Published online Nov 6, 2025. doi: 10.12998/wjcc.v13.i31.112023
Revised: July 28, 2025
Accepted: September 12, 2025
Published online: November 6, 2025
Processing time: 106 Days and 20.6 Hours
Mantle cell lymphoma (MCL) is an aggressive B-cell non-Hodgkin lymphoma that rarely presents with cutaneous involvement, which typically occurs in advanced disease stages. Primary cutaneous manifestations are particularly uncommon and frequently misdiagnosed. We report a diagnostically challenging case of MCL that first appeared as a nodule on the lower leg.
An elderly female had a painless red nodule on the front of her right lower leg for six months. Positron emission tomography-computed tomography showed a soft tissue mass at the same site and increased activity in the right groin lymph nodes. Skin and lymph node biopsies, along with immunostaining, confirmed MCL. The patient began combination chemotherapy, resulting in a marked improvement of skin lesions and lymphadenopathy after two weeks.
Cutaneous manifestations, though uncommon, may serve as the initial clinical presentation of MCL. Dermatologists and pathologists should maintain heigh
Core Tip: Mantle cell lymphoma (MCL) is an aggressive subtype of B-cell lymphoma that rarely involves the skin. When cutaneous involvement occurs, it often indicates advanced systemic disease and is associated with a poor prognosis. This case emphasizes the difficulty in differentiating cutaneous MCL from other similar lymphomas, such as diffuse large B-cell lymphoma, leg-type. Accurate diagnosis relies heavily on immunophenotypic markers, particularly cyclin D1 and SOX11. Therefore, a comprehensive approach integrating clinical findings, histopathology, and molecular studies is vital for timely diagnosis, appropriate treatment, and improved patient outcomes.
- Citation: Zhou PY, Chen W, Wang L. Mantle cell lymphoma presenting primarily as cutaneous lesions: A case report. World J Clin Cases 2025; 13(31): 112023
- URL: https://www.wjgnet.com/2307-8960/full/v13/i31/112023.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i31.112023
Mantle cell lymphoma (MCL) is an aggressive subtype of non-Hodgkin lymphoma derived from mature B cells, representing 3%-10% of all such cases[1]. While MCL predominantly affects lymph nodes and frequently involves extranodal sites-such as the bone marrow, spleen, and liver-cutaneous manifestations are exceedingly rare[2]. We describe an unusual case of MCL that first presented as a solitary skin nodule on the lower leg. Furthermore, we conducted a review of published cases to summarize the clinical characteristics of MCL with skin involvement.
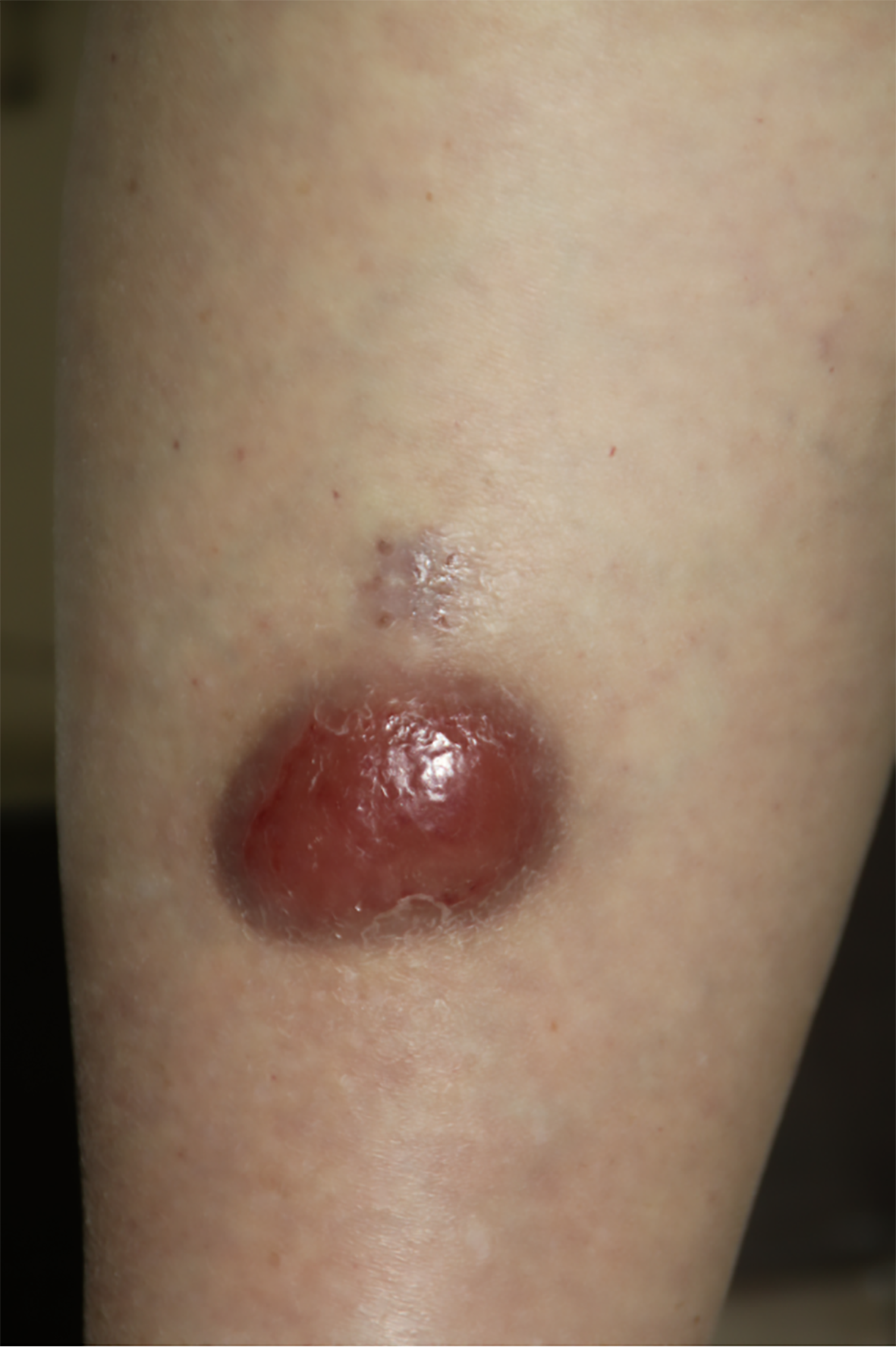
A 74-year-old Chinese woman presented to the dermatology department with a solitary, asymptomatic red nodule on the front of her right lower leg, present for six months.
The lesion initially was small but progressively enlarged to approximately 6 cm × 4 cm. The nodule had a smooth surface, a firm consistency, and no signs of ulceration, itching, or tenderness.
Seven years earlier, the patient underwent bilateral thyroidectomy for papillary thyroid carcinoma, with subsequent neck lymph node dissection one year later for metastatic disease.
No relevant family history of malignancy was noted.
On examination, a solitary, asymptomatic red nodule, approximately 6 cm × 4 cm, was found on the front of her right lower leg. The nodule exhibited a smooth surface and firm consistency, without ulceration, itching, or tenderness (Figure 1).
No abnormalities were detected in routine blood tests, urinalysis, or liver/kidney function tests.
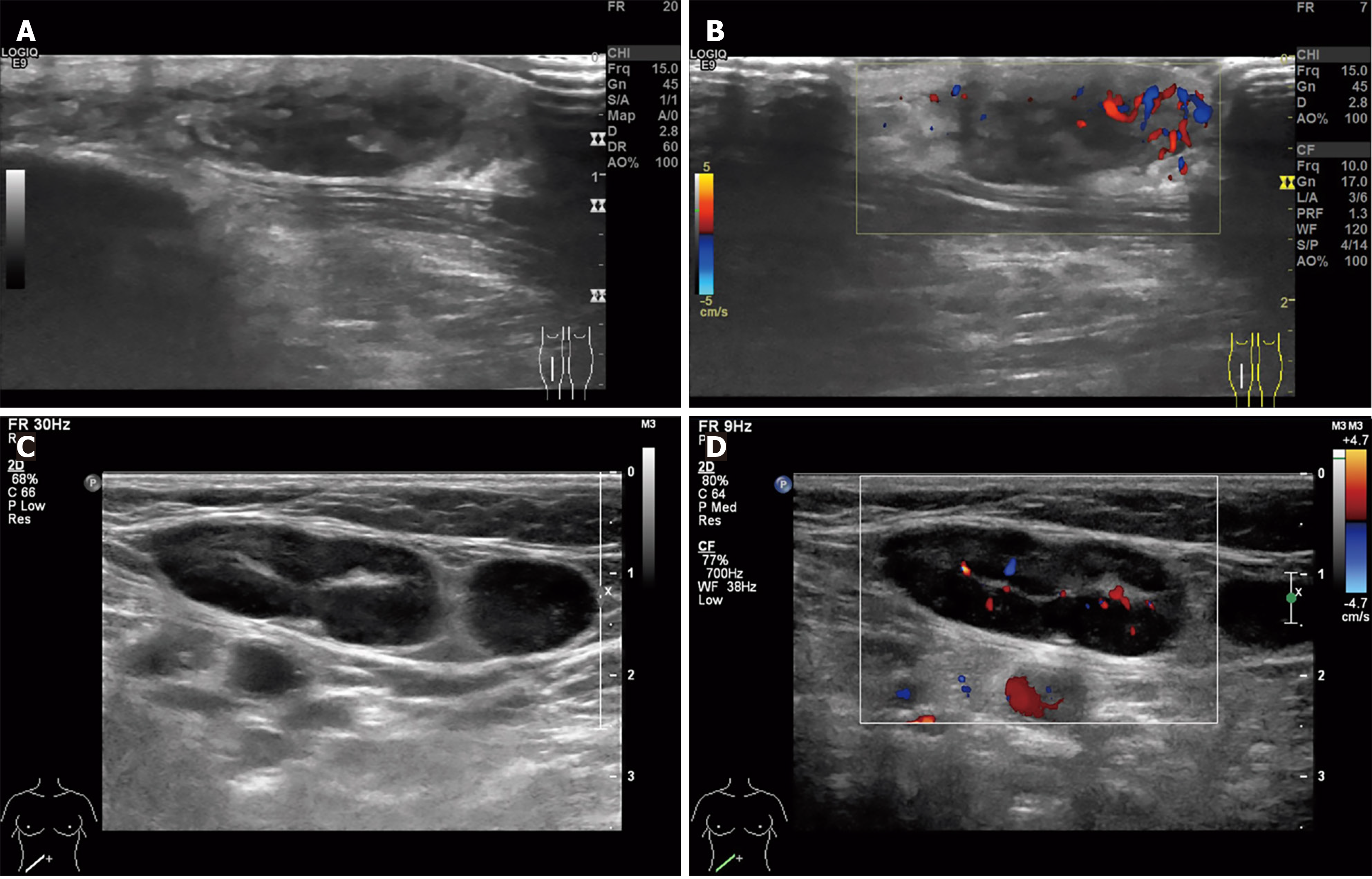
Ultrasound examination identified a 25 mm × 9 mm × 25 mm hypoechoic subcutaneous mass in the mid-right lower leg displaying poorly defined borders and irregular shape. The surrounding area showed enhanced echogenicity with abundant peripheral blood flow signals (Figure 2). Concurrently, imaging studies revealed multiple enlarged lymph nodes in the right inguinal region, with the largest measuring 30 mm × 13 mm. These nodes showed asymmetric cortical thickening and punctate/Linear vascular signals (Figure 2).
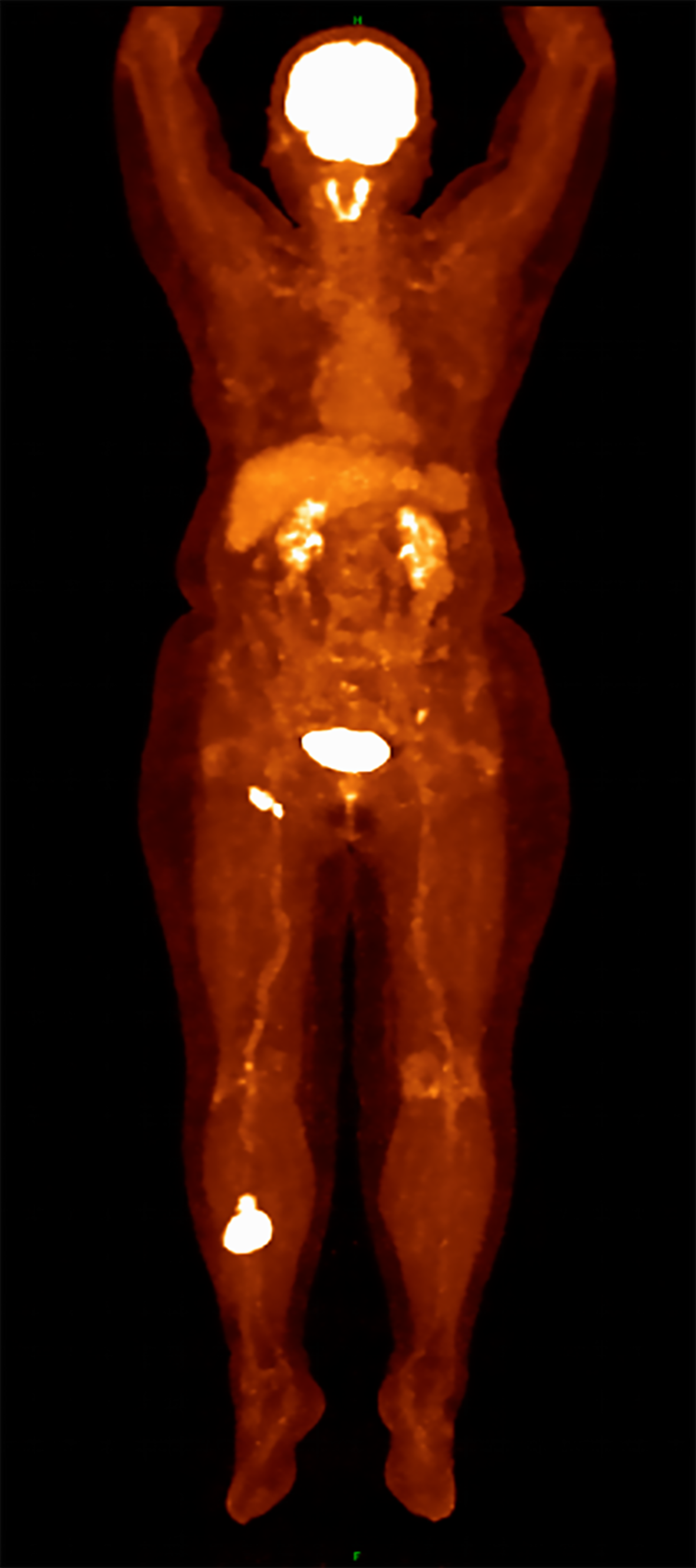
Positron emission tomography- computed tomography (PET-CT) imaging demonstrating intense fluorodeoxyglucose uptake in both the cutaneous lesion and right inguinal lymph nodes, consistent with lymphoma involvement. There was no abnormal uptake or reduction in other soft tissues or bones (Figure 3).
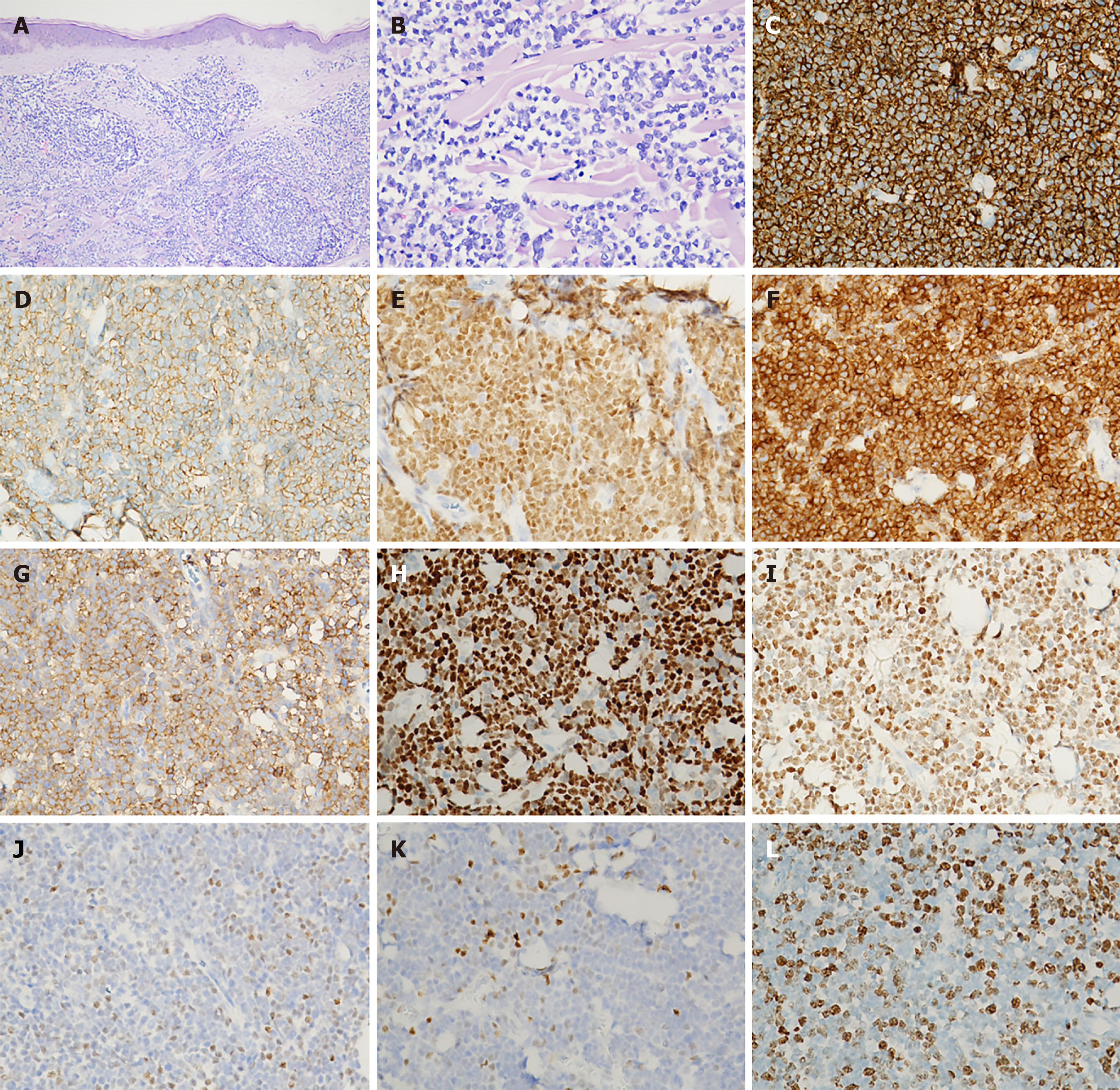
Biopsy of the skin nodule on the right lower leg revealed mild thickening of the epidermal stratum spinosum, with a clear separation between the epidermis and the dermis. The dermis and subcutaneous fat were densely infiltrated by lymphoid cells. These cells exhibited large, irregular nuclei with abnormal features and occasionally underwent cell division. Immunohistochemical analysis demonstrated positivity for CD20, CD79a, PAX5, Bcl-2, CD5, Cyclin D1, and SOX11. Partial positivity was observed for Bcl-6, while C-MYC was positive in scattered small areas. Markers including CD3, CD10, CD21, CD43, and MUM-1 were negative. The proliferation rate, indicated by Ki-67, reached about 60% in the most active areas. Testing for Epstein-Barr virus was negative (Figure 4). Genetic analysis confirmed clonal rearrangements for TCRG, IgH, and IgK.
A fine needle biopsy of the right inguinal lymph node showed a proliferative lymphoid lesion mainly composed of medium-sized lymphoid cells, some with atypical features. Immunohistochemistry confirmed positivity for CD20, CD79a, CD5, Cyclin D1, and SOX-11, with approximately 70% of cells positive for Bcl-2. Other markers including CD3, CD10, CD21, CD23, CD43, and Bcl-6 were negative. The Ki-67 proliferation index was about 70%. Epstein-Barr virus testing was again negative. Genetic studies identified clonal rearrangements involving IgH and IgK genes.
Bone marrow examination showed no abnormalities.
Considering the clinical findings and pathological analysis, the patient was diagnosed with mantle cell lymphoma, classified as Ann Arbor stage IIE/A[3].
The patient was referred to the Oncology to receive R-CHOP chemotherapy (rituximab 700 mg d1, cyclophosphamide 1300 mg d1, pirarubicin 85 mg d1, vincristine 2 mg d1, prednisone 100 mg d1-5 q3w). However, after two cycles, significant chemotherapy-related side effects prompted a change in therapy to orelabrutinib (150 mg qd). A follow-up PET-CT scan conducted after four months of orelabrutinib treatment showed mixed responses. The lesion in the lower leg demonstrated reduced size and decreased glucose metabolism (Deauville score: 5). Meanwhile, the right inguinal lymph node lesions exhibited a partial increase in size but a reduction in metabolic activity. Additionally, a new lesion emerged near the right external iliac vessels. In response to these findings, the treatment regimen was adjusted to R-Len (rituximab 620 mg d1, lenalidomide 20 mg qd d1-21 q4w). The patient has now completed 2 cycles of this regimen.
Most recent PET-CT evaluation revealed stable disease in the right lower leg tumor lesion (Deauville score: 5), while demonstrating complete metabolic response in both the right inguinal and external iliac lymph node lesions with no detectable F-18 fluorodeoxyglucose avidity, suggesting successful treatment response at these sites. The patient continues regular follow-up monitoring in our outpatient clinic for planned periodic reassessments.
This case demonstrates MCL presenting as a smooth, firm, red nodule on the anterior right lower leg of an elderly woman. Such cutaneous manifestations are frequently misdiagnosed as diffuse large B-cell lymphoma, leg-type (DLBCL-LT), given their overlapping clinical and histopathological features. Accurate diagnosis requires careful immunophenotypic analysis. MCL typically expresses B-cell markers (CD20, CD79a, PAX5) alongside CD43, CD5, cyclin D1, and SOX11. While CD10 and BCL-6 are usually negative, they may be present in more aggressive variants. Although CD5 po
Cutaneous involvement in MCL is rare, occurring in approximately 1% of cases, and typically signifies advanced disease with poor prognosis[2]. A comprehensive review of published English-language cases reveals that MCL can manifest in any area of the skin, most frequently on the trunk, followed by the lower limbs, upper limbs, and face. Cutaneous lesions in MCL may present as solitary or multiple lesions, most commonly appearing as nodules, papules, macules, or plaques. In contrast, bullae[8], ulcers[9], and melanocytic lesions[10] are rare. Most patients already have systemic involvement when skin lesions appear, with only seven reported cases of skin-only MCL without evidence of systemic disease (Table 1). However, due to limited follow-up duration, the existence of primary cutaneous MCL remains to be debated.
| Case | Ref. | Sex/age | Localization | Type of skin lesion |
| 1 | Bertero et al[13], 1994 | Female/78 | Breast, back | Nodules |
| 2 | Bertero et al[13], 1994 | Male/22 | Breast | Nodules |
| 3 | Sen et al[14], 2002 | Male/76 | Thigh | Solitary nodule |
| 4 | Estrozi et al[15], 2009 | Male/72 | Temporal region | Nodule |
| 5 | Zattra et al[16], 2011 | Male/77 | All body areas | Nodules, plaques |
| 6 | Lynch et al[17], 2012 | Male/83 | Thighs | Nodules |
| 7 | Zheng et al[18], 2020 | Male/74 | Trunk, scrotum | Nodules, plaques |
The current standard care for MCL relies on combination chemotherapy, with common regimens including hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone). The addition of anti-CD20 therapy (rituximab) has demonstrated a marked survival benefit in a study of orbital MCL (5-year overall survival rates of 83% vs 8%)[11]. For relapsed or refractory disease, novel therapeutic options such as Bruton’s tyrosine kinase inhibitors, proteasome inhibitors, immunomodulatory drugs, purine analogs, and CAR-T cell therapy have expanded the treatment landscape[12]. However, clinical outcomes remain suboptimal. Among patients with cutaneous involvement, the median overall survival is 69 months, with a median interval of 24.3 months from skin manifestation to death or last follow-up. Notably, survival outcomes do not differ significantly between patients who initially present with cutaneous lesions and those who develop them during disease relapse or progression[10].
In summary, MCL represents an aggressive malignancy where cutaneous involvement typically signifies advanced systemic disease and carries an unfavorable prognosis. While exceptionally uncommon, as illustrated by our case, skin manifestations may occasionally serve as the presenting feature. The pathological distinction between MCL and DLBCL-LT poses significant diagnostic challenges, necessitating careful immunohistochemical analysis for accurate differentiation.
The authors thank the patient and her family for their assistance.
| 1. | Campo E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC, Brousset P, Cerroni L, de Leval L, Dirnhofer S, Dogan A, Feldman AL, Fend F, Friedberg JW, Gaulard P, Ghia P, Horwitz SM, King RL, Salles G, San-Miguel J, Seymour JF, Treon SP, Vose JM, Zucca E, Advani R, Ansell S, Au WY, Barrionuevo C, Bergsagel L, Chan WC, Cohen JI, d'Amore F, Davies A, Falini B, Ghobrial IM, Goodlad JR, Gribben JG, Hsi ED, Kahl BS, Kim WS, Kumar S, LaCasce AS, Laurent C, Lenz G, Leonard JP, Link MP, Lopez-Guillermo A, Mateos MV, Macintyre E, Melnick AM, Morschhauser F, Nakamura S, Narbaitz M, Pavlovsky A, Pileri SA, Piris M, Pro B, Rajkumar V, Rosen ST, Sander B, Sehn L, Shipp MA, Smith SM, Staudt LM, Thieblemont C, Tousseyn T, Wilson WH, Yoshino T, Zinzani PL, Dreyling M, Scott DW, Winter JN, Zelenetz AD. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood. 2022;140:1229-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 1047] [Article Influence: 261.8] [Reference Citation Analysis (0)] |
| 2. | Zanelli M, Sanguedolce F, Zizzo M, Fragliasso V, Broggi G, Palicelli A, Loscocco GG, Cresta C, Caprera C, Corsi M, Martino G, Bisagni A, Marchetti M, Koufopoulos N, Parente P, Caltabiano R, Ascani S. Skin Involvement by Hematological Neoplasms with Blastic Morphology: Lymphoblastic Lymphoma, Blastoid Variant of Mantle Cell Lymphoma and Differential Diagnoses. Cancers (Basel). 2023;15:3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin's Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2850] [Cited by in RCA: 4191] [Article Influence: 349.3] [Reference Citation Analysis (8)] |
| 4. | Räty R, Franssila K, Jansson SE, Joensuu H, Wartiovaara-Kautto U, Elonen E. Predictive factors for blastoid transformation in the common variant of mantle cell lymphoma. Eur J Cancer. 2003;39:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Liu Z, Dong HY, Gorczyca W, Tsang P, Cohen P, Stephenson CF, Berger CS, Wu CD, Weisberger J. CD5- mantle cell lymphoma. Am J Clin Pathol. 2002;118:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 2409] [Article Influence: 602.3] [Reference Citation Analysis (0)] |
| 7. | Hsiao SC, Cortada IR, Colomo L, Ye H, Liu H, Kuo SY, Lin SH, Chang ST, Kuo TU, Campo E, Chuang SS. SOX11 is useful in differentiating cyclin D1-positive diffuse large B-cell lymphoma from mantle cell lymphoma. Histopathology. 2012;61:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Darji K, Bahram-Ahi E, Dhandha M, Guo M. Mantle cell lymphoma presenting with exaggerated skin reaction to insect bites. BMJ Case Rep. 2019;12:e227590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Motegi S, Okada E, Nagai Y, Tamura A, Ishikawa O. Skin manifestation of mantle cell lymphoma. Eur J Dermatol. 2006;16:435-438. [PubMed] |
| 10. | Kim DH, Medeiros LJ, Aung PP, Young KH, Miranda RN, Ok CY. Mantle Cell Lymphoma Involving Skin: A Clinicopathologic Study of 37 Cases. Am J Surg Pathol. 2019;43:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Rasmussen P, Sjö LD, Prause JU, Ralfkiaer E, Heegaard S. Mantle cell lymphoma in the orbital and adnexal region. Br J Ophthalmol. 2009;93:1047-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, McSweeney PA, Miklos DB, Pagel JM, Kersten MJ, Milpied N, Fung H, Topp MS, Houot R, Beitinjaneh A, Peng W, Zheng L, Rossi JM, Jain RK, Rao AV, Reagan PM. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382:1331-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1385] [Article Influence: 230.8] [Reference Citation Analysis (0)] |
| 13. | Bertero M, Novelli M, Fierro MT, Bernengo MG. Mantle zone lymphoma: an immunohistologic study of skin lesions. J Am Acad Dermatol. 1994;30:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Sen F, Medeiros LJ, Lu D, Jones D, Lai R, Katz R, Abruzzo LV. Mantle cell lymphoma involving skin: cutaneous lesions may be the first manifestation of disease and tumors often have blastoid cytologic features. Am J Surg Pathol. 2002;26:1312-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Estrozi B, Sanches JA Jr, Varela PC, Bacchi CE. Primary cutaneous blastoid mantle cell lymphoma-case report. Am J Dermatopathol. 2009;31:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Zattra E, Zambello R, Marino F, Bordignon M, Alaibac M. Primary cutaneous mantle cell lymphoma. Acta Derm Venereol. 2011;91:474-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Lynch DW, Verma R, Larson E, Geis MC, Jassim AD. Primary cutaneous mantle cell lymphoma with blastic features: report of a rare case with special reference to staging and effectiveness of chemotherapy. J Cutan Pathol. 2012;39:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Zheng XD, Zhang YL, Xie JL, Zhou XG. Primary cutaneous mantle cell lymphoma: Report of a rare case. World J Clin Cases. 2020;8:1507-1514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/