Published online Nov 16, 2025. doi: 10.12998/wjcc.v13.i32.109094
Revised: May 30, 2025
Accepted: October 9, 2025
Published online: November 16, 2025
Processing time: 197 Days and 15.4 Hours
Pediatric complicated community-acquired pneumonia (CCAP) is on the rise. The three subtypes include para-pneumonic effusion (PPE), necrotizing pneumonia (NP), and empyema.
To study different sub-types of pediatric CCAP, and compare their etiology, clinical profile, and outcome in the post-pneumococcal vaccination era.
This prospective observational study was conducted over a 2-year period. All details (demographics, clinical, management, and outcomes) were recorded. Con
Of the 80 cases included (71% aged 4-8 years), the distribution was as follows: PPE (42%), empyema (39%), and NP (19%). Bacterial etiology was identified in 28% (empyema 63%, P = 0.012). Staphylococcus aureus (45%) was most common followed by Escherichia coli (E. coli) (22.7%), and Streptococcus pneumoniae (13.6%). Patients with empyema, compared to PPE and NP, were less likely to receive prior antibiotics (32% vs 56% and 58%, respectively, P = 0.03). Duration (days, mean ± SD) of hospitalization was longer in children with NP compared to empyema and PPE (17.7 ± 9.8, 16.1 ± 7.5, and 13.6 ± 4.2, respectively). All children recovered with the medical management except 2 children requiring decor
Staphylococcus aureus and E. coli are the most common bacterial etiology in the post-pneumococcal vaccination era. Empyema might be related to a delay in antibiotics administration. NP is the most severe pediatric CCAP with pro
Core Tip: Complicated community-acquired pneumonia (CCAP) follows a severe course, and involve complications such as para-pneumonic effusion, necrotizing pneumonia (NP), empyema, or lung abscess. Present study conducted 5 years after introduction of pneumococcal vaccine in India found Staphylococcus aureus and Escherichia coli as the most common bacterial etiology of pediatric CCAP. In addition, empyema was found to be due to a delay in antibiotics administration, and NP was the most severe pediatric CCAP with prolonged hospitalization. This is important as far as the empirical antibiotics as well as further management of pediatric CCAP cases are concerned.
- Citation: Kanhar P, Agarwalla SK, Das RR. Clinical profile and outcome of complicated community-acquired pneumonia in children in the post-pneumococcal vaccination era. World J Clin Cases 2025; 13(32): 109094
- URL: https://www.wjgnet.com/2307-8960/full/v13/i32/109094.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i32.109094
Pediatric complicated community-acquired pneumonia (CCAP) refers to pneumonia cases in children that are more severe and involve complications such as para-pneumonic effusion (PPE), necrotizing pneumonia (NP), empyema, or lung abscess[1,2]. It typically requires hospitalization, aggressive management, and sometimes surgical intervention[3-5]. The common presentations include high fever, dry cough, chest pain, respiratory distress, and features of disseminated infection (sepsis)[1-5]. The common risk factors are young age (infants or young children), underlying chronic diseases, immunodeficiency, and infections by aggressive pathogens like Streptococcus pneumoniae or Staphylococcus aureus[6]. Sometimes pediatric CCAP can occur even in apparently healthy children.
PPE means the collection of fluid in the pleural space due to inflammation, which can progress to empyema if infected[7,8]. Empyema is an advanced or severe form of PPE where the pus accumulates in the pleural cavity, often requiring drainage through an intercostal chest drain (ICD)[3-5]. NP is a rare but serious complication where the lung tissue undergoes necrosis or destruction[9,10]. It is often caused by highly virulent organisms like Staphylococcus aureus (meth
In a developing country setting like India, Staphylococcus aureus is the most common bacterial etiology of pediatric CCAP, and in developed countries, Streptococcus pneumoniae is the most common organism[5]. In 2017, a universal pneumococcal vaccination (PCV) strategy was adopted in India[11]. No study from India has specifically reported the etiological and clinical profile as well as outcomes of different types of pediatric CCAP five years after the PCV adoption[3,7,12,13]. This is important as far as the empirical antibiotics, as well as further management of pediatric CCAP cases are concerned.
This prospective observational study was conducted in the pediatrics department (a 200-bedded hospital dedicated to children), which is attached to a tertiary care teaching institute in Eastern India. The study duration was from August 2022 to July 2024. The study was approved by the Institutional Ethics Committee. Written informed consents were taken from the parents or caregivers, and assents were taken from children > 7 years age. The study is reported as per the declaration of Helsinki.
Inclusion criteria included children of 1 month to 14 years of age with a diagnosis of CCAP and admitted to the inpatient department. Pneumonia was defined as per the World Health Organization’s revised 2014 definition of pneumonia in children. CCAP was defined as pneumonia along with the presence of complications like pleural effusion, empyema, and necrotizing pneumonia. PPE was diagnosed in cases not fitting to the criteria of empyema. Empyema was defined by one of the following criteria: Presence of purulent fluid or frank pus, pleural fluid leucocyte count > 50000/µL, or bacteria (identified by gram stain or culture) in the pleural fluid[5]. NP was a radiological diagnosis and was made by one of the following criteria - chest X ray showing cavitations inside the consolidation or computerized tomography (CT) scan showing liquefaction of lung tissue as reported by the radiologist[9,10]. Children with underlying chronic conditions, suspected or confirmed immunodeficiency, prior thoracic surgery, malignancy or post-chemotherapy, hospital acquired pneumonia, and refusal of consent were excluded.
After history and clinical examinations, the cases underwent a series of investigations including complete blood count, liver function tests, renal function tests, blood culture, inflammatory markers (C-reactive protein, proclacitonin, erythrocyte sedimentation rate), imaging (chest x ray, ultrasonography, and CT scan), and thoracentesis for pleural fluid (PF) analysis (total and differential count, protein, glucose, lactate dehydrogenase, gram stain and culture). ICD was put, and intravenous empirical antibiotics (ceftriaxone plus vancomycin or teicoplanin or linezolid) were started. If symptoms (fever & respiratory distress) persisted beyond 48-72 hours, ultrasonography was done to identify fibrin strands or loculations, or septations. If any of these were present, a fibrinolytic (streptokinase - 15000 units/kg diluted in 50 mL of normal saline, once daily dose) for 3-5 days was used. Fibrinolytic was not used if necrotizing pneumonia or broncho-pleural fistula, or air-leak was detected on the imaging. In case of poor or no response to empirical antibiotics, up-gradation was done based on the culture report or hospital protocol (in case culture was sterile). Patients were monitored clinically. Patients were discharged with oral antibiotics (mostly Linezolid) to complete a total duration of 6 weeks (intravenous plus oral). They were planned for follow-up for 1 year to see any long-term complications (not part of the present study).
All the data pertaining to demography, clinical presentation, final diagnosis, investigations, management, and outcome were recorded in the case record forms.
The sample size was calculated to be 80, considering the mean prevalence of complications in pneumonia to be 1% with an absolute precision of 0.4% and a 95% confidence interval. The data were entered into the Microsoft Excel sheet. Data were analyzed by using the IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, United States). Continuous data were presented either as mean and standard deviation (SD), or as median and inter-quartile range (IQR). Categorical data were presented as frequencies and percentages (%). Categorical data (nominal) were analyzed by using the χ2 test or Fisher’s exact test. Continuous data were analyzed by using the Student t-test or Mann-Whitney U test. For the calculation of the difference between the groups, either a linear regression model (for continuous variables) or a logistic regression model (for nominal categorical variables) was used. Bonferroni correction was used (for assessing paired comparisons when performing multiple comparisons). A P value of < 0.05 was considered statistically significant.
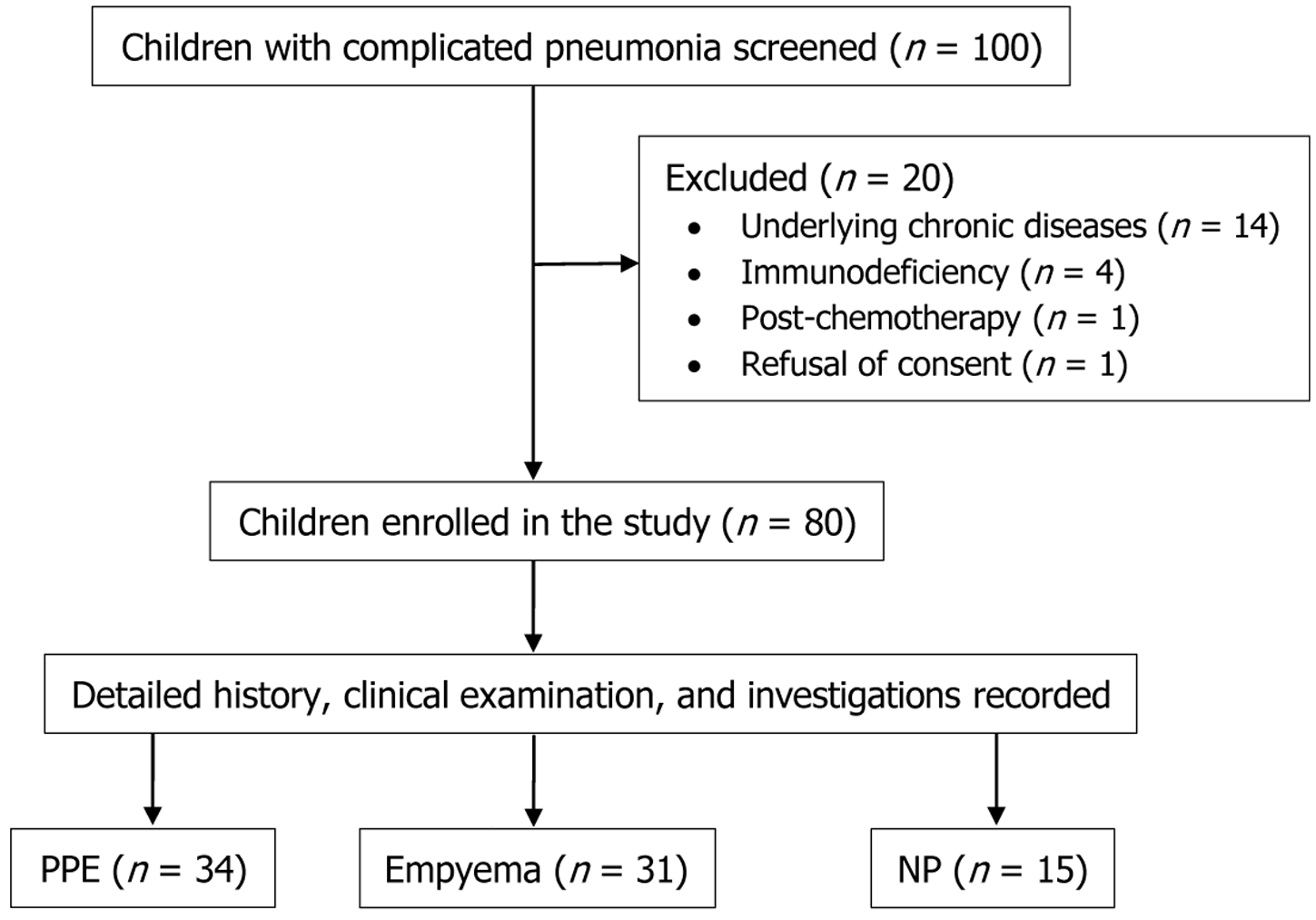
A total of 100 children with CCAP were screened, and 20 were excluded. The details of the study flow have been mentioned in Figure 1. Of the 80 included children, the majority (n = 56, 71%) were between 4 to 8 years of age. Of the rest 29% children, 24% (n = 19) were 1 month to 4 years (3 were infants, and all had NP), and 5% (n = 5) were > 8 years of age. Majority was male (n = 46) with a ratio of 1.35. The most common age group affected with empyema and NP was 2 to 8 years, and with PPE was 4 to 8 years. All the types of CCAP were common in males. Fever, cough, and breathing difficulty were the most common presenting complaints. The mean (± SD) duration of illness prior to admission was 7 (± 2.2) days. Data on prior antibiotic use were available in 38 (47.5%) cases. Patients with empyema, compared to PPE and NP, were less likely to receive empirical antibiotics (32% vs 56% and 58%, respectively, P = 0.03). ICD was inserted in 19 (23.8%) cases prior to referral (empyema and NP = 1, PPE = 6, and empyema = 12). Immunization history was not available in 14 (17.5%) cases. Regarding malnutrition, 12 (15%) were severely malnourished, and 28 (35%) had moderate acute malnutrition. Severe malnutrition was common in children with empyema (P = 0.01). Six children (7.5%) had a skin infection (pustular lesion or boil, or cellulitis) anywhere in the body. Thirteen children (16.2%) had a preceding history of viral infection (chickenpox = 6, measles = 4, and Influenza = 3). The baseline characteristics and management approaches are provided in Table 1.
| Characteristics | PPE (n = 34) | Empyema (n = 31) | NP (n = 15) | P value |
| Age (years), mean ± SD | 5.9 ± 2.8 | 4.7 ± 2.3 | 4.1 ± 2.1 | 0.12 |
| Age sub-group | ||||
| < 1 year | 1 (2.9) | 1 (3.3) | 3 (20) | 0.01a |
| 1-5 years | 16 (47.1) | 18 (58) | 8 (53.3) | 0.26 |
| > 5 years | 17 (50) | 12 (38.7) | 4 (26.7) | 0.1 |
| Male | 17 | 15 | 14 | 0.36 |
| Duration of illness prior to admission (days), mean ± SD | 6.5 ± 2.8 | 7.3 ± 2.2 | 6.9 ± 2.4 | 0.47 |
| Not immunized | 5 (14.7) | 6 (19.3) | 3 (20) | 0.33 |
| Severe malnutrition | 2 (5.8) | 9 (29) | 1 (6.7) | 0.01a |
| Clinical feature | ||||
| Cough | 30 (88.2) | 28 (90.3) | 12 (80) | 0.56 |
| Fever | 33 (97) | 31 (100) | 15 (100) | 0.49 |
| Rapid breathing | 29 (85.3) | 28 (90.3) | 15 (100) | 0.62 |
| Chest retraction | 25 (73.5) | 26 (83.9) | 14 (93.3) | 0.39 |
| Cyanosis | 3 (8.8) | 5 (19.3) | 2 (13.3) | 0.25 |
| Lethargy | 3 (8.8) | 4 (12.9) | 2 (13.3) | 0.31 |
| Sepsis | 2 (5.8) | 2 (6.4) | 2 (13.3) | 0.09 |
| Shock | - | 2 (6.4) | 1 (6.7) | 0.44 |
| Prior antibiotic use | 19 (56) | 10 (32) | 9 (58) | 0.03a |
| Prior ICD insertion | 6 (17.6) | 12 (38.7) | 1 (6.7) | 0.02a |
| New ICD inserted | 5 (14.7) | 19 (61.3) | 1 (6.7) | 0.01a |
| Fibrinolytic use | - | 10 (32.2) | 2 (13.3) | 0.07 |
| PICU admission | 2 (5.8) | 9 (29) | 3 (20) | 0.042a |
| Invasive mechanical ventilation | 1 (2.9) | 5 (19.3) | 2 (13.3) | 0.036a |
The distribution of different types of CCAP was as follows: PPE (n = 34, 42%), empyema (n = 31, 39%), and NP (n = 15, 19%). The consolidation was right-sided in the majority (n = 41, 51.2%), followed by left-sided (n = 32, 40%) and bilateral (n = 7, 8.8%). Empyema was right-sided in 18 cases (58%) and left sided in 13 cases (42%). Empyema necessitans was present in 2 cases (2.5%). Other pathologies were – pneumatoceles (n = 11, 13.8%), air leaks (n = 6, 7.5%), and broncho-pleural fistula (n = 2, 2.5%).
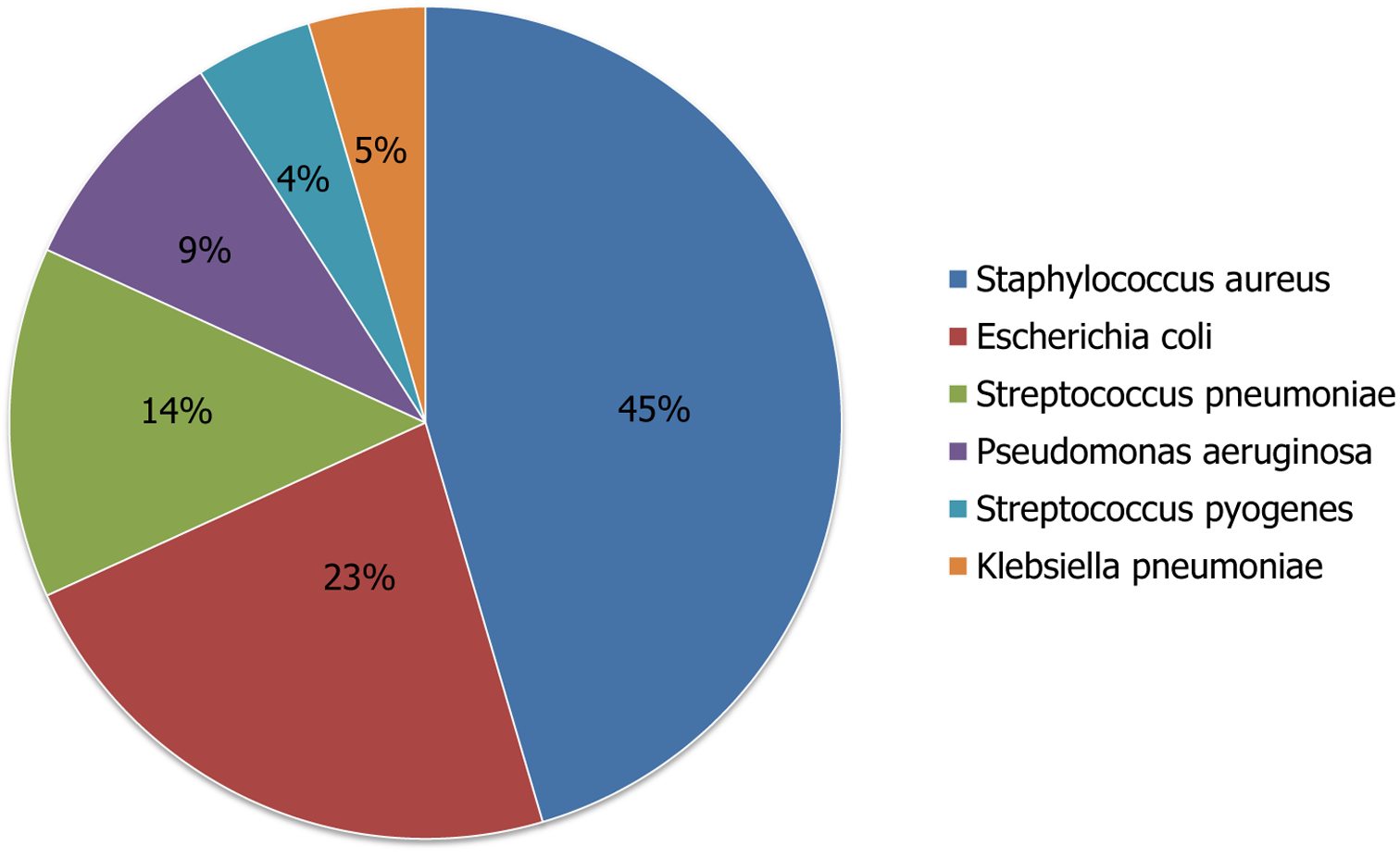
Bacterial etiology was identified in 28% (n = 22) [blood culture = 7/22 (32%), PF culture = 13/22 (59%), both = 2/22 (9%)]. The isolation of a bacterium was higher in PF (59%) compared to blood culture (32%) (P = 0.037). Of the 22 culture-positive cases, the majority were empyema cases (P = 0.012). Of the 7 cases with only positive blood culture, 4 were PPE, and 3 were NP. In the majority of the cases of NP and PPE, the blood culture was sterile. PF culture was positive in 13 of 31 cases (38.7%) of empyema, and in 2 cases with both empyema and NP. The 2 cases with empyema and NP had both blood and PF culture positive. Staphylococcus aureus was the most common organism isolated in 45% cases (n = 10, MSSA = 5, MRSA = 5). Escherichia coli (E. coli) was the next most common organism isolated in 22.7% cases (n = 5). Streptococcus pneumoniae was surprisingly the third most common organism isolated in 13.6% cases (n = 3). Other organisms were Pseudomonas aeruginosa (n = 2), Streptococcus pyogenes (n = 1), and Klebsiella pneumoniae (n = 1). The detailed breakups of the isolated organisms are provided in Figure 2. Prior antibiotics (before admission) use were associated with a significant negative yield on culture (64%) compared to no antibiotics (29%) (P = 0.01).
The clinical courses and outcomes are described in Table 2. Fourteen children (17.5%) required intensive care unit admission, of which 8 (57.1%) required invasive mechanical ventilation. This was significant in the empyema and/or NP group. Antibiotics were upgraded in 17 (21.2%) children as the recovery was getting delayed, and this was significant in the empyema and/or NP group. Ten (32.2%) children with empyema and 2 children with NP plus empyema required the fibrinolytic. The total duration (days, mean ± SD) of hospitalization was longer in children with NP compared to empyema and PPE (17.7 ± 9.8, 16.1 ± 7.5, and 13.6 ± 4.2, respectively) (P = 0.09). All children recovered with the medical management, except 2 children (both had empyema and NP) who required decortication. No child required video assisted thoracoscopic surgery.
| Characteristics | PPE (n = 34) | Empyema (n = 31) | NP (n = 15) | P value |
| Time to resolution of fever (days), mean ± SD | 10.1 ± 2.4 | 12.4 ± 2 | 13.3 ± 2.9 | 0.26 |
| Time to resolution of illness (days), mean ± SD | 10.7 ± 2.9 | 13.2 ± 2.2 | 13.9 ± 3.1 | 0.43 |
| Duration of ICD stay (days), mean ± SD | 4.5 ± 1.9 | 7.1 ± 2.8 | 6.8 ± 2.3 | 0.37 |
| Duration of IV antibiotics use (days), mean ± SD | 11.3 ± 3.1 | 14.1 ± 2.8 | 15.5 ± 3.7 | 0.08 |
| Duration of hospitalization (days), mean ± SD | 13.6 ± 4.2 | 16.1 ± 7.5 | 17.7 ± 9.8 | 0.09 |
| Required change or upgrade of antibiotics | 3 (8.8) | 9 (26.5) | 5 (33.3) | 0.03a |
| Required inotropes for shock | - | 2 (6.4) | 1 (6.7) | 0.51 |
In this prospective observational study including 80 children with CCAP, PPE was found in 42%, empyema in 39%, and NP in 19% cases. Bacterial etiology was identified in 28% (most common from the pleural fluid in empyema). Staphylococcus aureus (45%) was the most common, followed by E. coli (22.7%), and Streptococcus pneumoniae (13.6%). Patients with empyema, compared to PPE and NP, were less likely to receive prior antibiotics. The duration of hospitalization was longer in children with NP compared to empyema and PPE, and all the children recovered with the medical mana
In a previous study including 144 children, the authors found that 40% had PPE, 40% had empyema, and 20% had NP[1]. A bacterium was isolated in 42% (most common being in empyema) with Streptococcus pneumoniae being the most common (32%), followed by Group A Streptococcus (9%). Children with empyema compared to PPE and NP were less likely to receive empirical antibiotics. The mean duration of hospitalization was longer in children with NP compared to those in the empyema and PPE group. The authors concluded that NP follows a more severe course with high morbidity and a longer duration of hospitalization. The present study findings mostly agree with this study. However, in the present study, Staphylococcus aureus was the most common, followed by E. coli, causing CCAP. In addition, NP and empyema followed a more severe course compared to PPE, with NP plus empyema being the worst.
In another study from India, the authors included 205 patients (1 month to 12 years) of empyema thoracis admitted over 5 years[3]. The pleural fluid cultures were positive in 40% with Staphylococcus aureus being the most common organism (66.7%). ICD was inserted in 97.5% cases, and fibrinolytics were administered in 33.6% cases. The mean (± SD) duration of hospital stay was 17.2 (± 6.3) days, and the mortality rate was 4%. The only similarities between this study and the present study were - Staphylococcus aureus being the most common organism, and duration of hospital stay. However, in another study from India, the authors included 150 cases and found Streptococcus pneumoniae to be the most common organism[13].
NP is characterized by the necrosis of lung tissue, leading to the formation of cavities or abscesses in the affected lung. This form of pneumonia is usually caused by bacterial infections and is more likely to occur in severe cases of com
Empyema refers to the accumulation of pus in the pleural space, typically as a complication of pneumonia. In children, empyema is frequently associated with bacterial pneumonia, especially with pathogens such as Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae[14,15]. Empyema results from an untreated or inadequately treated bacterial infection that spreads from the lung tissue into the pleural space. The pleura becomes inflamed, and fluid accumulates in the pleural cavity. As the infection progresses, the pleural fluid becomes purulent, and the pleural membranes may become fibrotic, leading to pleural thickening and, in severe cases, lung collapse. The diagnosis of empyema requires a high index of suspicion. Clinical features such as fever, respiratory distress, pleuritic chest pain, and persistent pneumonia unresponsive to initial treatment suggest the possibility of empyema. Empirical antibiotics to cover both Staphylococcus aureus and Streptococcus pneumoniae in addition to ICD cure most of the children with surgery, requiring only in few[1,5,16,17].
The incidence of necrotizing pneumonia and empyema in children varies depending on geographic location and the prevalence of specific pathogens. Studies have shown that while most pneumonia cases in children are uncomplicated, a small percentage progress to more severe forms such as empyema and necrotizing pneumonia. Studies have found that the incidence of complicated pneumonia in children, including necrotizing pneumonia and empyema, is increasing[18]. Risk factors for developing these complications include younger age, immunocompromised status, underlying lung disease, no vaccination against pneumococcus, and delayed initiation of appropriate antibiotic therapy[7,8,13].
In the present study, we found E. coli as the second most common cause of CCAP. It’s not an unusual finding, as E. coli has been shown to cause empyema[19-22]. It can cause empyema even in neonates[19,20]. In a previous study from India, including 70 children, E. coli was the second most common cause (4.3%) after Staphylococcus aureus (34.2%)[21]. In a study from China including 63 children, E. coli was reported in 3.2% cases[22]. With the widespread vaccination against Streptococcus pneumoniae and irrational use of antibiotics, we are going to see a surge of gram-negative organisms causing CCAP.
The limitations of present study include the following: (1) Being a referral hospital, selection bias of cases cannot be ruled out; (2) The study findings may not be generalized to primary and secondary health care centres; (3) Prior anti
Staphylococcus aureus and E. coli are the most common bacterial etiology in the post-pneumococcal vaccination era. Empyema might be related to a delay in antibiotic administration. NP is the most severe pediatric CCAP with prolonged hospitalization.
| 1. | Erlichman I, Breuer O, Shoseyov D, Cohen-Cymberknoh M, Koplewitz B, Averbuch D, Erlichman M, Picard E, Kerem E. Complicated community acquired pneumonia in childhood: Different types, clinical course, and outcome. Pediatr Pulmonol. 2017;52:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Yavuz S, Sherif A, Amirrad M, Sabet K, Hassan M, Abuelreish M, Langawi N, Almanasir M, Francis N. A Retrospective Chart Review of Pediatric Complicated Community-Acquired Pneumonia: An Experience in the Al Qassimi Women and Children Hospital. Cureus. 2022;14:e31119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Angurana SK, Kumar R, Singh M, Verma S, Samujh R, Singhi S. Pediatric empyema thoracis: What has changed over a decade? J Trop Pediatr. 2019;65:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Buonsenso D, Cusenza F, Passadore L, Bonanno F, Calanca C, Mariani F, Di Martino C, Rasmi S, Esposito S. Parapneumonic empyema in children: a scoping review of the literature. Ital J Pediatr. 2024;50:136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Kuru M, Altinok T. Empyema in children. Turk Gogus Kalp Damar Cerrahisi Derg. 2024;32:S29-S36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Tuğcu GD, Özsezen B, Türkyılmaz İ, Pehlivan Zorlu B, Eryılmaz Polat S, Özkaya Parlakay A, Cinel G. Risk factors for complicated community-acquired pneumonia in children. Pediatr Int. 2022;64:e15386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | P M, Awasthi S. Predicting Complicated Parapneumonic Effusion in Community Acquired Pneumonia: Hospital Based Case-Control Study. Indian J Pediatr. 2019;86:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Mahon C, Walker W, Drage A, Best E. Incidence, aetiology and outcome of pleural empyema and parapneumonic effusion from 1998 to 2012 in a population of New Zealand children. J Paediatr Child Health. 2016;52:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Teresinha Mocelin H, Bueno Fischer G, Danezi Piccini J, de Oliveira Espinel J, Feijó Andrade C, Bush A. Necrotizing Pneumonia In Children: A Review. Paediatr Respir Rev. 2024;52:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Chen Y, Li L, Wang C, Zhang Y, Zhou Y. Necrotizing Pneumonia in Children: Early Recognition and Management. J Clin Med. 2023;12:2256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 11. | Kumar P, Ray A, Kumari A, Sultana A, Hora R, Singh K, Mehra R, Kaur A, Koshal SS, Quadri SF, Singh SK, Roy AD. Chronicling the Journey of Pneumococcal Conjugate Vaccine Introduction in India. Vaccines (Basel). 2025;13:432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Narayanappa D, Rashmi N, Prasad NA, Kumar A. Clinico-bacteriological profile and outcome of empyema. Indian Pediatr. 2013;50:783-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Dass R, Deka NM, Barman H, Duwarah SG, Khyriem AB, Saikia MK, Hoque R, Mili D. Empyema thoracis: analysis of 150 cases from a tertiary care centre in North East India. Indian J Pediatr. 2011;78:1371-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Amin M, Yousef Pour S, Navidifar T. Detection of the major bacterial pathogens among children suffering from empyema in Ahvaz city, Iran. J Clin Lab Anal. 2019;33:e22855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Stankey CT, Spaulding AB, Doucette A, Hamre KES, Wheeler W, Pomputius WF, Kurachek S. Blood Culture and Pleural Fluid Culture Yields in Pediatric Empyema Patients: A Retrospective Review, 1996-2016. Pediatr Infect Dis J. 2018;37:952-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Stockmann C, Ampofo K, Pavia AT, Byington CL, Sheng X, Greene TH, Korgenski EK, Hersh AL. Comparative Effectiveness of Oral Versus Outpatient Parenteral Antibiotic Therapy for Empyema. Hosp Pediatr. 2015;5:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Proesmans M, Gijsens B, Van de Wijdeven P, De Caluwe H, Verhaegen J, Lagrou K, Van Even E, Vermeulen F, De Boeck K. Clinical outcome of parapneumonic empyema in children treated according to a standardized medical treatment. Eur J Pediatr. 2014;173:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Gautam A, Wiseman GG, Goodman ML, Ahmedpour S, Lindsay D, Heyer A, Stalewski H, Norton RE, White AV. Paediatric thoracic empyema in the tropical North Queensland region of Australia: Epidemiological trends over a decade. J Paediatr Child Health. 2018;54:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Gustavson EE. Escherichia coli empyema in the newborn. Am J Dis Child. 1986;140:408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Gupta R, Faridi MM, Gupta P. Neonatal empyema thoracis. Indian J Pediatr. 1996;63:704-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Goyal V, Kumar A, Gupta M, Sandhu HP, Dhir S. Empyema thoracis in children: Still a challenge in developing countries. Afr J Paediatr Surg. 2014;11:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Zhang X, Zhang H. Microbiological characteristics and outcomes of children with pleural empyema admitted to a tertiary hospital in southeast China, 2009-2018. Turk J Pediatr. 2021;63:994-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/