Published online Jan 26, 2025. doi: 10.12998/wjcc.v13.i3.98110
Revised: September 28, 2024
Accepted: October 21, 2024
Published online: January 26, 2025
Processing time: 146 Days and 19.8 Hours
The issue of plastic pollutants has become a growing concern. Both microplastics (MPs) (particle size < 5 mm) and nanoplastics (NPs) (particle size < 1 µm) can cause DNA damage, cytotoxicity, and oxidative stress in various organisms. The primary known impacts of microplastic/nanoplastic are observed in the liver and respiratory system, leading to hepatotoxicity and chronic obstructive pulmonary disease. Although research on the effects of MPs and NPs on diabetes is still in its early stages, there are potential concerns. This editorial highlights the risk to diabetics from co-exposure to contaminants and MPs/NPs, supported by evi
Core Tip: Laboratory studies provide strong evidence of the impacts of microplastic and nanoplastic exposure on glycolysis, blood glucose levels, insulin secretion, and insulin resistance. However, the lack of long-term human studies results in inconclusive conclusions regarding the direct effects of microplastics/nanoplastics on the risk of developing diabetes.
- Citation: Hsiao HY, Nien CY, Shiu RF, Chin WC, Yen TH. Microplastic and nanoplastic exposure and risk of diabetes mellitus. World J Clin Cases 2025; 13(3): 98110
- URL: https://www.wjgnet.com/2307-8960/full/v13/i3/98110.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i3.98110
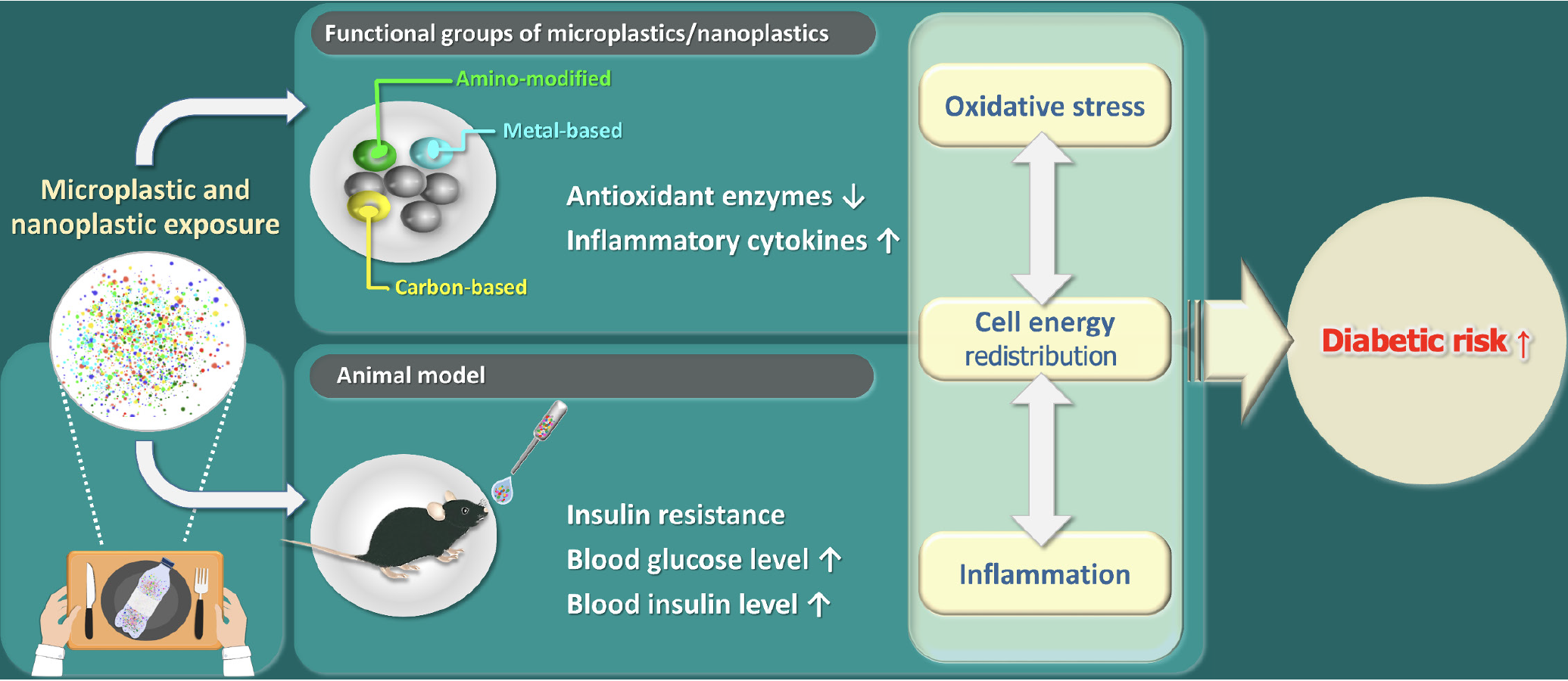
Microplastics (MPs) (MPs < 5 mm) and nanoplastics (NPs) (NPs < 1 μm) are small plastic particles produced by industry or degraded by physical and chemical activities[1]. Negative influences of the exposure of MPs or NPs on human health include oxidative stress, inflammation, endocrine disturbance, liver metabolism, kidney damage, and even carcinogenicity[2]. Most of the studies focus on hepatoxicity and immunotoxicity since the liver and immune system are highly susceptible to potential toxicity from environmental pollutants. In contrast, the findings on the impacts of MPs/NPs causing the development of diabetic mellitus are limited. When MPs or NPs contain endocrine-disrupting compounds, such as bisphenol A or phthalates, their impacts on rapid elevation in plasma insulin and increase the risk of developing type 2 diabetes are foreseeable[3]. Mostly, like plastics, MPs/NPs can be made of polyethylene, polypropylene, polystyrene, and polyvinyl chloride[4]. Polystyrene MPs/NPs have been found to accumulate in the food chain and have exhibited various impacts on human organs[5]. Here, we summarize the shreds of evidence on the potential risks of diabetes mellitus under the exposure of MPs/NPs (Figure 1).
Diabetes mellitus is caused by a combination of genetic and environmental factors. The environmental factors that might increase the risk of diabetes mellitus encompass lack of physical activity, obesity, exposure to medicines and toxins, virus infection, geographic areas, etc.[6,7]. MPs are released from takeaway containers, and individuals who order takeaway food 5–10 times a month may consume 145–5520 microplastic particles from these containers[8]. NPs are defined as the size ranging between 1 nm and 1 μm[9]. MPs/NPs are evenly distributed within the aquatic environment. The effect of MPs/NPs influences the growth rate of the shrimps, that might due to the change of their gut microbial diversity[10]. In addition, exposure to MPs/NPs presented endocrine-disrupting effects, reproductive hormones, or even gene expression patterns[11]. NP exposure impaired hepatic function and lipid metabolism. NPs affected the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling pathway, disrupting glucose metabolism[12]. The increased phos
Environmental pollution caused by the suspension of MPs/NPs only is usually accompanied by metal or toxic chemicals. The high surface ratio and lipophilicity of polystyrene NPs make them easy to absorb heavy metals in the environment[18]. Cadmium is a common contaminant found in soil and water[19]. In addition to cadmium treatment, combined exposure to MPs enhanced zebrafish's toxicity, growth, and reactive oxygen species accumulation[18]. Furthermore, the combined treatment of cadmium and polystyrene NPs exhibited synergistic effects on oxidative stress and kidney damages[20]. The mice that were co-exposure to lead and MPs experienced insulin resistance and damaged glucose tolerance via the nuclear factor erythroid 2-related factor 2 (Nrf2)/nuclear factor-kappa B (NF-κB) signaling pathway by increase in phosphorylated p-NF-κB protein levels and reduction of Nrf2 mRNA and protein level[21].
Here are listed couples of studies that exhibited different functional group of NPs also brings different effects on diabetic symptoms.
Polystyrene nanoparticles (PS-NH2) nanoparticles are known to induce inflammation[22,23]. The nanoparticles (NH2)-based nanoparticles not only induced NOD-like receptor family pyrin domain containing 3 inflammasome and proinflammatory IL-1β release but also medicated apoptosis through the release of cathepsin B and damage to the mitochondrial membrane[23]. Besides, PS-NH2 nanoparticles particularly inhibited the phagocytosis of Escherichia coli by M1 and M2 microphage[24]. In liver slides, the downregulation of inflammation markers, IL-1β and IL-10, was observed, and the viability of the cells significantly decreased after exposure to NH2-based nanoparticles[25]. The NH2 group exhibited the most significant fasting blood glucose elevation, glycogen accumulation, and insulin resistance[26]. Those effects might be due to the positive group of NH2 based nanoparticles, which makes it easy to enter the cell by reducing the thickness of the lipid layer[27].
Metal-based NPs: Research has shown that exposure to certain metal-based nanoparticles, such as titanium dioxide or zinc oxide, can lead to oxidative stress and inflammation in animal models[28]. These effects can disrupt glucose homeostasis and potentially contribute to the development of insulin resistance and diabetes. MPs/NPs can act as vectors for heavy metals like copper and zinc to form a complex because they are easily absorbed by MPs/NPs[29]. The oxygen groups on NPs increase surface polarity, enhancing the absorption of metal ion[30]. The co-localization of silver and NPs in various cell compartments of Caco-2 cells (a human intestinal carcinoma cell line) was further confirmed by confocal analysis, suggesting the tendency of association between metal and NPs[31]. Zhong et al[32] found that co-exposure to metals and NPs elevated ferroptosis-related genes, leading to renal damage in mice. Ferroptosis, a novel form of programmed cell death, is involved in the development of diabetics[33]. In diabetic mice, there was an observed increase in the expression of ferroptosis markers, specifically acyl-coenzyme A synthetase long-chain family member, and a decrease in the expression of glutathione peroxidase 4. These changes further contributed to the dysfunction of pancreatic β cells[34].
Carbon-based NPs: Carbon-based nanoparticles, such as carbon nanotubes and graphene oxide, have also been studied for their impact on metabolic health[35]. Some animal studies suggest that exposure to these nanoparticles can lead to liver damage, changes in lipid metabolism, and impaired glucose tolerance, which are risk factors for diabetes[36]. The acute exposure of carboxyl NPs exhibited toxicity in daphnids and elevated the expression of metabolic enzymes, such as lactate dehydrogenase (LDH)[37]. LDH, involved in catalyzing pyruvate into lactic acid during glycolysis, has been determined to be an assessment of the progression of diabetics[38]. The level expression of LDH presented a positive correlation with glucose level, suggesting that exposure to carboxyl NPs presents the risk of developing diabetics.
The mice exposed to polystyrene NPs for nine weeks presented the elevation in the fasting blood glucose levels, glucose intolerance, and insulin resistance[26]. Those conditions were more severe in diabetic mice. Blood glucose, glucose intolerance, and insulin resistance were increased under the condition of oral exposure to different dosages of polystyrene NPs for 8 weeks in a mouse model[39]. Even though the exposure time was shortened to two weeks, the liver tissue structure was abnormal and filled with inflammatory cells[40]. Using ultra-high-performance liquid chromatography coupled with orbitrap mass spectrometry analysis, 353 metabolites were identified in hepatic samples exposed to polystyrene NPs for two weeks[40]. Notably, levels of cortisol and acetylcholine were significantly reduced. Cortisol was found to be linked to insulin secretion[41], while acetylcholine played a direct role in mediating insulin secretion[42]. Besides oral exposure to NPs, airborne NPs also presented a similar effect on animal metabolism. Mice were kept in an airborne nanoplastic (NP) chamber with NP exposure doses ranging from 0.1 mg/day to 0.8 mg/day. Under two weeks of airborne NP exposure, the eating, drinking, and body weight were decreased while blood glucose was elevated[16]. The effect of elevated glucose was the consequence of insulin signaling impairment, in which FoxO1, the transcript factor of gluconeogenesis, was activated and subsequently promoted the expression of downstream genes, such as phosphoenolpyruvate carboxykinase 1, glucose-6-phosphatas, and peroxisome proliferator-activated receptor-γ coactivator-1α[16]. Consequently, exposure to polystyrene-NPs through oral or airborne exposure led to dysregulated insulin secretion and insulin resistance.
Synergistic effects of polystyrene NPs were observed in diabetic animals. The oral exposure of polystyrene NPs in the mice treated with streptozocin injection, which is commonly used to induce diabetic situations. The oxidative stress, glucose intolerance, and insulin resistance were aggravated than that of their health counterpart[39]. The oxidative stress could be alleviated by treating with Akt activator suggesting polystyrene NPs involving manipulating the level of reactive oxygen species through Akt phosphorylation pathway[39]. In type 2 diabetic mice, exposure to 0.5 mm (500 nm) NPs resulted in impairment in glucose tolerance and an increase in blood glucose level[43]. Furthermore, the hepatic lipid metabolism was distributed, and liver fibrosis was observed by activating the Wnt/β-catenin signaling pathway.
Animal studies provide strong evidence of the impacts of MPs and NPs exposure on glycolysis, blood glucose levels, insulin secretion, and insulin resistance. However, the lack of long-term human studies results in ambiguous conclusions regarding the direct effects of MPs/NPs on the risk of developing diabetes. Further research is essential to comprehensively understand the mechanisms of MPs/NPs toxicity in diabetic patients and to develop strategies for preventing these risks. With the risks of diabetes due to MPs/NPs exposure have been underscored, the significance and urgency of addressing MPs/NPs pollution for human health should be prioritized.
| 1. | Chiang CC, Yeh H, Shiu RF, Chin WC, Yen TH. Impact of microplastics and nanoplastics on liver health: Current understanding and future research directions. World J Gastroenterol. 2024;30:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (4)] |
| 2. | Ali N, Katsouli J, Marczylo EL, Gant TW, Wright S, Bernardino de la Serna J. The potential impacts of micro-and-nano plastics on various organ systems in humans. EBioMedicine. 2024;99:104901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 168] [Article Influence: 84.0] [Reference Citation Analysis (1)] |
| 3. | Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 457] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 4. | Li M, Pan Y, Hou Z, Wu Z, Zeng Z, Wang B. Plastic or plastic-free life: From formation to removal. Sci Total Environ. 2023;890:164359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 5. | Kik K, Bukowska B, Sicińska P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ Pollut. 2020;262:114297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 325] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 6. | Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 7. | Chung YL, Hou YC, Wang IK, Lu KC, Yen TH. Organophosphate pesticides and new-onset diabetes mellitus: From molecular mechanisms to a possible therapeutic perspective. World J Diabetes. 2021;12:1818-1831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 8. | Zhou X, Wang J, Ren J. Analysis of Microplastics in Takeaway Food Containers in China Using FPA-FTIR Whole Filter Analysis. Molecules. 2022;27:2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Joseph TM, Kar Mahapatra D, Esmaeili A, Piszczyk Ł, Hasanin MS, Kattali M, Haponiuk J, Thomas S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials (Basel). 2023;13:574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 167] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 10. | Li H, Chen H, Wang J, Li J, Liu S, Tu J, Chen Y, Zong Y, Zhang P, Wang Z, Liu X. Influence of Microplastics on the Growth and the Intestinal Microbiota Composition of Brine Shrimp. Front Microbiol. 2021;12:717272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Yang WS, Nevin DN, Peng R, Brunzell JD, Deeb SS. A mutation in the promoter of the lipoprotein lipase (LPL) gene in a patient with familial combined hyperlipidemia and low LPL activity. Proc Natl Acad Sci U S A. 1995;92:4462-4466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Fan X, Wei X, Hu H, Zhang B, Yang D, Du H, Zhu R, Sun X, Oh Y, Gu N. Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice. Chemosphere. 2022;288:132607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 13. | Huang D, Zhang Y, Long J, Yang X, Bao L, Yang Z, Wu B, Si R, Zhao W, Peng C, Wang A, Yan D. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. Sci Total Environ. 2022;838:155937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 14. | Yin XH, Xu YM, Lau ATY. Nanoparticles: Excellent Materials Yet Dangerous When They Become Airborne. Toxics. 2022;10:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Yang S, Zhang T, Ge Y, Yin L, Pu Y, Liang G. Inhalation exposure to polystyrene nanoplastics induces chronic obstructive pulmonary disease-like lung injury in mice through multi-dimensional assessment. Environ Pollut. 2024;347:123633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 16. | Yang Z, Dong H, Gao Y, Liu S, Chen L, Ni G, Guo X, Wang M, Wang C, Chen Y, Chen L. Airborne Nanoplastics Exposure Inducing Irreversible Glucose Increase and Complete Hepatic Insulin Resistance. Environ Sci Technol. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Marfella R, Prattichizzo F, Sardu C, Fulgenzi G, Graciotti L, Spadoni T, D'Onofrio N, Scisciola L, La Grotta R, Frigé C, Pellegrini V, Municinò M, Siniscalchi M, Spinetti F, Vigliotti G, Vecchione C, Carrizzo A, Accarino G, Squillante A, Spaziano G, Mirra D, Esposito R, Altieri S, Falco G, Fenti A, Galoppo S, Canzano S, Sasso FC, Matacchione G, Olivieri F, Ferraraccio F, Panarese I, Paolisso P, Barbato E, Lubritto C, Balestrieri ML, Mauro C, Caballero AE, Rajagopalan S, Ceriello A, D'Agostino B, Iovino P, Paolisso G. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N Engl J Med. 2024;390:900-910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 541] [Article Influence: 270.5] [Reference Citation Analysis (0)] |
| 18. | Yang H, Zhu Z, Xie Y, Zheng C, Zhou Z, Zhu T, Zhang Y. Comparison of the combined toxicity of polystyrene microplastics and different concentrations of cadmium in zebrafish. Aquat Toxicol. 2022;250:106259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Guo H, Hu R, Huang G, Pu W, Chu X, Xing C, Zhang C. Molybdenum and cadmium co-exposure induces endoplasmic reticulum stress-mediated apoptosis by Th1 polarization in Shaoxing duck (Anas platyrhyncha) spleens. Chemosphere. 2022;298:134275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Bhagat J, Zang L, Kaneco S, Nishimura N, Shimada Y. Combined exposure to nanoplastics and metal oxide nanoparticles inhibits efflux pumps and causes oxidative stress in zebrafish embryos. Sci Total Environ. 2022;835:155436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Zhu M, Li P, Xu T, Zhang G, Xu Z, Wang X, Zhao L, Yang H. Combined exposure to lead and microplastics increased risk of glucose metabolism in mice via the Nrf2/NF-κB pathway. Environ Toxicol. 2024;39:2502-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Kim JA, Åberg C, Salvati A, Dawson KA. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat Nanotechnol. 2011;7:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 477] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 23. | Lunov O, Syrovets T, Loos C, Nienhaus GU, Mailänder V, Landfester K, Rouis M, Simmet T. Amino-functionalized polystyrene nanoparticles activate the NLRP3 inflammasome in human macrophages. ACS Nano. 2011;5:9648-9657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 24. | Fuchs AK, Syrovets T, Haas KA, Loos C, Musyanovych A, Mailänder V, Landfester K, Simmet T. Carboxyl- and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials. 2016;85:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Bartucci R, van der Meer AZ, Boersma YL, Olinga P, Salvati A. Nanoparticle-induced inflammation and fibrosis in ex vivo murine precision-cut liver slices and effects of nanoparticle exposure conditions. Arch Toxicol. 2021;95:1267-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Xu K, Gao X, Wei Z, Han Q, Wang S, Du W, Chen M. Polystyrene nanoplastics with different functional groups and charges have different impacts on type 2 diabetes. Part Fibre Toxicol. 2024;21:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 27. | Liu S, Wu X, Gu W, Yu J, Wu B. Influence of the digestive process on intestinal toxicity of polystyrene microplastics as determined by in vitro Caco-2 models. Chemosphere. 2020;256:127204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Wang S, Alenius H, El-Nezami H, Karisola P. A New Look at the Effects of Engineered ZnO and TiO(2) Nanoparticles: Evidence from Transcriptomics Studies. Nanomaterials (Basel). 2022;12:1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 29. | Liu S, Shi J, Wang J, Dai Y, Li H, Li J, Liu X, Chen X, Wang Z, Zhang P. Interactions Between Microplastics and Heavy Metals in Aquatic Environments: A Review. Front Microbiol. 2021;12:652520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Holmes LA, Turner A, Thompson RC. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ Pollut. 2012;160:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 600] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 31. | Domenech J, Cortés C, Vela L, Marcos R, Hernández A. Polystyrene Nanoplastics as Carriers of Metals. Interactions of Polystyrene Nanoparticles with Silver Nanoparticles and Silver Nitrate, and Their Effects on Human Intestinal Caco-2 Cells. Biomolecules. 2021;11:859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Zhong G, Qiao B, He Y, Liu H, Hong P, Rao G, Tang L, Tang Z, Hu L. Co-exposure of arsenic and polystyrene-nanoplastics induced kidney injury by disrupting mitochondrial homeostasis and mtROS-mediated ferritinophagy and ferroptosis. Pestic Biochem Physiol. 2024;201:105904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 33. | Wang Y, Bi R, Quan F, Cao Q, Lin Y, Yue C, Cui X, Yang H, Gao X, Zhang D. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur J Pharmacol. 2020;888:173574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 34. | Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, Tang Y, Gao C, Yao P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients. 2020;12:2954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 35. | Strojny B, Kurantowicz N, Sawosz E, Grodzik M, Jaworski S, Kutwin M, Wierzbicki M, Hotowy A, Lipińska L, Chwalibog A. Long Term Influence of Carbon Nanoparticles on Health and Liver Status in Rats. PLoS One. 2015;10:e0144821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Haldar S, Yhome N, Muralidaran Y, Rajagopal S, Mishra P. Nanoplastics Toxicity Specific to Liver in Inducing Metabolic Dysfunction-A Comprehensive Review. Genes (Basel). 2023;14:590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 37. | Panagiotidis K, Engelmann B, Krauss M, Rolle-Kampczyk UE, Altenburger R, Rochfort KD, Grintzalis K. The impact of amine and carboxyl functionalised microplastics on the physiology of daphnids. J Hazard Mater. 2023;458:132023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 38. | Dmour HH, Khreisat EF, Khreisat AF, Hasan SA, Atoom O, Alkhatib AJ. Assessment of Lactate Dehydrogenase Levels Among Diabetic Patients Treated in the Outpatient Clinics at King Hussein Medical Center, Royal Medical Services, Jordan. Med Arch. 2020;74:384-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Wang Y, Wei Z, Xu K, Wang X, Gao X, Han Q, Wang S, Chen M. The effect and a mechanistic evaluation of polystyrene nanoplastics on a mouse model of type 2 diabetes. Food Chem Toxicol. 2023;173:113642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 40. | Shi C, Han X, Guo W, Wu Q, Yang X, Wang Y, Tang G, Wang S, Wang Z, Liu Y, Li M, Lv M, Guo Y, Li Z, Li J, Shi J, Qu G, Jiang G. Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ Int. 2022;164:107273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 41. | Schernthaner-Reiter MH, Wolf P, Vila G, Luger A. The Interaction of Insulin and Pituitary Hormone Syndromes. Front Endocrinol (Lausanne). 2021;12:626427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Molina J, Rodriguez-Diaz R, Fachado A, Jacques-Silva MC, Berggren PO, Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes. 2014;63:2714-2726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 43. | Li X, Feng L, Kuang Q, Wang X, Yang J, Niu X, Gao L, Huang L, Luo P, Li L. Microplastics cause hepatotoxicity in diabetic mice by disrupting glucolipid metabolism via PP2A/AMPK/HNF4A and promoting fibrosis via the Wnt/β-catenin pathway. Environ Toxicol. 2024;39:1018-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/