant pleural mesothelioma. Radiographics. 2014;34:1692-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Metintas M, Ucgun I, Elbek O, Erginel S, Metintas S, Kolsuz M, Harmanci E, Alatas F, Hillerdal G, Ozkan R, Kaya T. Computed tomography features in malignant pleural mesothelioma and other commonly seen pleural diseases. Eur J Radiol. 2002;41:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Shiba N, Kusumoto M, Tsuta K, Watanabe H, Watanabe S, Tochigi N, Arai Y. A case of malignant pleural mesothelioma with osseous and cartilaginous differentiation. J Thorac Imaging. 2011;26:W30-W32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Rao D P, Vijayan S, Vananjakar SS, T PK. Malignant pleural mesothelioma. BMJ Case Rep. 2025;18:e263562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | de Fonseka D, Underwood W, Stadon L, Rahman N, Edey A, Rogers C, Maskell NA. Randomised controlled trial to compare the diagnostic yield of positron emission tomography CT (PET-CT) TARGETed pleural biopsy versus CT-guided pleural biopsy in suspected pleural malignancy (TARGET trial). BMJ Open Respir Res. 2018;5:e000270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Bianco A, Valente T, De Rimini ML, Sica G, Fiorelli A. Clinical diagnosis of malignant pleural mesothelioma. J Thorac Dis. 2018;10:S253-S261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Martini K, Frauenfelder T. Old Borders and New Horizons in Multimodality Imaging of Malignant Pleural Mesothelioma. Thorac Cardiovasc Surg. 2022;70:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Lim JH, Choi JY, Im Y, Yoo H, Jhun BW, Jeong BH, Park HY, Lee K, Kim H, Kwon OJ, Han J, Ahn MJ, Kim J, Um SW. Prognostic value of SUVmax on 18F-fluorodeoxyglucose PET/CT scan in patients with malignant pleural mesothelioma. PLoS One. 2020;15:e0229299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Terada T, Tabata C, Tabata R, Okuwa H, Kanemura S, Shibata E, Nakano T. Clinical utility of 18-fluorodeoxyglucose positron emission tomography/computed tomography in malignant pleural mesothelioma. Exp Ther Med. 2012;4:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Griffeth LK. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc (Bayl Univ Med Cent). 2005;18:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Fortin M, Cabon E, Berbis J, Laroumagne S, Guinde J, Elharrar X, Dutau H, Astoul P. Diagnostic Value of Computed Tomography Imaging Features in Malignant Pleural Mesothelioma. Respiration. 2020;99:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Abdel Rahman AR, Gaafar RM, Baki HA, El Hosieny HM, Aboulkasem F, Farahat EG, Nouh AM, Mansour KA. Prevalence and pattern of lymph node metastasis in malignant pleural mesothelioma. Ann Thorac Surg. 2008;86:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Soussan M, Brillet PY, Mekinian A, Khafagy A, Nicolas P, Vessieres A, Brauner M. Patterns of pulmonary tuberculosis on FDG-PET/CT. Eur J Radiol. 2012;81:2872-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Özmen Ö, Tatci E, Demiröz ŞM, Tazeler Z, Demirağ F. Is 18F-FDG PET/CT capable of differential diagnosis from tuberculous pleurisy from malignant mesothelioma? Nucl Med Commun. 2021;42:672-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Yan J, Ahl KL, Manning KA, Mann FA, Lewis DH. Radiology-Pathology Conference: 18F FDG PET-CT imaging of solitary fibrous tumor of the pleura. Clin Imaging. 2013;37:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Zhao L, Wang H, Shi J. (18)F-FDG PET/CT characteristics of solitary fibrous tumour of the pleura: single institution experience. Ann Nucl Med. 2022;36:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/