Published online Oct 26, 2025. doi: 10.12998/wjcc.v13.i30.110314
Revised: July 9, 2025
Accepted: August 20, 2025
Published online: October 26, 2025
Processing time: 129 Days and 19.6 Hours
Plasmacytoid urothelial carcinoma (PUC) is a rare histologic variant of urothelial carcinoma. PUC is characterized by aggressiveness and poor prognosis. Since the first reported case of PUC in 1991, approximately 100 cases have been docu
A 65-year-old Taiwanese man presented to our emergency department with a 3-month history of progressive abdominal fullness. A month before presentation, he had visited our hospital for gross hematuria with hydronephrosis and was su
Our case highlights diagnostic challenges posed by atypical imaging findings and unusual etiologies of gas
Core Tip: This report describes a case of colorectal metastasis in a 65-year-old man with plasmacytoid urothelial carcinoma. The patient had hospitalization with the impression of colitis with ileus initially for the image disclosed diffused bowel distension and long segmental rectosigmoid colonic wall thickening, with clear vesicorectal fat plane. And the colorectal metastasis with an unusal hypothesized pathogenesis was detected and confirmed by endoscopy and pathologic examinations with immunohistochemical staining. This case underscores the uncommon image findings and atypical pathway of me
- Citation: Chen GY, Tsai HL, Chen PJ, Chen YC, Wang JY, Lin CY, Yin HL. Plasmacytoid urothelial carcinoma of the urinary bladder with colorectal metastasis and mimicking colitis: A case report. World J Clin Cases 2025; 13(30): 110314
- URL: https://www.wjgnet.com/2307-8960/full/v13/i30/110314.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i30.110314
Plasmacytoid urothelial carcinoma (PUC) was first reported in 1991 as a rare histologic variant of urothelial carcinoma. PUC is characterized by discohesive tumor cells that morphologically resemble plasma cells, with eccentric nuclei and eosinophilic cytoplasm[1,2]. These cells express CD138, which reflects their plasmacytoid morphology, and typically exhibit strong immunopositivity for CK7, CK20, and GATA-3, which serve as biomarkers of urothelial cancers including PUC. These features help differentiate PUC from signet ring cell carcinoma, which has similar histomorphology, characterized by discohesive tumor cells, eccentric hyperchromatic nuclei, and eosinophilic cytoplasm. Furthermore, p63 immunostaining can help differentiate urothelial carcinoma from prostatic adenocarcinoma in complex cases[3]. PUC is characterized by aggressiveness, with a propensity for diffuse infiltration rather than discrete mass formation and a strong tendency for intraperitoneal dissemination[4]. Clinically, patients with PUC often present with hematuria and nonspecific urinary symptoms such as dysuria, frequency, urgency, or urinary retention; however, initial manifestations may also include distant metastases or peritoneal dissemination[5]. PUC can manifest as unusual gastrointestinal symptoms rather than those typical of urothelial carcinoma. Thus, a comprehensive histologic examination is required to determine the presence and extent of metastasis. Given the rarity of PUC, very few autopsy reports are available[6-8]. Herein, we report the case of a patient with urinary bladder PUC who initially presented with progressive abdominal fullness, nausea, and reduced appetite due to tumor infiltration of the colon and rectum and eventually received a diagnosis of colorectal metastasis. Our case features atypical imaging findings and unusual etiologies of gastrointestinal wall thickening with peripheral infiltration.
A 65-year-old Taiwanese man presented to our emergency department with a 3-month history of progressive abdominal fullness.
The patient first noted abdominal fullness, particularly after food intake, approximately 3 months before their pre
The patient had a history of hypertension managed with medication. Approximately 1 month before presentation, he visited our urology department for painless gross hematuria, which developed approximately 2 months after the onset of the abdominal symptoms. He denied having any other urinary symptoms. Imaging examinations revealed a bladder tumor on the left side. He was subsequently hospitalized in the urology ward for transurethral resection of the bladder tumor. Postoperative histopathologic examination confirmed PUC.
The patient had a healthy lifestyle without relevant occupational or social risk factors. Furthermore, he had no known family history of major illnesses or genetic disorders.
Physical examination revealed a distended abdomen with generalized tympany and hyperactive bowel sounds.
Laboratory tests revealed leukocytosis (29640/μL) and an elevated C-reactive protein level (161.46 mg/L). However, the patient’s hemoglobin level, liver and renal function test results, and serum electrolyte levels were within normal limits.
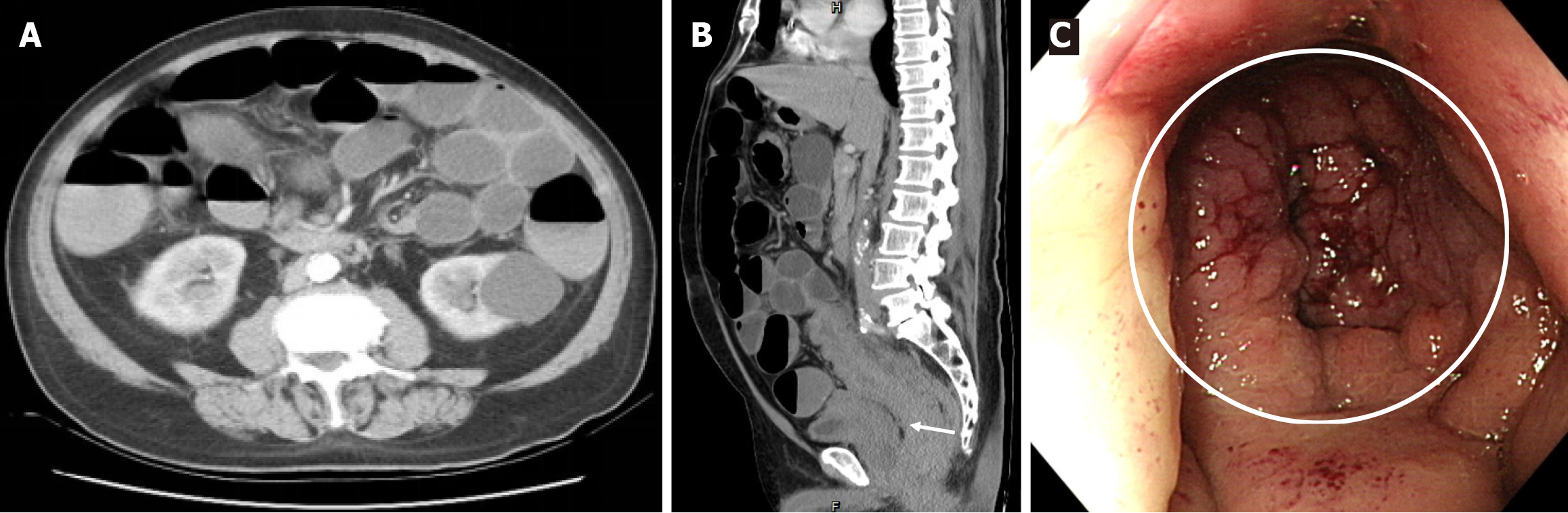
Abdominal computed tomography (CT) revealed diffuse distension of the small and large intestines and long segmental thickening of the rectosigmoid colonic wall, with a preserved vesicorectal fat plane (Figure 1A and B). Colonoscopy revealed luminal obstruction with edematous mucosal changes up to 5 cm from the anal verge (Figure 1C). Multiple deep biopsies were conducted.
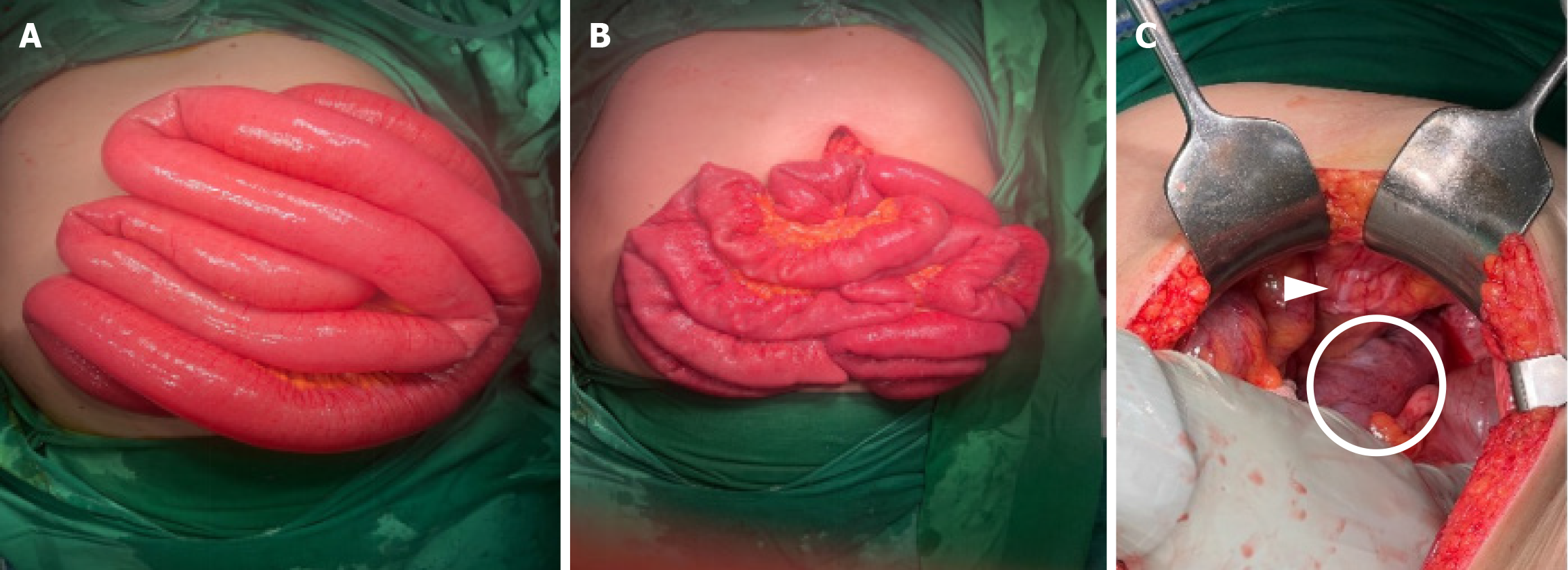
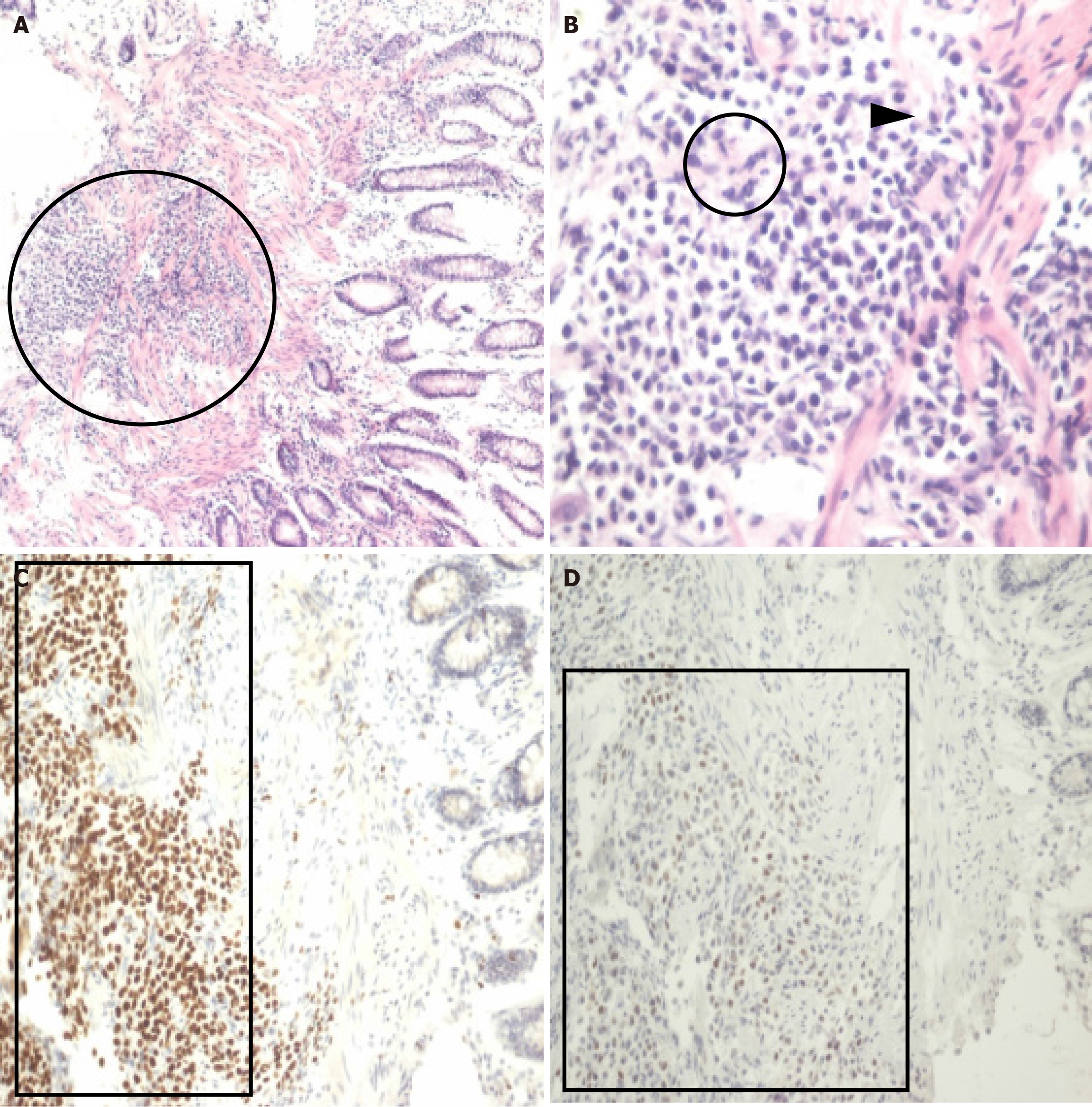
Given the patient’s inadequate response to conservative management—empirical antibiotics, bowel rest, nasogastric decompression, and intravenous fluid support—an exploratory laparotomy was performed. Intraoperatively, the small intestine was found to be distended from the ligament of Treitz to the ileocecal valve and ascending colon, without any evidence of adhesions or a clear obstructive lesion. Long segmental wall thickening and marked pericolic inflammation were observed from the rectum to the sigmoid colon. A diverting loop ileostomy was performed to decompress the distended intestinal tract (Figure 2). After surgery, the abdominal fullness resolved and normal stool passage through the ileostomy was noted. Histopathologic examination of the colonoscopic biopsies revealed infiltration by PUC cells (Figure 3A and B), which were positive for GATA-3 and p63 on immunostaining (Figure 3C and D), consistent with the histology and immunohistochemistry after transurethral resection of the bladder tumor.
The patient received a final diagnosis of PUC with rectosigmoid colonic metastasis.
The patient was referred to a urologist for further management. After a multidisciplinary conference was held, a radical cystectomy, to be followed by systemic chemotherapy plus targeted therapy, was scheduled.
During radical cystectomy, peritoneal carcinomatosis was observed; hence, the surgery was aborted. The urologist informed the patient and family of the unfavorable operative findings, poor prognosis, and limited response expected from systemic therapy at this advanced stage. The patient and family opted for comfort care and declined further aggressive or invasive interventions. Thus, the patient was referred to the palliative care unit for hospice management. He passed away approximately 3 months after the PUC diagnosis was made.
PUC accounts for 1%–3% of all bladder cancer cases. It is associated with adverse clinicopathologic features, advanced stage at cystectomy, and poor prognosis[2,9-13]. Some reports have reported that PUC invades along the perirectal or perivesical fascial plane[9,12,13], a pattern that is difficult to detect through conventional CT or magnetic resonance imaging[14]. The median duration of survival in patients with PUC is 17.7 months, with the duration being lower in patients with stage IV disease (13.3 months) than in those with stage I–III disease (45.8 months)[15]. In our case, the patient died approximately 3 months after the confirmed diagnosis of bladder cancer was made. This duration is shorter than that reported in the literature, likely attributable to the fact that our patient received only hospice care without systemic therapy. A similar trend was observed in a study exploring treatment patterns and the benefits of neoadjuvant chemotherapy for patients with PUC, which reported a significant difference in survival duration between patients receiving neoadjuvant chemotherapy and those not receiving it[16]. In our case, PUC was diagnosed following a presentation of painless gross hematuria. The patient’s history of intermittent abdominal fullness over 3 months indicated colonic involvement. The rarity and atypical presentation of PUC complicate its early diagnosis. The colon and rectum are less common sites of metastasis compared with other organs, such as the lungs, liver, and brain. Metastases to the colon and rectum are rarely observed in other cancers. Only one study reported a case of gastric cancer with metastases to the colon[17]. In that case, the metastatic gastric cancer mimicked primary colon cancer on imaging examinations. The final diagnosis was made through histologic examination and immunohistochemical staining for CDX-2, CK7, and CK20.
PUC rarely metastasizes to the gastrointestinal tract. A study investigating the correlations between metastatic patterns and primary bladder tumor characteristics reported that only 3% of all primary bladder cancer cases involve metastasis to the intestines[18]. Lesions may spread to the colon through lymphovascular or direct extension invasion routes. Lymphovascular spread is generally associated with regional lymphadenopathy, which was not observed in our patient, making this pathway unlikely. The mechanisms of extension invasion through which urologic malignancies metastasize to the colon remain unclear. Several studies have proposed different hypotheses. One study suggested that a history of surgical exposure can induce malignant deposition[19]. However, the absence of a history of pelvic surgery in our patient precludes this route. Another study proposed directional invasion, wherein cancer cells breach the bladder wall and extend to the colon through Denonvilliers’ fascia[20]. This route was also unlikely in our case because no directional invasion between the colon and bladder was detected. Kobayashi et al[21] suggested metastasis from the bladder’s lateral pedicles to the posterior rectal wall, followed by permeation into the colorectal wall, resulting in wall infiltration[21]. This route appears most plausible in our case because it aligns with the CT findings. However, our case is unique because of the sequence of symptom presentation. Most cases of bladder cancer with colorectal metastases initially manifest with urinary symptoms, such as gross hematuria, dysuria, or urinary retention. This observation is consistent with the findings of a study reporting that rectal involvement was detected, on average, 13.5 ± 11.8 months after the initial diagnosis of bladder urothelial carcinoma[22]. The initial gastrointestinal presentation in our case could have led to misdiagnosis and inappropriate treatment.
The present case highlights factors that may lead to misdiagnosis in cases of PUC. Abdominal CT images revealed segmental thickening of the colorectal wall, accompanied by pericolic inflammation. Differentiating colitis from tumor infiltration solely on the basis of imaging data—without endoscopic and histologic examinations—can be challenging. A study reported a case of recurrent colon cancer diagnosed 13 years after resection, presenting with gross hematuria[23]. In that case, diagnosis was confirmed through CT and histologic examination. Given the risk of metastasis, patients with a history of cancer or unexplained symptoms should be comprehensively evaluated, including through CT, endoscopy, and histopathology. Immunohistochemical staining can also aid in establishing the diagnosis and guiding therapeutic strategies. Metastasis to the colon may occur through embolic, lymphovascular, or direct invasion routes. In our case, metastasis was initially excluded because of the absence of regional lymphadenopathy and directional invasion to the rectum on CT. Nevertheless, a review of the literature indicated that metastasis from the bladder’s lateral pedicles to the posterior rectal wall was the most likely route.
Although the colon and rectum are less commonly affected by metastatic lesions than are other organs, colonic and rectal metastases should still be considered in urothelial carcinoma and PUC. Our case involved PUC with metastasis to the rectosigmoid colon, mimicking the colitis symptoms of colorectal wall thickening and pericolic infiltration on CT. Given the high risk of misdiagnosis, clinicians must maintain a high index of suspicion when encountering cases that resemble colitis.
| 1. | Sahin AA, Myhre M, Ro JY, Sneige N, Dekmezian RH, Ayala AG. Plasmacytoid transitional cell carcinoma. Report of a case with initial presentation mimicking multiple myeloma. Acta Cytol. 1991;35:277-280. [PubMed] |
| 2. | Ro JY, Shen SS, Lee HI, Hong EK, Lee YH, Cho NH, Jung SJ, Choi YJ, Ayala AG. Plasmacytoid transitional cell carcinoma of urinary bladder: a clinicopathologic study of 9 cases. Am J Surg Pathol. 2008;32:752-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Brustmann H. Plasmacytoid Urothelial Carcinoma of the Urinary Bladder Metastatic to the Duodenum: A Case Report-Diagnostic Relevance of GATA3 Immunohistochemistry. Case Rep Pathol. 2017;2017:5209059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Ricardo-Gonzalez RR, Nguyen M, Gokden N, Sangoi AR, Presti JC Jr, McKenney JK. Plasmacytoid carcinoma of the bladder: a urothelial carcinoma variant with a predilection for intraperitoneal spread. J Urol. 2012;187:852-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Kohada Y, Kaiho Y, Ito J, Mikami J, Anan G, Asano K, Yaegashi T, Murakami K, Nakamura Y, Sato M. Progressive plasmacytoid variant bladder cancer with retroperitoneal dissemination: An autopsy case report. IJU Case Rep. 2020;3:166-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Simon CT, Skala SL, Killen PD, Siddiqui J, Cao X, Qiao Y, Al-Ahmadie H, Camelo-Piragua SI, Jentzen J, Chinnaiyan AM, Dhanasekaran SM, Reichert ZR, Mehra R. Plasmacytoid urothelial carcinoma: a rapid autopsy case report with unique clinicopathologic and genomic profile. Diagn Pathol. 2019;14:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Ando T, Watanabe K, Takahashi K, Mizusawa T, Sakai T, Katagiri A. Duodenal and rectal obstructions due to urothelial cancer infiltration from recurrent renal pelvic cancer in the bladder wall: An autopsy case. Urol Case Rep. 2019;27:100903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Tanaka A, Ohori M, Hashimoto T, Hamada R, Nomura M, Kusama H, Nagao T, Tachibana M. [A case of plasmacytoid urothelial carcinoma of the bladder: rapid progression after transurethral resection]. Hinyokika Kiyo. 2012;58:101-103. [PubMed] |
| 9. | Dayyani F, Czerniak BA, Sircar K, Munsell MF, Millikan RE, Dinney CP, Siefker-Radtke AO. Plasmacytoid urothelial carcinoma, a chemosensitive cancer with poor prognosis, and peritoneal carcinomatosis. J Urol. 2013;189:1656-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Lopez-Beltran A, Requena MJ, Montironi R, Blanca A, Cheng L. Plasmacytoid urothelial carcinoma of the bladder. Hum Pathol. 2009;40:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Monn MF, Kaimakliotis HZ, Pedrosa JA, Cary KC, Bihrle R, Cheng L, Koch MO. Contemporary bladder cancer: variant histology may be a significant driver of disease. Urol Oncol. 2015;33:18.e15-18.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Cockerill PA, Cheville JC, Boorjian SA, Blackburne A, Thapa P, Tarrell RF, Frank I. Outcomes Following Radical Cystectomy for Plasmacytoid Urothelial Carcinoma: Defining the Need for Improved Local Cancer Control. Urology. 2017;102:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Li Q, Assel M, Benfante NE, Pietzak EJ, Herr HW, Donat M, Cha EK, Donahue TF, Bochner BH, Dalbagni G. The Impact of Plasmacytoid Variant Histology on the Survival of Patients with Urothelial Carcinoma of Bladder after Radical Cystectomy. Eur Urol Focus. 2019;5:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Chung AD, Schieda N, Flood TA, Cagiannos I, Mai KT, Malone S, Morash C, Hakim SW, Breau RH. Plasmacytoid urothelial carcinoma (PUC): Imaging features with histopathological correlation. Can Urol Assoc J. 2017;11:E50-E57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | da Fonseca LG, Souza CE, Mattedi RL, Girardi DM, Sarkis ÁS, Hoff PMG. Plasmacytoid urothelial carcinoma: a case of histological variant of urinary bladder cancer with aggressive behavior. Autops Case Rep. 2014;4:57-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Rahman S, Kong V, Jalfon M, Hesse D, Kim J, Wright JL, Adeniran A, Humphrey P, Martin DT, Ghali F. Evaluating Treatment Patterns and the Role of Neoadjuvant Chemotherapy in Plasmacytoid Urothelial Carcinoma: Insights from a Combined National and Institutional Series. Cancers (Basel). 2024;16:3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Su WC, Tsai HL, Wu CC, Tsai SY, Yeh YS, Ma CJ, Wang JY. Two rare cases of synchronous and metachronous colonic metastases in patients with advanced gastric cancer. World J Surg Oncol. 2018;16:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol. 2011;196:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Langenstroer P, Zacharias A, Almagro U, Dewire D. Annular constriction of the rectum secondary to transitional cell carcinoma of the bladder. Urology. 1996;47:442-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Stillwell TJ, Rife CC, Lieber MM. Bladder carcinoma presenting with rectal obstruction. Urology. 1989;34:238-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Kobayashi S, Kato H, Iijima K, Kinebuchi Y, Igawa Y, Nishizawa O. Annular rectal constriction due to infiltration by bladder cancer. Hinyokika Kiyo. 2006;52:569-572. [PubMed] |
| 22. | Aneese AM, Manuballa V, Amin M, Cappell MS. Bladder urothelial carcinoma extending to rectal mucosa and presenting with rectal bleeding. World J Gastrointest Endosc. 2017;9:282-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Huang YH, Tsai HL, Chai CY, Wang JY. Relapsed colon cancer patient presenting with hematuria 13 years after primary tumor resection: a case report. Kaohsiung J Med Sci. 2010;26:211-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/