Published online Oct 26, 2025. doi: 10.12998/wjcc.v13.i30.110330
Revised: July 4, 2025
Accepted: August 13, 2025
Published online: October 26, 2025
Processing time: 123 Days and 1.2 Hours
High microsatellite instability (MSI-H) colorectal cancer (CRC), caused by deficient mismatch repair, accounts for about 15% of all CRC cases and is more common in right-sided tumors. While early-stage MSI-H CRC has a relatively good prognosis, advanced cases often respond poorly to standard chemotherapy. Immune checkpoint inhibitors, such as pembrolizumab, have shown strong and lasting effects in MSI-H CRC. Pembrolizumab is now approved as a first-line treatment for metastatic MSI-H CRC due to its superior outcomes compared to traditional chemotherapy.
A 44-year-old male with MSI-H transverse colon cancer presented with hema
Immune checkpoint inhibitors may cause delayed structural damage to bowel tissue even after apparent complete tumor regression.
Core Tip: We report here the first case of spontaneous colonic transection in a patient with high microsatellite instability colon cancer who achieved pathologic complete response after long-term pembrolizumab therapy. Despite no residual tumor on imaging or pathology, intraoperative findings revealed a discontinuity of the bowel at the original tumor site. Immune-mediated remodeling can compromise bowel integrity and may not be apparent on imaging. Clinicians should consider potential anatomic changes when planning surgery in patients treated with immunotherapy, even in the context of a complete response.
- Citation: Lee C, Kim MH, Choi ET, Park IJ, Lim SB, Yoon YS, Kim CW, Lee JL, Park EJ. Spontaneous colonic transection following pathologic complete response to pembrolizumab in high microsatellite instability colorectal cancer: A case report and review of literature. World J Clin Cases 2025; 13(30): 110330
- URL: https://www.wjgnet.com/2307-8960/full/v13/i30/110330.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i30.110330
The introduction of immune checkpoint inhibitors for the treatment of high microsatellite instability (MSI-H) or mismatch repair-deficient (dMMR) colorectal cancer (CRC) brought a paradigm shift in the medical field. Pembrolizumab emerged as a key therapeutic option for patients with advanced-stage disease[1-3]. Recently, studies have demonstrated the durable antitumor efficacy of pembrolizumab, with some patients achieving a complete response (CR)[4,5].
Bowel obstruction associated with immune checkpoint inhibitor therapy has been reported in a limited number of cases[6]. It typically occurs during or after treatment. However, spontaneous colonic transection in the setting of complete tumor regression has not been documented in the literature previously.
We report herein a case of a patient with MSI-H transverse colon cancer who achieved a pathologic CR (pCR) following long-term pembrolizumab therapy. During surgical resection an unexpected spontaneous transection of the colon at the site of the original tumor was discovered. To our knowledge this is the first reported case of colonic transection associated with immune checkpoint inhibitor therapy in the setting of complete tumor regression.
A 44-year-old male presented with intermittent hematochezia and right upper quadrant abdominal pain.
The patient reported a 6-month history of intermittent hematochezia and loose stools occurring every 1-2 days. These symptoms were accompanied by anorexia and significant weight loss of approximately 25 kg.
The patient had a history of liver cirrhosis due to chronic HBV infection. Viral suppression was attained by tenofovir therapy.
The patient’s mother had a history of hepatocellular carcinoma, and his father had been diagnosed with CRC. No other notable personal or familial conditions were reported.
The patient’s vital signs were stable. Abdominal examination revealed a soft, non-tender abdomen without rebound tenderness or palpable mass.
Initial laboratory investigations showed severe anemia with a hemoglobin level of 9.0 g/dL (normal range: 13.0-17.0 g/dL) and carcinoembryonic antigen of 3.5 ng/mL (normal range: 0-6.0 ng/mL). Liver function was classified as Child-Pugh B. HBV DNA level was normalized at 1.9 × 107 IU/mL (normal range: < 10 × 107 IU/mL).
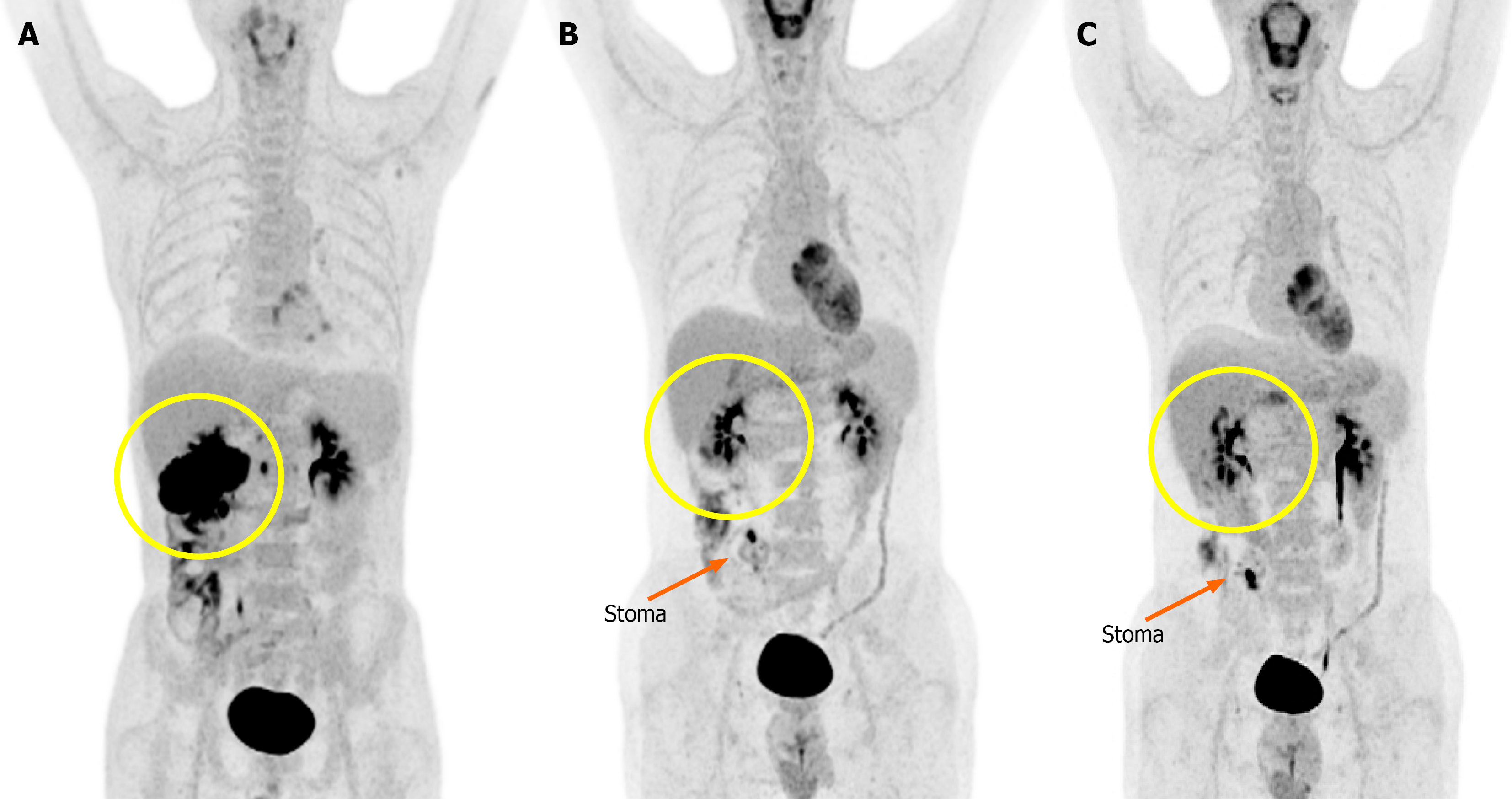
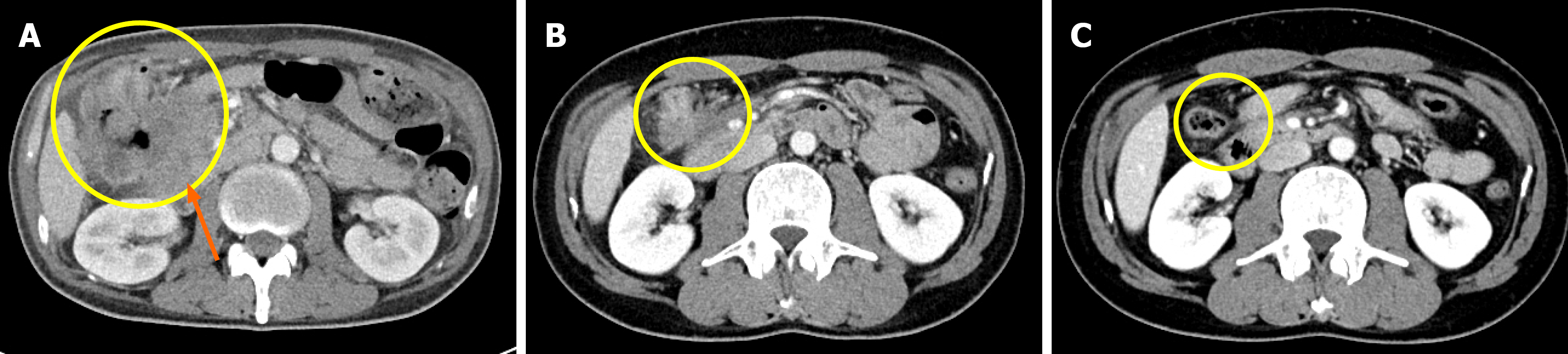
Abdominal CT demonstrated a mass in the proximal transverse colon invading the second portion of the duodenum, pancreatic head, and superior mesenteric vein. There were multiple low-attenuation lesions in the liver and ascites. Positron emission tomography-CT showed intense fluorodeoxyglucose uptake in the hepatic flexure and suggested possible peritoneal involvement (Figure 1A).
The patient was diagnosed with MSI-H CRC, specifically a poorly differentiated adenocarcinoma of the transverse colon. Molecular testing revealed dMMR with loss of MLH1 and PMS2 and KRAS G13D mutation.
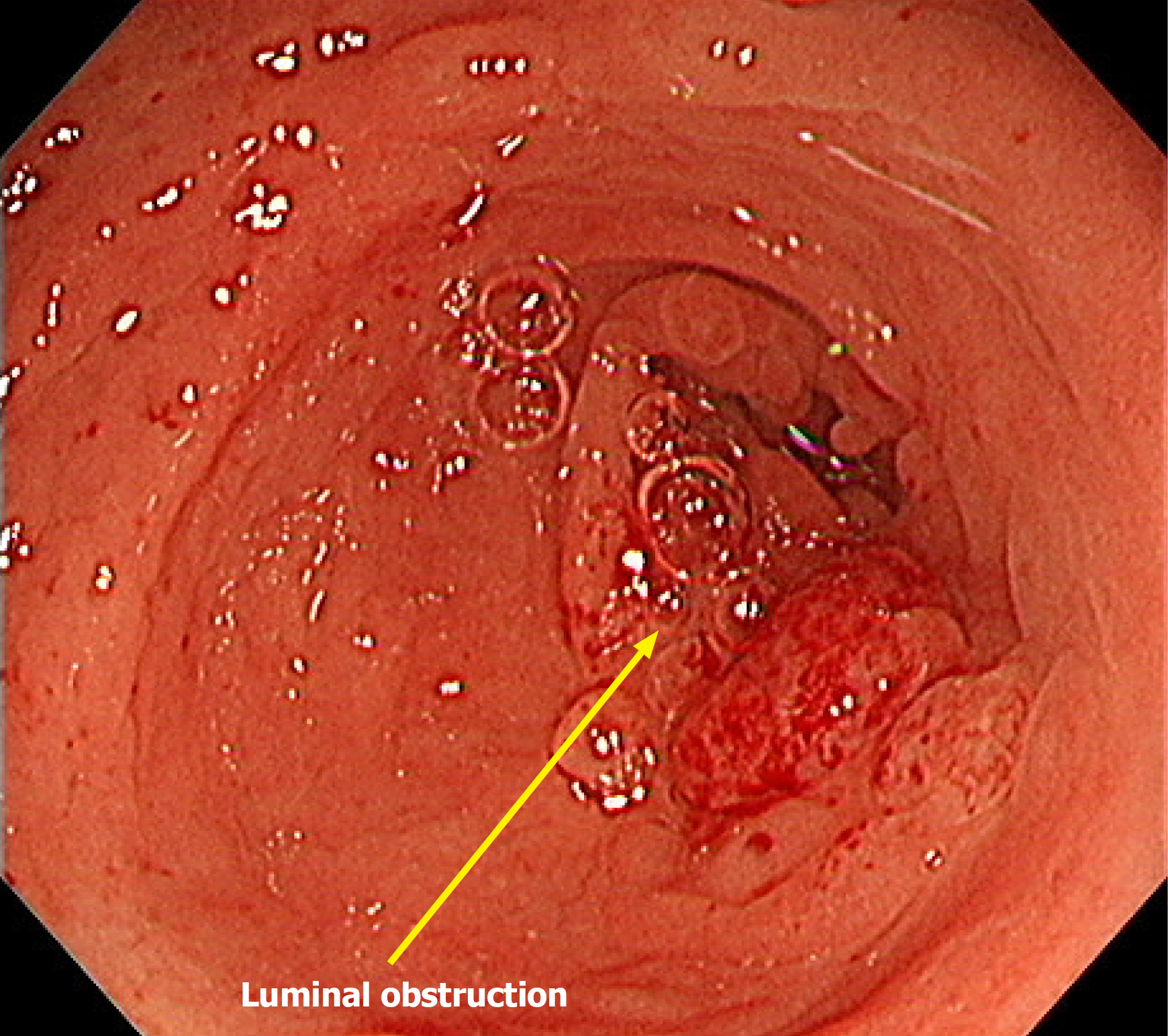
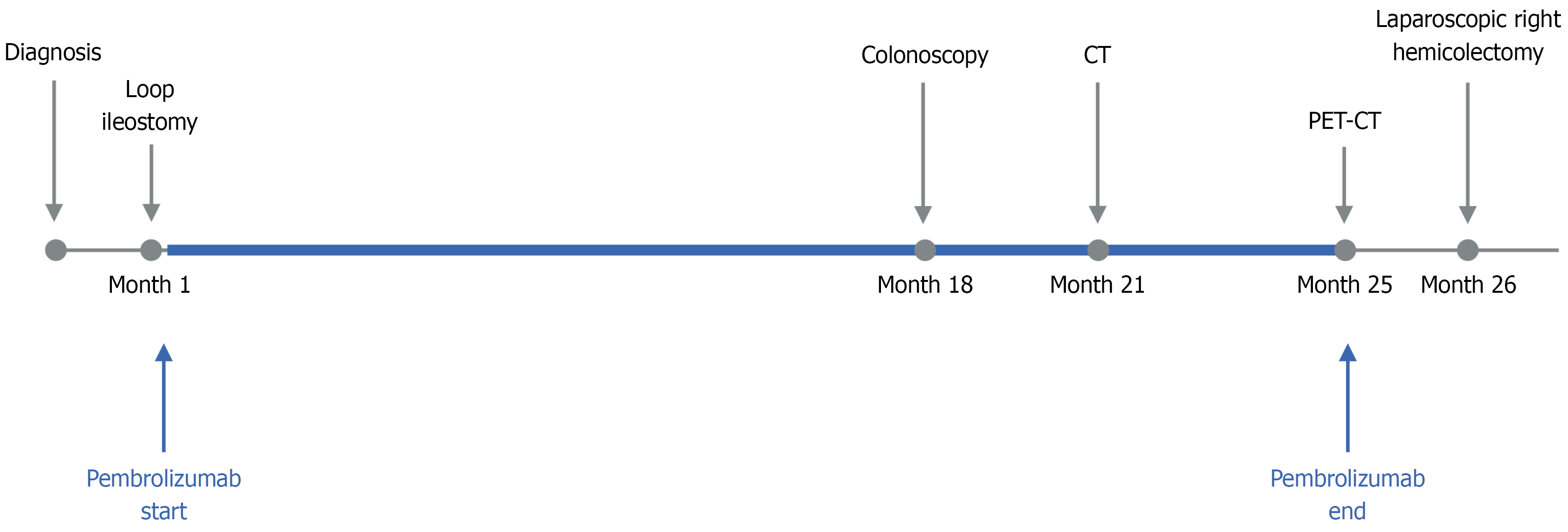
Due to a high risk of obstruction, a laparoscopic loop ileostomy was performed 1 month after diagnosis. Immunotherapy with pembrolizumab (200 mg every 3 weeks) was initiated shortly thereafter and continued for approximately 2 years (36 cycles in total). The patient initially showed a partial response to treatment. Seventeen months after initiation of immunotherapy, a colonoscopy was attempted but could not be advanced beyond the lesion due to persistent obstruction (Figure 2). No additional endoscopic evaluations were performed thereafter.
During the treatment course interval CT demonstrated a marked reduction in tumor burden, including near-complete resolution of the primary lesion (Figure 3). Positron emission tomography-CT performed at the same time confirmed the disappearance of the initial mass but revealed new hypermetabolic foci near the stoma site and gallbladder bed, suspicion for inflammatory changes (Figure 1).
After completion of immunotherapy, the patient showed a clinical CR. Surgery was scheduled approximately 1 month later, considering both the imaging findings and coordination of the surgical schedule between the patient and the hospital (Figure 4). Intraoperatively, a spontaneous transection of the colon was identified at the hepatic flexure. Postoperatively, the patient experienced colitis due to Clostridium difficile infection that was successfully treated with oral vancomycin. The remainder of the patient’s recovery was uneventful, and he was discharged in stable condition on postoperative day 14.
A follow-up CT and laboratory tests were performed 1 month after surgery and showed no evidence of recurrence and no remarkable findings. Final pathology confirmed a pCR (ypT0N0, 0/45 Lymph nodes). Histopathologic examination of the hepatic flexure revealed transmural fibrosis and calcification, accompanied by mild microvascular damage and chronic inflammatory response, suggesting ongoing tissue remodeling. Additionally, a granulomatous abscess with actinomycosis was identified near the stoma site. Although these findings support the hypothesis of immune-related structural changes, additional histologic characterization such as immunohistochemical staining was not performed.
Immune checkpoint inhibitors, particularly those targeting programmed death-1 (PD-1), have become the primary treatment for patients with MSI-H or dMMR CRC[7-10]. The most well-known PD-1 inhibitors include pembrolizumab, nivolumab, and dostarlimab. These agents restore the function of exhausted T cells and enhance antitumor immune responses[11-13].
Among these, pembrolizumab is the most widely used PD-1 inhibitor. It has been approved as a first-line treatment for MSI-H/dMMR metastatic CRC based on the KEYNOTE-177 trial. Pembrolizumab showed superior outcomes compared with conventional chemotherapy with a progression-free survival of 16.5 months vs 8.2 months (hazard ratio: 0.60). The incidence of grade 3 or higher adverse events was only 22%[14,15].
Although PD-1 blockade was initially introduced for use in patients with metastatic or treatment-refractory disease[16], its application has rapidly expanded into the neoadjuvant setting, particularly for patients with locally advanced MSI-H/dMMR tumors[11,17,18]. Recent trials demonstrated high rates of pCR, paving the way for organ-preserving strategies and reduced treatment intensity in selected patients[19]. These findings reflect a broader shift in CRC treatment toward personalized, biomarker-driven approaches to optimize therapy according to individual tumor biology and treatment response[19-22].
Recent reports have shown an increasing number of cases achieving pCR[23,24]. The first report of a patient achieving a pCR with pembrolizumab monotherapy was initially diagnosed with metastatic ascending colon cancer[5]. Several other cases have since demonstrated CR even in high-risk patients with liver or peritoneal metastases[4,25-27]. However, PD-1 inhibitors are not effective in all patients. Approximately 20%-30% of patients exhibit primary resistance, showing no initial response, while others develop acquired resistance during treatment. These resistance mechanisms may involve mutations in B2M or JAK1/2, reduced antigen presentation, activation of alternative immune checkpoints such as LAG-3 and TIM-3, or an immunosuppressive tumor microenvironment enriched with regulatory T cells or myeloid-derived suppressor cells[11,28-30].
Recently, gastrointestinal complications related to immune-related adverse events have been identified during PD-1 inhibitor therapy. A multicenter case series reported 9 cases of bowel obstruction that occurred during pembrolizumab treatment[6]. Notably, most of these patients had achieved pCR. However, obstruction developed due to fibrosis or strictures induced by immune-mediated reactions.
However, the present case was differs significantly from previously reported cases. Our patient was diagnosed with MSI-H transverse colon cancer. He achieved radiologic CR after long-term pembrolizumab treatment, and a spontaneous colonic transection was identified intraoperatively. To our knowledge this highly unusual finding has not been previously reported in the literature. While prior reports have described findings such as mucin pools, necrotic tissue, or fibrosis, none have documented structural disruption severe enough to cause a loss of anatomical continuity.
Immune checkpoint inhibitor-related tissue remodeling has been observed across various organs, including the lung and heart, where structural damage may persist even after cessation of therapy[31,32]. Similar to pneumonitis or myocarditis, where chronic inflammation and fibrosis can remain despite clinical resolution, our case suggests that bowel wall fragility may reflect a parallel mechanism of immune-mediated injury[33].
Immune-mediated tissue remodeling processes are complex biological phenomena that involve a range of pathological alterations, including but not limited to fibrosis and microvascular damage[34,35]. These processes can lead to significant and lasting changes in the architecture of the bowel wall. Even in cases where tumor regression has been achieved, the effects of these immune-mediated alterations may persist, continuing to compromise the structural integrity of the bowel. Furthermore, although clinical CR may be observed, the actual mechanical integrity of the bowel may remain compromised. This case represents the first surgical confirmation of such a risk. Therefore, in patients with MSI-H CRC receiving immune checkpoint inhibitors, surgical planning should carefully consider the risk of bowel obstruction or perforation. In select cases the need for prophylactic stoma formation may warrant consideration.
This study presented a rare case of MSI-H CRC in which a spontaneous colonic transection was identified during surgery following pembrolizumab treatment. It suggested that immune checkpoint inhibitors may exert structural effects beyond the tumor itself and provided important clinical insight for surgical planning in patients undergoing immunotherapy.
| 1. | Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1066] [Article Influence: 266.5] [Reference Citation Analysis (0)] |
| 2. | Kanani A, Veen T, Søreide K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br J Surg. 2021;108:1417-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 3. | Saberzadeh-Ardestani B, Jones JC, Hubbard JM, McWilliams RR, Halfdanarson TR, Shi Q, Sonbol MB, Ticku J, Jin Z, Sinicrope FA. Association Between Survival and Metastatic Site in Mismatch Repair-Deficient Metastatic Colorectal Cancer Treated With First-line Pembrolizumab. JAMA Netw Open. 2023;6:e230400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Tan-Garcia A, Wang LM, Ngu JC, Goh L. Complete pathologic response (pCR) following neoadjuvant pembrolizumab monotherapy in treatment-naive locally advanced, mismatch repair protein-deficient (dMMR) colonic cancer: a case report and literature review. Acta Oncol. 2022;61:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Tominaga T, Nonaka T, Fukuda A, Moriyama M, Oyama S, Ishii M, Sawai T, Okano S, Nagayasu T. Pathological complete response to pembrolizumab in patients with metastatic ascending colon cancer with microsatellite instability. Clin J Gastroenterol. 2022;15:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Platt JR, Allotey J, Alouani E, Glasbey J, Intini R, Lonardi S, Mazzoli G, Militello AM, Modest DP, Palle J, Pietrantonio F, Riyad K, Samuel L, Schulze AV, Shiu KK, Taieb J, Tolan DJM, West NP, Westwood AC, Williams CJM, Seligmann JF. Risk of bowel obstruction in patients with colon cancer responding to immunotherapy: an international case series. ESMO Open. 2024;9:103698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Chakrabarti S, Grewal US, Vora KB, Parikh AR, Almader-Douglas D, Mahipal A, Sonbol MBB. Outcome of Patients With Early-Stage Mismatch Repair Deficient Colorectal Cancer Receiving Neoadjuvant Immunotherapy: A Systematic Review. JCO Precis Oncol. 2023;7:e2300182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 5165] [Article Influence: 573.9] [Reference Citation Analysis (0)] |
| 9. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7482] [Article Influence: 680.2] [Reference Citation Analysis (2)] |
| 10. | Pei F, Wu J, Zhao Y, He W, Yao Q, Huang M, Huang J. Single-Agent Neoadjuvant Immunotherapy With a PD-1 Antibody in Locally Advanced Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer. Clin Colorectal Cancer. 2023;22:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 11. | Ozer M, Vegivinti CTR, Syed M, Ferrell ME, Gonzalez Gomez C, Cheng S, Holder-Murray J, Bruno T, Saeed A, Sahin IH. Neoadjuvant Immunotherapy for Patients with dMMR/MSI-High Gastrointestinal Cancers: A Changing Paradigm. Cancers (Basel). 2023;15:3833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 12. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 2039] [Article Influence: 339.8] [Reference Citation Analysis (0)] |
| 13. | Kavun A, Veselovsky E, Lebedeva A, Belova E, Kuznetsova O, Yakushina V, Grigoreva T, Mileyko V, Fedyanin M, Ivanov M. Microsatellite Instability: A Review of Molecular Epidemiology and Implications for Immune Checkpoint Inhibitor Therapy. Cancers (Basel). 2023;15:2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 14. | Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fourchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zhong WY, Fogelman D, Marinello P, Andre T; KEYNOTE-177 Investigators. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 601] [Article Influence: 150.3] [Reference Citation Analysis (0)] |
| 15. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJA, Smith D, Garcia-Carbonero R, Alcaide-Garcia J, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zuo Y, Fogelman D, Adelberg D, Diaz LA. Pembrolizumab versus chemotherapy in microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer: 5-year follow-up from the randomized phase III KEYNOTE-177 study. Ann Oncol. 2025;36:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 47] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 16. | Trullas A, Delgado J, Genazzani A, Mueller-Berghaus J, Migali C, Müller-Egert S, Zander H, Enzmann H, Pignatti F. The EMA assessment of pembrolizumab as monotherapy for the first-line treatment of adult patients with metastatic microsatellite instability-high or mismatch repair deficient colorectal cancer. ESMO Open. 2021;6:100145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Ludford K, Ho WJ, Thomas JV, Raghav KPS, Murphy MB, Fleming ND, Lee MS, Smaglo BG, You YN, Tillman MM, Kamiya-Matsuoka C, Thirumurthi S, Messick C, Johnson B, Vilar E, Dasari A, Shin S, Hernandez A, Yuan X, Yang H, Foo WC, Qiao W, Maru D, Kopetz S, Overman MJ. Neoadjuvant Pembrolizumab in Localized Microsatellite Instability High/Deficient Mismatch Repair Solid Tumors. J Clin Oncol. 2023;41:2181-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 126] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 18. | Shimozaki K, Nakayama I, Hirota T, Yamaguchi K. Current Strategy to Treat Immunogenic Gastrointestinal Cancers: Perspectives for a New Era. Cells. 2023;12:1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Ros J, Baraibar I, Saoudi N, Rodriguez M, Salvà F, Tabernero J, Élez E. Immunotherapy for Colorectal Cancer with High Microsatellite Instability: The Ongoing Search for Biomarkers. Cancers (Basel). 2023;15:4245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 20. | Taieb J, Svrcek M, Cohen R, Basile D, Tougeron D, Phelip JM. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur J Cancer. 2022;175:136-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 21. | Damato A, Rotolo M, Caputo F, Borghi E, Iachetta F, Pinto C. New Potential Immune Biomarkers in the Era of Precision Medicine: Lights and Shadows in Colorectal Cancer. Life (Basel). 2022;12:1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Zhu J, Lian J, Xu B, Pang X, Ji S, Zhao Y, Lu H. Neoadjuvant immunotherapy for colorectal cancer: Right regimens, right patients, right directions? Front Immunol. 2023;14:1120684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Zhang J, Cai J, Deng Y, Wang H. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology. 2019;8:e1663108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C, Beets GL, Snaebjornsson P, Maas M, Mertz M, Veninga V, Bounova G, Broeks A, Beets-Tan RG, de Wijkerslooth TR, van Lent AU, Marsman HA, Nuijten E, Kok NF, Kuiper M, Verbeek WH, Kok M, Van Leerdam ME, Schumacher TN, Voest EE, Haanen JB. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 1007] [Article Influence: 167.8] [Reference Citation Analysis (0)] |
| 25. | Yang J, Bi F, Gou H. Complete Pathologic Response After Concurrent Treatment with Pembrolizumab and Radiotherapy in Metastatic Colorectal Cancer: A Case Report. Onco Targets Ther. 2021;14:2555-2561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Krekeler C, Wethmar K, Mikesch JH, Kerkhoff A, Menck K, Lenz G, Schildhaus HU, Wessolly M, Hoffmann MW, Pascher A, Asmus I, Wardelmann E, Bleckmann A. Complete Metabolic Response to Combined Immune Checkpoint Inhibition after Progression of Metastatic Colorectal Cancer on Pembrolizumab: A Case Report. Int J Mol Sci. 2023;24:12056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Hosokawa A, Tamura H, Ichihara A, Imamura N, Kai K, Fukushima T, Nanashima A, Komohara Y. Pathological Complete Response to Liver Metastasis With Pembrolizumab in a Previously Treated Patient With Microsatellite Instability-high Colorectal Cancer. Anticancer Res. 2024;44:4119-4125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, Burge M, O'Neil B, Kavan P, Yoshino T, Guimbaud R, Taniguchi H, Elez E, Al-Batran SE, Boland PM, Crocenzi T, Atreya CE, Cui Y, Dai T, Marinello P, Diaz LA Jr, André T. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 746] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 29. | Mulet-Margalef N, Linares J, Badia-Ramentol J, Jimeno M, Sanz Monte C, Manzano Mozo JL, Calon A. Challenges and Therapeutic Opportunities in the dMMR/MSI-H Colorectal Cancer Landscape. Cancers (Basel). 2023;15:1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 55] [Reference Citation Analysis (1)] |
| 30. | Fabrizio DA, George TJ Jr, Dunne RF, Frampton G, Sun J, Gowen K, Kennedy M, Greenbowe J, Schrock AB, Hezel AF, Ross JS, Stephens PJ, Ali SM, Miller VA, Fakih M, Klempner SJ. Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J Gastrointest Oncol. 2018;9:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 31. | Leo F, Pelosi G, Sonzogni A, Chilosi M, Bonomo G, Spaggiari L. Structural lung damage after chemotherapy fact or fiction? Lung Cancer. 2010;67 (3):306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 602] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 33. | Miao K, Zhang L. Pathogenesis, pathological characteristics and individualized therapy for immune-related adverse effects. Chin Med J Pulm Crit Care Med. 2023;1 (4):215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 34. | Jancar S, Sánchez Crespo M. Immune complex-mediated tissue injury: a multistep paradigm. Trends Immunol. 2005;26 (1):48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Lupher ML, Jr. , Gallatin WM. Regulation of fibrosis by the immune system. Adv Immunol. 2006;89:245-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/