Published online Oct 26, 2025. doi: 10.12998/wjcc.v13.i30.112419
Revised: August 3, 2025
Accepted: August 22, 2025
Published online: October 26, 2025
Processing time: 76 Days and 19.4 Hours
Acute disseminated encephalomyelitis (ADEM), which is rare, primarily affects children. It usually manifests as acute encephalopathy and multifocal neurological impairments after infection or vaccination. Diagnosis is still difficult due to the clinical and radiological similarity to other central nervous system disorders. Adult-onset ADEM calls for thorough reporting in order to improve diagnosis and treatment.
A 55-year-old man with hypertension had a high fever, intense headache and a steady decline in his neurological function after two weeks. Left facial paralysis was the initial symptom, which progressed to left hemiparesis, reduced consciousness level, photophobia, phonophobia, vomiting, and a focal seizure in the right leg. He had no history of autoimmune disease, vaccinations, or infections. Investigations showed negative infectious/autoimmune serology, mild cerebrospinal fluid lymphocytic pleocytosis (protein 76 mg/dL), and lymph
This case shows an uncommon, idiopathic, cerebellar-predominant ADEM var
Core Tip: Acute disseminated encephalomyelitis (ADEM) is typically a pediatric, post-infectious demyelinating disease of the central nervous system. We report a rare case of idiopathic, adult-onset ADEM in a 55-year-old man with no preceding infection, vaccination, or autoimmune history. This case underscores the diagnostic complexity of ADEM in older adults, particularly when presenting with atypical symptoms and radiological findings. Early recognition and a high index of suspicion are critical for timely intervention, even in resource-limited settings. Our findings emphasize the need to broaden diagnostic considerations for encephalopathy and multifocal neurological deficits in adults.
- Citation: Faisal A, Tariq Z, Latif RU, Amir S, Basit A, Basil AM. Silent triggers and symmetric peduncles - a rare presentation of adult-onset acute disseminated encephalomyelitis: A case report. World J Clin Cases 2025; 13(30): 112419
- URL: https://www.wjgnet.com/2307-8960/full/v13/i30/112419.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i30.112419
Acute demyelinating encephalomyelitis, also often referred to as acute disseminated encephalomyelitis (ADEM), is a debilitating, immune-mediated disease of the central nervous system. It is an inflammatory demyelinating disease and is thought to be a rare illness, with reports of around 0.07 to 0.9 per 100000 children being affected every year[1]. The disease is characterized by an acute onset and a plethora of neurological symptoms consistent with demyelination. The disease is often preceded by a viral infection and this is often used as a diagnostic tool by physicians. These diagnostic tools are often deemed necessary since ADEM can present a clinically similar picture to other demyelinating disorders such as multiple sclerosis.
The predominant features in the pathogenesis of ADEM include periventricular inflammation and predominant inflammation of the white matter of the spinal cord. Preceding viral infections may trigger an autologous T-cell response to myelin basic protein and other antigens[2]. Timely diagnosis for ADEM is deemed essential. If started timely, most patients can respond well to high-dose corticosteroids. However, due to the potential clinical overlap that ADEM has with other demyelinating disorders, it warrants further investigations. In this report we present the case of a 55-year-old hypertensive man who was diagnosed with ADEM after development of several neurologic deficits and other symptoms. This report highlights the atypical nature of this case, as ADEM is usually a diagnosis made in children. Nuanced cases like these are pivotal in understanding more about this disease.
High-grade fever, severe headache, and progressive neurological deterioration.
A 55-year-old man with a history of well-managed hypertension arrived with constitutional symptoms, a high-grade intermittent fever, and a severe headache that had been persistent for two weeks. He experienced sudden onset left facial paralysis shortly after the fever started. His left arm and leg became weaker over the course of the following 48 hours, making it impossible for him to walk or feed himself without help. His family observed that throughout the first week of his sickness, he grew more reclusive, irritated, and unresponsive. During this time, he also suffered from phonophobia, photophobia, nausea, vertigo, and several episodes of vomiting. On the seventh day of his sickness, he experienced a focal seizure that only affected his right leg (Table 1).
| Day of illness | Event |
| 0 | Onset of high-grade fever and severe headache |
| 2 | Acute left facial paralysis |
| 4 | Progressive left hemiparesis (unable to walk/self-feed) |
| 7 | Focal seizure (right leg); hospital admission |
| 8 | Brain MRI + lumbar puncture performed |
| 8-14 | Persistent encephalopathy; fever resolution by Day 14 |
| 21 | Spontaneous neurological improvement noted (strength/alertness) |
| 22 | High-dose IV methylprednisolone initiated (1 g/day) |
| 22-26 | IV methylprednisolone continued (5-day course) |
| 27 | Transition to oral prednisone taper (60 mg/day starting dose) |
| 28 | Transferred to rehabilitation ward; physiotherapy started |
| 42 | Motor strength 4/5; independent ambulation; discharged |
No history of joint pain, rash, recent vaccination, or recent infection.
No relevant family history of neurological, autoimmune, or demyelinating disorders. He wasn't on any herbal or immunosuppressive drugs.
Upon examination, his Glasgow Coma Scale score was 10 (E4V1M5), indicating that he was drowsy and febrile. Vital signs remained steady. After a neurological examination, the patient had normal reflexes and down going plantar responses, but a left-sided lower motor neuron facial palsy and left hemiparesis (upper limb Medical Research Council grade 3/5, lower limb 2/5). There was no papilledema or nuchal stiffness, and his pupils were equal and responsive. Additionally, a mild left-sided ptosis was observed (Table 1). Overall, the clinical picture showed encephalopathy and developing multifocal neurological abnormalities.
According to preliminary lab results, there was relative lymphopenia and mild leukocytosis (white blood cell count approximately 13 × 109/L). With the exception of slightly increased urea and creatinine (caused by dehydration), renal and liver function tests were within normal ranges. Albumin, calcium, and electrolytes were all within normal limits. A lumbar puncture was done on the second day of the hospital; the cerebrospinal fluid (CSF) was colorless and transparent, with normal glucose, a slight lymphocytic pleocytosis (a few leukocytes per mm3, all lymphocytes), and high protein (approximately 76 mg/dL) (Table 2). Viral polymerase chain reaction (herpes simplex virus, varicella zoster virus, Japanese encephalitis virus), regular CSF cultures, and Gram stain results were all negative. Instead of bacterial meningitis, these results were in line with an aseptic, most likely post-infectious inflammatory condition.
| Presenting symptoms | High-grade fever, severe headache, left facial paralysis, hemiparesis, reduced level of consciousness, photophobia, phonophobia, nausea, vomiting, focal seizure (right leg) |
| Neurological exam | Left LMN facial palsy, left hemiparesis (UL 3/5, LL 2/5), encephalopathy, mild ptosis |
| CSF analysis | Clear; elevated protein (76 mg/dL); lymphocytic pleocytosis; normal glucose; negative Gram stain, culture, and viral PCR (HSV, VZV, Japanese encephalitis virus) |
| MRI brain | Bilateral, symmetric T2/FLAIR hyperintensities in middle cerebellar peduncles; subtle changes in pons and periventricular white matter; no diffusion restriction, no enhancement |
| Other workup | EEG: Diffuse slowing (encephalopathy); Normal renal/Liver function; relative lymphopenia; mild transaminitis |
| Negative serology | ANA, aquaporin-4, MOG-Ab, HIV, syphilis, TB (ADA) |
| Treatment & outcome | Delayed IV methylprednisolone (5-day course) followed by oral taper; spontaneous improvement before treatment; complete motor recovery |
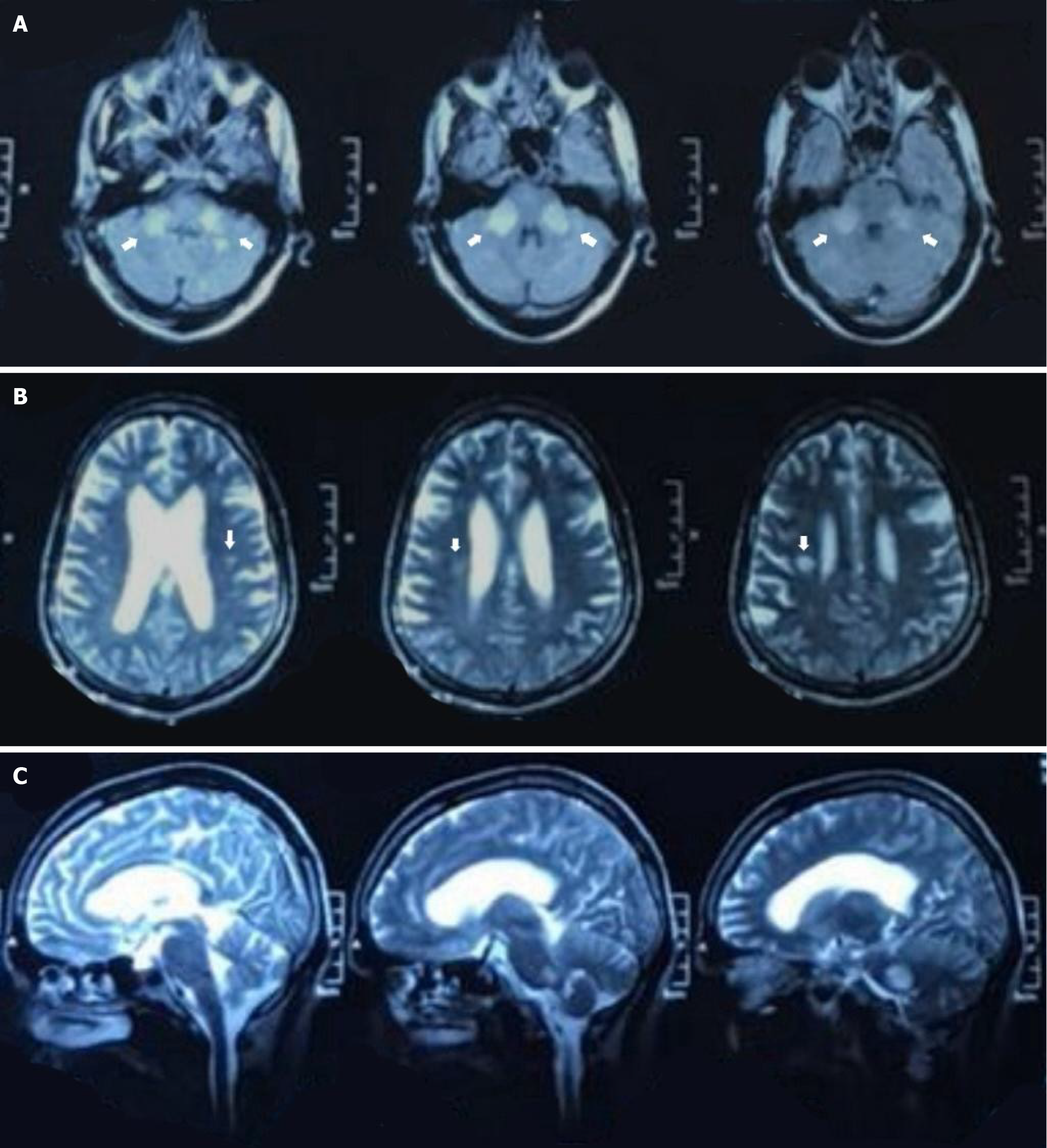
A demyelinating process was strongly suggested by neuroimaging. The middle cerebellar peduncles and surrounding cerebellar white matter were affected by bilateral symmetrical T2/fluid-attenuated inversion recovery hyper intense lesions (Figure 1A), but the right pons and right periventricular white matter were more subtly affected (Figure 1B and C), according to an earlier brain magnetic resonance imaging (MRI) (done on day 8 of the illness). Diffusion restriction, mass effect, and contrast-induced enhancement were absent from these poorly defined lesions. The MRI results showed a multifocal white matter inflammatory process in conjunction with the clinical condition (Figure 1). His disorientation and seizure history was evaluated using electroencephalography, which supported an encephalopathic state by demonstrating diffuse slowing without epileptiform activity (Table 2).
Neurology, radiology, and internal medicine teams reviewed the case. A demyelinating process was suspected, with ADEM favored over infectious or vascular causes. Due to logistical issues, treatment initiation was delayed.
Idiopathic adult-onset ADEM.
The patient initially received conservative care, including supportive measures and close monitoring. By the third week of illness, spontaneous neurological improvement was noted. High-dose intravenous methylprednisolone (1 g/day) was initiated and given for five days, followed by an oral prednisone taper. Early physiotherapy and interdisciplinary rehabilitation were started post- intensive care unit transfer.
By week 6, the patient regained strength (4/5) in all affected limbs and was able to walk independently. He was discharged home after functional recovery and continued follow-up in outpatient neurology.
ADEM is an inflammatory demyelinating disorder of the central nervous system (CNS), first described by Clifton in 1724 during his doctoral thesis on pox at the University of Leiden, The Netherlands[3,4]. Epidemiologically, this demyelination syndrome may occur at any age but it is most prevalent during childhood, with the average incidence in children ranging from 0.07 to 0.4 per 100000 persons annually. Definite incidence data hasn’t been documented in adults, making our case a viable focus for further investigation and analysis[5,6]. Most studies report ADEM as more common in males than females, with a male: Female ratio ranging from 1:0.8 to 2.3:1. In terms of causation, ADEM has been recognised as a predominantly post-infectious CNS disorder, with known triggers in 50%-85% cases[7]. Initially, it was linked exclusively to viral infections like smallpox and measles, but it was found to be associated with other infections as well, including bacterial infections[8]. This led to previous infection being a requisite for ADEM diagnosis, but it is not included in the current inclusion criteria[3]. Vaccination has also been recognised as a possible cause of ADEM, but has been observed to be relevant in a smaller number of cases (5%-19% in the largest cohorts) than infection itself[4,9]. Unlike the typical paediatric or post-infectious presentations, our case involved a 55-year-old adult male with no preceding infection, vaccination, or autoimmune disease — emphasizing a rare adult-onset, idiopathic ADEM variant.
ADEM is characterised by a polysymptomatic clinical presentation, originating from different regions of CNS and involving multifocal neurological deficits, which is often used as an inclusion criterion[4]. The disease onset is subacute to acute, although prodromal phases like malaise and headache have also been reported, and the disease progresses within days[3]. Neurological signs include brainstem symptoms, ataxia, pyramidal signs, optic neuritis (ON) and transverse myelitis (TM), while atypical signs include extrapyramidal movements, tumor-like lesions and seizures[10]. In some rare cases, cardiac anomalies, like myocarditis, and peripheral nervous system involvement have also been reported[11,12]. Our case highlights the clinical presentation and progression of ADEM in adults, beginning with abrupt onset left-sided facial paralysis, which progressed to hemiparesis and global disorientation, resembling vascular or viral causes. The absence of meningeal symptoms or convulsions early in the course, as well as the development of ptosis, increased the diagnostic uncertainty. These findings on the atypical presentation of ADEM in adults are consistent with the reports by Khojah et al[12] and Iardino et al[13], both of which describe distinct clinical manifestations that differ not only from each other but also from the typical presentations observed in pediatric cases.
Three pathophysiological phenomena have been described regarding ADEM. First is perivascular demyelination, forming perivenous sleeves, while multiple sclerosis (MS) patients exhibit confluent demyelination[14,15]. However, both types of demyelination were observed in an ADEM case, suggesting a possibility of wrong categorisation[15]. Second is the presence of inflammatory cells, such as myelin-laden macrophages, T and B lymphocytes, occasional plasma cells and granulocytes. Third is the increase in inflammatory biomarkers in the CSF, predominantly interleukin-6, cytokines Th1 and Th2, macrophage/microglia-related cytokines showing innate immunity, oligoclonal IgG and antibodies against myelin oligodendrocyte glycoprotein (MOG-Ab)[16,17]. MOG protein is expressed exclusively in the CNS while the expression of serum MOG-Ab is age-dependent, with different disease presentations, including ADEM, ON and TM, either alone or combined[17]. The predominant clinical manifestation in young children is ADEM, while that in older children is ON, myelitis or brainstem symptoms[3].
ADEM is classically considered a monophasic illness, but the emergence of relapsing demyelinating disorders after at least 3 months, like multiphasic disseminated encephalomyelitis, ADEM-MS, ADEM neuromyelitis optica spectrum disorder, ADEM optic neuritis, has complicated accurate diagnosis[4,14]. As a result, ADEM remains a diagnosis of exclusion, having no defined biomarker[18]. For this reason, patients of ADEM require a comprehensive clinical assessment and neuroimaging, to differentiate it from other neurological disorders and to work towards appropriate management. First, most common diagnostic tool is MRI, showing multiple bilateral T2-enhancing supratentorial white matter lesions and accompanying lesions in the thalamus and/or basal ganglia (17%-63%) and the brainstem and/or cerebellum (22%-63%)[4,12]. In our case, MRI revealed symmetric hyperintensities in the middle cerebellar peduncles and subtle involvement of the pons and periventricular regions, a less common distribution seen in adult-onset ADEM, but may also mimic metabolic or paraneoplastic syndromes or rhombencephalitis, as seen in a case report by Hwang et al[19]; Second, CSF examination is performed to exclude CNS infections by checking for elevated inflammatory markers and pleocytosis[3,20]; Third, laboratory studies are required for checking for the presence of autoantibodies, anti-aquaporin-4 and MOG-Ab, for confirming NMOSD and anti-MOG disease, respectively[21]. Some studies also report the detection of oligoclonal bands and the usage of electroencephalogram for diagnosis, but it is not substantiated or confirmed enough[22]. In our case, CSF had increased protein and mild lymphocytic pleocytosis, but neither xanthochromia nor hypoglycorrhachia were present. This CSF pleocytosis has been recognised as a marker of meningoencephalitis[23]. However, our results of Gramme stain, cultures, and Adenosine deaminase were all negative, supporting an immune-mediated process and ruling out bacterial, tuberculous, and fungal causes of CNS inflammation. These laboratory findings prove our initial presentation to be misleading, as focal neurological deficit (facial palsy), fever, vomiting, and altered sensorium are likely to mimic infective, vascular, or neoplastic causes, which were excluded by these laboratory results.
Since there are no randomised trials for ADEM, treatment has been derived from other types of studies and expert opinions[3]. Corticosteroids are considered as first-line treatment, with intravenous methylprednisolone being administered at a dose of 30 mg/kg/day for 3-5 days, and then an oral prednisone taper for 4-6 weeks[14]. In case of a steroid-unresponsive ADEM, second-line treatment is prescribed as Intravenous immunoglobulin at a total dose of 2 g/kg for 2-5 days[24]. In refractory patients, Plasma exchange is used with 3 to 7 exchanges[25]. In studies where increased intracranial pressure was unresponsive to treatment protocols and immunotherapy, craniectomy was employed[26]. Since 33.8% patients show relapsing demyelinating events, B cell-targeted treatments and continuous oral steroid treatment was shown to be associated with reduced frequency of these relapsing forms[27]. In our case, due to logistical obstacles, the patient had to undergo conservative treatment for a while before showing signs of partial neurological recovery. In many adult ADEM patients, this spontaneous recovery, even prior to plasmapheresis or high-dose corticosteroids, is uncommon and may indicate a self-limiting course of the disease, although it must be viewed with caution. Observing the phenomenon of spontaneous recovery in children, Tenembaum[28] emphasize the importance of differentiating transient demyelinating events from chronic demyelinating disorders, which could possibly enable timely clinical intervention and ensure the efficient allocation of healthcare resources[29]. Additionally, our case illustrates the difficulties that arise in the real world when launching immunotherapy in regions with limited resources, specifically in tertiary settings in low- and middle-income countries.
This case demonstrates a rare presentation of adult-onset ADEM, with cerebellar-predominant lesions and a monophasic, spontaneously stabilising course, and without a known trigger. It highlights the diagnostic difficulties in differentiating ADEM from vascular, infectious, and neoplastic causes, particularly in the absence of conventional triggers and radiological patterns. Even in cases where initial resources are limited, appropriate management can be guided by early consideration of ADEM in adults with multifocal neurological deficits and prompt imaging. Improved and equitable access to healthcare, increased awareness among clinicians, tailored public healthcare strategies and further research in geriatric health and neurology are required to bridge the gaps in literature, diagnosis and treatment and work towards improved quality of life.
We would like to acknowledge the neurology and internal medicine teams involved in the patient’s care. We also thank the radiology department for their assistance in MRI acquisition and interpretation. Finally, we are grateful to the patient and his family for their cooperation and consent for publication.
| 1. | Shrestha AB, Mokbul MI, Chowdhury T, Shrestha S, Shrestha S, Raut R, Nuruzzaman M. Acute disseminated encephalomyelitis following the COVID-19 Vaccine Sinopharm in low- and middle-income country: a case report. Ann Med Surg (Lond). 2023;85:6182-6185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Paolilo RB, Deiva K, Neuteboom R, Rostásy K, Lim M. Acute Disseminated Encephalomyelitis: Current Perspectives. Children (Basel). 2020;7:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Koelman DL, Mateen FJ. Acute disseminated encephalomyelitis: current controversies in diagnosis and outcome. J Neurol. 2015;262:2013-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Pohl D, Hennemuth I, von Kries R, Hanefeld F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: results of a nationwide survey. Eur J Pediatr. 2007;166:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Torisu H, Kira R, Ishizaki Y, Sanefuji M, Yamaguchi Y, Yasumoto S, Murakami Y, Shimono M, Nagamitsu S, Masuzaki M, Amamoto M, Kondo R, Uozumi T, Aibe M, Gondo K, Hanai T, Hirose S, Matsuishi T, Shirahata A, Mitsudome A, Hara T. Clinical study of childhood acute disseminated encephalomyelitis, multiple sclerosis, and acute transverse myelitis in Fukuoka Prefecture, Japan. Brain Dev. 2010;32:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Parrish JB, Yeh EA. Acuted disseminated encephalomyelitis. Adv Exp Med Biol. 2012;724:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Berzero G, Cortese A, Ravaglia S, Marchioni E. Diagnosis and therapy of acute disseminated encephalomyelitis and its variants. Expert Rev Neurother. 2016;16:83-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Panicker JN, Nagaraja D, Kovoor JM, Nair KP, Subbakrishna DK. Lower urinary tract dysfunction in acute disseminated encephalomyelitis. Mult Scler. 2009;15:1118-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Tenembaum S, Chitnis T, Ness J, Hahn JS; International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology. 2007;68:S23-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 448] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 10. | Lademann H, Bertsche A, Petzold A, Zack F, Büttner A, Däbritz J, Hauenstein C, Bahn E, Spang C, Reuter D, Warnke P, Ehler J. Acute Disseminated Encephalomyelitis with Seizures and Myocarditis: A Fatal Triad. Medicina (Kaunas). 2020;56:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Marchioni E, Ravaglia S, Montomoli C, Tavazzi E, Minoli L, Baldanti F, Furione M, Alfonsi E, Bergamaschi R, Romani A, Piccolo L, Zardini E, Bastianello S, Pichiecchio A, Ferrante P, Delbue S, Franciotta D, Bono G, Ceroni M. Postinfectious neurologic syndromes: a prospective cohort study. Neurology. 2013;80:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 12. | Khojah IM. Adult Onset Acute Disseminated Encephalomyelitis (ADEM): A Comprehensive Case Repot. Saudi J Emerg Med. 2025;6:85-85. [DOI] [Full Text] |
| 13. | Iardino A, Garner O, Rajasekar S, Alexander A, Helekar A, Shim G, Loveman D, Chemitiganti R, Bhairavarasu K. Atypical Presentation of Acute Disseminated Encephalomyelitis (ADEM) in a Middle-Aged Adult. Am J Case Rep. 2019;20:361-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Pohl D, Alper G, Van Haren K, Kornberg AJ, Lucchinetti CF, Tenembaum S, Belman AL. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology. 2016;87:S38-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 15. | Young NP, Weinshenker BG, Parisi JE, Scheithauer B, Giannini C, Roemer SF, Thomsen KM, Mandrekar JN, Erickson BJ, Lucchinetti CF. Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain. 2010;133:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Ishizu T, Minohara M, Ichiyama T, Kira R, Tanaka M, Osoegawa M, Hara T, Furukawa S, Kira J. CSF cytokine and chemokine profiles in acute disseminated encephalomyelitis. J Neuroimmunol. 2006;175:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Hennes EM, Baumann M, Schanda K, Anlar B, Bajer-Kornek B, Blaschek A, Brantner-Inthaler S, Diepold K, Eisenkölbl A, Gotwald T, Kuchukhidze G, Gruber-Sedlmayr U, Häusler M, Höftberger R, Karenfort M, Klein A, Koch J, Kraus V, Lechner C, Leiz S, Leypoldt F, Mader S, Marquard K, Poggenburg I, Pohl D, Pritsch M, Raucherzauner M, Schimmel M, Thiels C, Tibussek D, Vieker S, Zeches C, Berger T, Reindl M, Rostásy K; BIOMARKER Study Group. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 272] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 18. | Boziki M, Sintila SA, Ioannidis P, Grigoriadis N. Biomarkers in Rare Demyelinating Disease of the Central Nervous System. Int J Mol Sci. 2020;21:8409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Hwang J, Lee AL, Chang KH, Hong HS. Acute Disseminated Encephalomyelitis Presenting as Rhombencephalitis: An Atypical Case Presentation. Investig Magn Reson Imaging. 2015;19:186. [DOI] [Full Text] |
| 20. | Singhi PD, Ray M, Singhi S, Kumar Khandelwal N. Acute disseminated encephalomyelitis in North Indian children: clinical profile and follow-up. J Child Neurol. 2006;21:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Chen JJ, Bhatti MT. Clinical phenotype, radiological features, and treatment of myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG-IgG) optic neuritis. Curr Opin Neurol. 2020;33:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 22. | Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123 Pt 12:2407-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 425] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 23. | Ketelslegers IA, Visser IE, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ. Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler. 2011;17:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Schneider JA. Neuropathology of Dementia Disorders. Continuum (Minneap Minn). 2022;28:834-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, Levy M. Treatment of acute relapses in neuromyelitis optica: Steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Pohl D, Tenembaum S. Treatment of acute disseminated encephalomyelitis. Curr Treat Options Neurol. 2012;14:264-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Hacohen Y, Wong YY, Lechner C, Jurynczyk M, Wright S, Konuskan B, Kalser J, Poulat AL, Maurey H, Ganelin-Cohen E, Wassmer E, Hemingway C, Forsyth R, Hennes EM, Leite MI, Ciccarelli O, Anlar B, Hintzen R, Marignier R, Palace J, Baumann M, Rostásy K, Neuteboom R, Deiva K, Lim M. Disease Course and Treatment Responses in Children With Relapsing Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. JAMA Neurol. 2018;75:478-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 332] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 28. | Tenembaum SN. Disseminated encephalomyelitis in children. Clin Neurol Neurosurg. 2008;110:928-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Absoud M, Parslow RC, Wassmer E, Hemingway C, Duncan HP, Cummins C, Lim MJ; UK & Ireland Childhood CNS Inflammatory Demyelination Working Group and the the Paediatric Intensive Care Audit Network. Severe acute disseminated encephalomyelitis: a paediatric intensive care population-based study. Mult Scler. 2011;17:1258-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/