Published online Oct 26, 2025. doi: 10.12998/wjcc.v13.i30.111020
Revised: July 18, 2025
Accepted: August 13, 2025
Published online: October 26, 2025
Processing time: 112 Days and 22.6 Hours
Dermatofibrosarcoma protuberans (DFSP) is a rare, low-grade, locally aggressive cutaneous sarcoma. DFSP in the periocular region is exceedingly rare, leading to diagnostic and surgical challenges due to anatomical constraints in the periocular region. Precise diagnosis is essential to guide appropriate surgical management and prevent recurrence.
A 32-year-old female presented with a recurrent tumor in the medial canthus, previously diagnosed as a solitary fibrous tumor in an outside institution. After complete radiological and systemic workup, she was scheduled for a wide local excision followed by reconstruction after getting tumor clear margins on frozen section. Histopathology confirmed DFSP, characterized by storiform spindle cell proliferation, diffuse cluster of differentiation 34 positivity, and signal transducer and activator of transcription 6 negativity.
This case highlights the challenges in the diagnostic and surgical management of DFSP in periocular tumors. Comprehensive surgical excision with appropriate reconstruction is critical for achieving oncological control while preserving aes
Core Tip: Periocular dermatofibrosarcoma protuberans (DFSP) of the lacrimal sac is extremely rare, often mimicking benign or inflammatory lesions, leading to delayed diagnosis. High clinical suspicion is warranted for slowly enlarging, firm, painless medial canthal masses. Histopathology with cluster of differentiation 34 immunopositivity confirms the diagnosis. Complete surgical excision with histologically clear margins is crucial to prevent recurrence, with consideration for Mohs micrographic surgery in select cases. Long-term follow-up is essential due to the locally aggressive nature of DFSP and potential for late recurrence. Early recognition and meticulous margin control are key to optimal functional and cosmetic outcomes.
- Citation: Panda BB, Gunasekar S, Agarwal U, Koppalu Lingaraju T, Adhya AK. Recurrent dermatofibrosarcoma protuberans involving the lacrimal sac: A case report. World J Clin Cases 2025; 13(30): 111020
- URL: https://www.wjgnet.com/2307-8960/full/v13/i30/111020.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i30.111020
Dermatofibrosarcoma protuberans (DFSP) is a rare, slow-growing, cutaneous soft tissue sarcoma of fibroblastic origin, representing less than 0.1% of all malignancies and approximately 1%-6% of all soft tissue sarcomas[1,2]. It predominantly affects young to middle-aged adults and shows a predilection for the trunk (50%-60%) and proximal extremities (20%-30%)[3]. Head and neck involvement accounts for 10%-15% of cases, but periocular DFSP, particularly involving the lacrimal sac or medial canthus, is exceedingly rare and sparsely documented in the literature[4,5].
Histologically, DFSP is characterized by a monotonous proliferation of spindle cells arranged in a storiform or cartwheel pattern, infiltrating the dermis and subcutaneous tissue[1,6]. The differential diagnosis includes a spectrum of benign and malignant spindle cell tumors, particularly solitary fibrous tumor (SFT), which shares overlapping morphological features. Both DFSP and SFT are cluster of differentiation 34 (CD34)-positive spindle cell neoplasms; however, SFT is distinguished by the nuclear expression of signal transducer and activator of transcription 6 (STAT6) resulting from the NGFI-A binding protein 2 (NAB2)-STAT6 fusion gene[7,8]. By contrast, DFSP harbors a specific chromosomal translocation t(17;22)(q22;q13) leading to the collagen type 1 alpha 1-platelet-derived growth factor beta gene fusion, which drives autocrine platelet-derived growth factor receptor beta activation[9,10].
Accurate diagnosis is critical as DFSP is locally aggressive and demonstrates a high propensity for local recurrence if not adequately excised, with recurrence rates ranging from 10% to 60% following standard wide local excision[11]. Mohs micrographic surgery has been advocated as the gold standard, particularly in functionally and cosmetically sensitive areas like the face and periocular region, due to its superior margin control and lower recurrence rate (0%-6.6%)[12].
Management of DFSP in the periocular region poses unique surgical challenges due to the proximity to critical anatomical structures such as the medial canthal tendon, lacrimal drainage system, and orbital contents. Achieving oncologic clearance while preserving ocular function and aesthetic integrity requires interdisciplinary collaboration and meticulous reconstructive planning[13]. This case report presents a rare instance of recurrent DFSP involving the lacrimal sac and medial canthus, initially misdiagnosed as SFT. The diagnostic pitfalls, surgical strategy involving wide local excision and Mustarde flap reconstruction, and the role of immunohistochemistry in definitive diagnosis are discussed, with a review of the current literature.
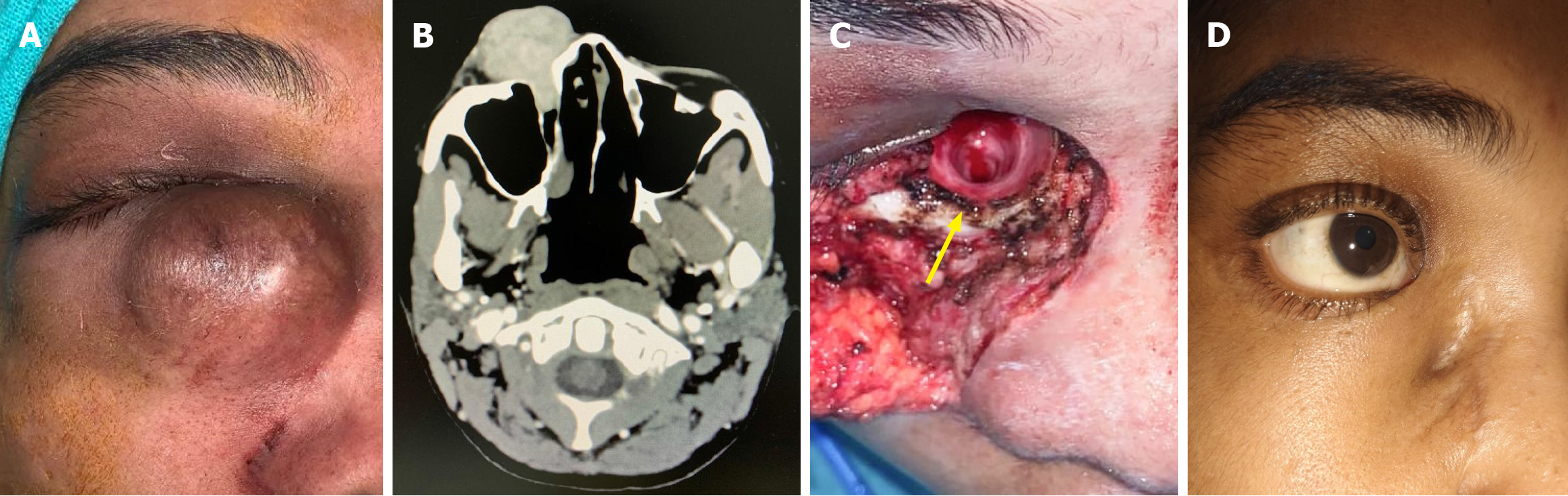
A 32-year-old female presented with a recurrent swelling located inferior to the right medial canthus, which she had first noticed in 2017 as a painless, slowly enlarging nodular lesion (Figure 1A).
The growing mass prompted the patient to seek further evaluation due to increasing cosmetic disfigurement, although she reported no associated symptoms such as pain, ulceration, bleeding, diplopia, or visual impairment.
Seven years prior, she underwent surgical excision at a local healthcare facility. Histopathological examination at that time suggested a diagnosis of SFT, although no immunohistochemical or molecular studies were performed to confirm the diagnosis. Two years following the initial surgery, the lesion recurred and demonstrated progressive enlargement over the subsequent 4 years, eventually reaching a size of approximately 5 cm × 4 cm. Her past medical history was unremarkable, with no known systemic comorbidities.
She had no relevant family history of soft tissue tumors.
On clinical examination, the patient had a best-corrected visual acuity of 20/20 in both eyes. The lesion measured approximately 5 cm × 4 cm, was soft, non-tender, non-compressible, and did not transilluminate. The overlying skin showed patchy hypopigmentation. There were no signs of orbital invasion, palpable lymphadenopathy, or other local complications.
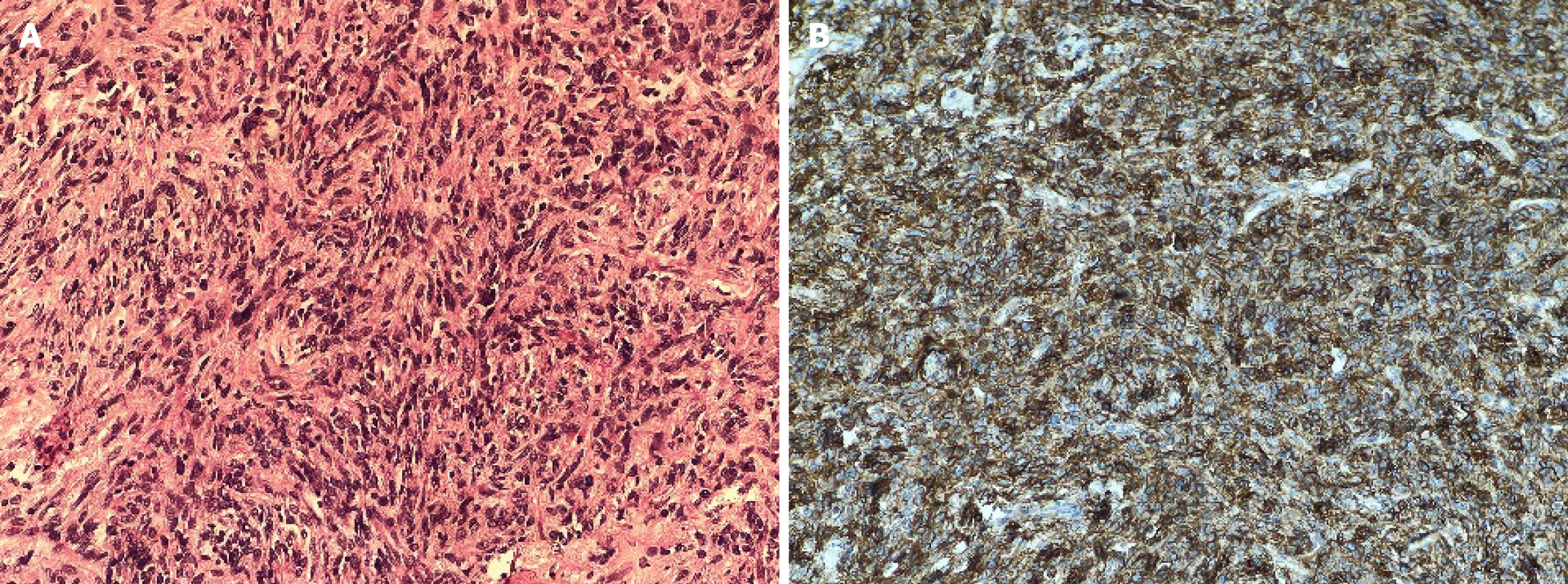
Her blood investigations of complete blood count, erythrocyte sedimentation rate, coagulation profile, blood sugar, liver function tests, and renal function tests were within normal limits. Histopathological examination: The wide local excision specimen measured 3.9 cm × 3.5 cm × 3.0 cm and included an overlying skin flap. On gross examination, the cut surface appeared homogenous, gray-white, and solid, with no areas of necrosis or hemorrhage. Microscopic analysis revealed a storiform arrangement of spindle cells with moderate eosinophilic cytoplasm, elongated nuclei, fine chromatin, and mild nuclear atypia, but no mitotic figures were detected (Figure 2A). The tumor infiltrated into the subcutaneous fat and was associated with hyalinized blood vessels, perivascular multinucleated giant cells, and interspersed mast cells. Immunohistochemical staining demonstrated strong, diffuse membranous positivity for CD34, while nuclear staining for STAT6 was negative (Figure 2B). The tumor was excised with clear margins, measured as follows: Anterior/skin: 0.2 cm, posterior: 0.1 cm, superior: 0.5 cm, inferior: 0.5 cm, lateral: 0.7 cm, and medial: 0.2 cm.
Contrast-enhanced computed tomography of the face demonstrated a well-defined soft tissue mass extending from the medial canthus to the nasolacrimal duct, with canal dilation but no bony erosion or orbital extension. Intense arterial enhancement suggested vascularity from external carotid branches (Figure 1B).
Following detailed preoperative evaluation, a multidisciplinary team comprising oculoplastic, maxillofacial, and oncologic surgeons formulated a surgical plan to address the tumor extent and anatomical complexity.
After complete clinico-radiological and histopathological examination and multidisciplinary consultation, a final diagnosis of DFSP was made.
Under general anesthesia, surgical access was achieved using a modified Weber-Ferguson incision with an infraciliary extension to allow optimal exposure. The tumor was excised en bloc with a 2 cm peripheral margin, which included a skin island to ensure oncological clearance. Given the tumor extension along the nasolacrimal duct, curettage of the lacrimal sac mucosa was performed to address potential microscopic invasion. Intraoperatively, a very wide nasolacrimal canal measuring 8 mm in diameter was visualized, which was probably due to the pressure effect of the tumor (Figure 1C). Reconstruction of the resultant defect, measuring in the medial canthal and cheek region, was accomplished using a Mustarde cheek advancement flap mobilized from the nasolabial fold, providing a tension-free and aesthetically favorable closure. A mini-Romovac drain was placed in the surgical cavity to prevent fluid accumulation. Layered wound closure was performed, with deep sutures placed using 3-0 Vicryl to anchor the flap to the periosteum, and interrupted skin sutures using 5-0 Ethilon.
The patient recovered uneventfully and has been under regular follow-up for the last 2 years. She had a good cosmetic and functional outcome without any recurrence (Figure 1D).
DFSP is a rare, low-grade dermal sarcoma of fibroblastic origin that exhibits slow but infiltrative growth and a significant propensity for local recurrence if incompletely excised. While the tumor most commonly involves the trunk and proximal extremities, head and neck presentations comprise only 10%-15% of cases, with periocular involvement being exceedingly rare[1-3]. In particular, DFSP involving the lacrimal sac is a diagnostic challenge, with only isolated case reports available in the literature[4,5]. Schittkowski and Wrede[4] reported an orbital presentation of DFSP managed surgically, under
| Ref. | Patient demographics | Clinical presentation | Imaging findings | Histopathology & IHC | Treatment | Outcome/follow-up |
| Erickson et al[5], 2015 | 71/male | Indolent growth above left medial canthus | CT: 2.1 cm × 2.2 cm × 2.5 cm mass extending into medial orbit and proximal nasolacrimal duct | Spindle cells in storiform pattern; CD34-positive; t(17;22) translocation absent | Surgical excision; partial intensity-modulated radiation therapy | No recurrence at 11 months |
| Schittkowski and Wrede[4], 2013 | 45/female | Mild epiphora; palpable mass in lacrimal sac | Not specified | Not specified | Surgical excision | Not specified |
| Sharma et al[14], 2017 | 50/female | Swelling in the medial aspect of the right eye associated with pain and diminution of vision | MRI: 5.4 cm × 3.8 cm × 4.1 cm in right orbit with extension to the ethmoid and frontal sinus, similar lesion in left medial canthus | Spindle tumor cells are uniform in appearance, with elongated nuclei, little or no pleomorphism, mitotic figures arranged in a storiform manner, CD34-positive and vimentin-positive | Palliative radiotherapy 20 Gy in five fractions, followed by tab Imatinib 400 mg per day increased to 800 mg per day after 2 years | Stable disease on tablet imatinib 800 mg per day on last follow-up |
| Rahman et al[15], 2013 | 70/female | Painless progressive swelling of left eye for 4 years | MRI: Well-defined lobulated soft tissue mass in the superolateral aspect of the left orbit measuring 58 mm × 42 mm × 38 mm, occupying the retro-orbital compartment with expansion of the bony wall and stretching of the optic nerve | Spindle cell tumor composed of slender to plump spindle cells arranged in storiform and focal pericytoma pattern, CD34-positive, S100-negative | Total orbital exenteration of the left eye with split-thickness skin graft from the thigh | No recurrence at 24 months |
| Present case | 32/female | Recurrent swelling inferior to right medial canthus for 6 years | CT: Soft tissue mass extending from medial canthus to nasolacrimal duct, causing canal widening | Storiform spindle cell proliferation; CD34-positive; STAT6-negative | Wide local excision with Mustarde flap reconstruction | No recurrence at 2-year follow-up |
Histologically, DFSP is composed of spindle cells arranged in a storiform pattern and infiltrating the dermis and subcutaneous fat. However, these features are shared by other spindle cell neoplasms, particularly SFT, which often leads to diagnostic ambiguity[6,8]. Immunohistochemistry plays a crucial role in resolving this overlap. Both DFSP and SFT are characteristically positive for CD34, but SFT exhibits nuclear expression of STAT6 due to the NAB2-STAT6 gene fusion - a marker absent in DFSP[9,10]. In the present case, the lesion was initially misclassified as SFT due to reliance solely on histomorphology, resulting in delayed definitive management. However, the immunohistochemistry confirmed CD34 positivity and STAT6 negativity, consistent with DFSP. Molecular analysis further enhances diagnostic accuracy. The defining genetic alteration in DFSP is a translocation t(17;22)(q22;q13), leading to fusion of the collagen type 1 alpha 1 and platelet-derived growth factor beta genes, resulting in autocrine activation of platelet-derived growth factor receptor beta signaling[1,7,11]. This molecular signature not only serves as a diagnostic tool but also has therapeutic implications, as it renders DFSP responsive to targeted therapy with imatinib, particularly in advanced or unresectable cases[12]. Management of periocular DFSP is inherently complex due to the proximity of vital structures such as the medial canthal tendon, lacrimal sac, and orbital contents. While Mohs micrographic surgery is considered the gold standard due to superior margin control and lower recurrence rates (0%-6.6%), its availability is limited in many settings[13,16]. Wide local excision with at least 4 cm margins remains a widely accepted alternative, although anatomical constraints in the periocular region often necessitate modification of this principle[1,7]. In this case, oncologic resection with a 2 cm margin was achieved, followed by defect reconstruction using a Mustarde cheek advancement flap. This technique provides well-vascularized tissue and allows tension-free closure while preserving facial symmetry, making it an ideal option for medial canthal and midfacial reconstruction. The success of this reconstructive strategy was evident in the patient’s satisfactory cosmetic outcome and the absence of recurrence over a two-year follow-up. Despite clear surgical margins, DFSP is associated with local recurrence rates ranging from 10% to 60%, with increased risk when margins are narrower than 1 cm or in high-risk histologic variants such as fibrosarcomatous DFSP[8,13]. Our patient’s closest margin was 0.1 cm posteriorly, reflecting the anatomical limitations of the region. The patient didn’t consent to chemotherapy or radio
Consequently, long-term surveillance with annual magnetic resonance imaging and patient education for self-monitoring were instituted, with recommended follow-up strategies[7,12]. Overall, this case reinforces the necessity of a multidisciplinary approach involving precise diagnosis using immunohistochemistry and molecular tools, tailored surgical resection, and advanced reconstructive techniques in managing DFSP of the lacrimal sac.
This case emphasizes the critical role of immunohistochemistry and molecular diagnostics in evaluating periocular spindle cell tumors. When correctly diagnosed, DFSP can be effectively managed with wide local excision and appropriate reconstructive techniques. Given the high risk of recurrence, long-term surveillance is essential. Interdisciplinary collaboration and awareness of rare presentations are key to achieving favorable outcomes.
| 1. | Llombart B, Serra-Guillén C, Monteagudo C, López Guerrero JA, Sanmartín O. Dermatofibrosarcoma protuberans: a comprehensive review and update on diagnosis and management. Semin Diagn Pathol. 2013;30:13-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Calonje E, Brenn T, Lazar A, McKee PH. McKee's pathology of the skin. 4th ed. United States: Saunders, 2011. |
| 3. | Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatol Surg. 2016;42 Suppl 1:S24-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Schittkowski MP, Wrede A. Dermatofibrosarcoma protuberans with primary orbital manifestation. Orbit. 2013;32:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Erickson BP, Henry C, Alabiad CR. Recurrent Dermatofibrosarcoma Protuberans Masquerading as a Lacrimal Sac Neoplasm: A Case Report and Review. Ophthalmic Plast Reconstr Surg. 2015;31:e135-e138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Chawla B, Pushker N, Sen S, Bajaj MS, Khuraijam N, Ghose S. Recurrent bilateral dermatofibrosarcoma protuberans of eyelids. Ophthalmic Plast Reconstr Surg. 2011;27:e167-e168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Hao X, Billings SD, Wu F, Stultz TW, Procop GW, Mirkin G, Vidimos AT. Dermatofibrosarcoma Protuberans: Update on the Diagnosis and Treatment. J Clin Med. 2020;9:1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 8. | Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 268] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, Wang WL. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 10. | Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 528] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 11. | Nakamura I, Kariya Y, Okada E, Yasuda M, Matori S, Ishikawa O, Uezato H, Takahashi K. A Novel Chromosomal Translocation Associated With COL1A2-PDGFB Gene Fusion in Dermatofibrosarcoma Protuberans: PDGF Expression as a New Diagnostic Tool. JAMA Dermatol. 2015;151:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Rutkowski P, Van Glabbeke M, Rankin CJ, Ruka W, Rubin BP, Debiec-Rychter M, Lazar A, Gelderblom H, Sciot R, Lopez-Terrada D, Hohenberger P, van Oosterom AT, Schuetze SM; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group; Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol. 2010;28:1772-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 13. | DuBay D, Cimmino V, Lowe L, Johnson TM, Sondak VK. Low recurrence rate after surgery for dermatofibrosarcoma protuberans: a multidisciplinary approach from a single institution. Cancer. 2004;100:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Sharma D, Singh G, Kakkar N, Jha V. Orbital dermatofibrosarcoma protuberans with frontal and ethmoid sinus involvement: A case report and brief review of literature. Indian J Ophthalmol. 2017;65:892-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Rahman T, Bhattacharjee K, Sarma JD, Dey D, Kuri G. Primary dermatofibrosarcoma protuberans of orbit--a rare entity. Orbit. 2013;32:127-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35:355-74; quiz 375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 263] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Fionda B, Loperfido A, Di Stefani A, Lancellotta V, Paradisi A, De Angeli M, Cappilli S, Rossi E, Caretto AA, Zinicola T, Schinzari G, Gentileschi S, Morganti AG, Rembielak A, Peris K, Tagliaferri L. The Role of Postoperative Radiotherapy in the Management of Dermatofibrosarcoma Protuberans: A Multidisciplinary Systematic Review. J Clin Med. 2024;13:1798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Noujaim J, Thway K, Fisher C, Jones RL. Dermatofibrosarcoma protuberans: from translocation to targeted therapy. Cancer Biol Med. 2015;12:375-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/