Published online Sep 26, 2025. doi: 10.12998/wjcc.v13.i27.105415
Revised: April 19, 2025
Accepted: June 19, 2025
Published online: September 26, 2025
Processing time: 196 Days and 7.4 Hours
Low-density lipoprotein cholesterol (LDL-C) is the most causal risk factor for atherosclerotic cardiovascular disease (ASCVD). Red yeast rice (RYR) is a nutraceutical widely used as a lipid-lowering dietary supplement. The main cholesterol-lower agents in RYR are monacolins, particularly monacolin K, a weak reversible inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, whose daily consumption (up to 10 mg/day) reduces LDL-C plasma levels up to 34% within 6-8 weeks when compared to placebo. The reduction in LDL-C is often accompanied by lower levels of plasma apolipoprotein B, total cholesterol, matrix metalloproteinases 2 and 9, high-sensitivity C-reactive protein, non-high-density lipoprotein cholesterol, and blood pressure. RYR has also demonstrated favorable reductions of up to 45% compared to placebo in the risk of ASCVD events in secondary prevention studies. The mechanism of action is similar to statins. When consumed appropriately, RYR is associated with only minimal side effects. Mild myalgia may be seen in patients who cannot tolerate low-dose statins. In individuals with no additional ASCVD risk factors, RYR is a safe and effective supplement in treating mild to moderate hyperlipidemia.
Core Tip: Red yeast rice (RYR) has cholesterol-lowering properties due to its active ingredient, monacolin K (MK), which mainly inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase. It is an excellent option for patients with mild to moderate dyslipidemia and low atherosclerotic cardiovascular disease risk factors who otherwise would not qualify for statin therapy. RYR can also serve as a valuable alternative in patients with statin intolerance or patients who otherwise are against medical treatment. Although effective, it is noteworthy to mention that the quantity of MK in commercial RYR products varies by brand. This article narratively reviews some existing data on RYR in the treatment of mild to moderate dyslipidemia.
- Citation: English K. Red yeast rice with monacolin K for the improvement of hyperlipidemia: A narrative review. World J Clin Cases 2025; 13(27): 105415
- URL: https://www.wjgnet.com/2307-8960/full/v13/i27/105415.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i27.105415
Elevated low-density lipoprotein cholesterol (LDL-C) is an alterable risk factor for atherosclerotic cardiovascular disease (ASCVD)[1,2]. Current clinical standards recommend lifestyle modifications, including diet and exercise, and may include lipid-lowering therapy based on ASCVD scoring[2,3]. In several cases, there are patients with mild to moderate cholesterol imbalance and less than average ASCVD risk who would not qualify for lipid-lowering therapy and otherwise would not be able to achieve normal lipid values via diet and exercise alone. Nutraceuticals such as red yeast rice (RYR) are an excellent option for management in these patients and individuals with mild to moderate dyslipidemia and high ASCVD risk who decline pharmacotherapy[4,5].
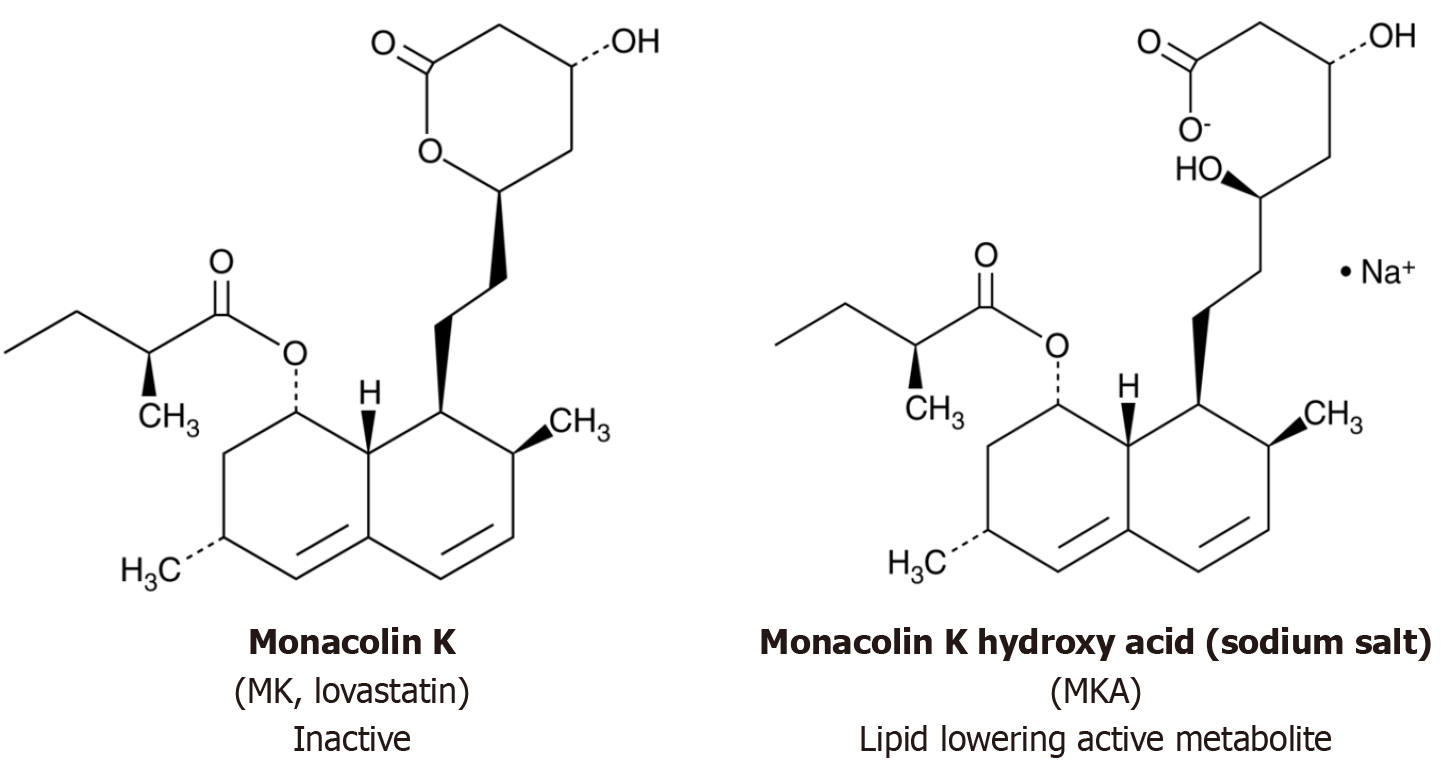
RYR is a Chinese folk medicine created by the process of fermenting white rice with yeast, mainly Monascus purpureus[6]. The fermentation process produces numerous chemicals, including the ones responsible for its typical red color[6,7]. This process creates monacolins, including monacolin K (MK), a subtype of monacolin similar in structure to lovastatin (Figure 1) with lipid lowering properties, which serves as the active ingredient in RYR[6-8]. Under acidic states, MK deploys one of two configurations: Acidic or lactone[8,9]. The acidic version plays a part in cholesterol reduction[9,10]. For decades, RYR has been used in Asia as a medicine and a coloring agent for food[9-11]. More recently, it has been extensively used as a cholesterol-lowering dietary supplement. The quality of RYR products can differ and are regulated variously. In Europe, commodities with MK are treated as food supplements, while in the United States, they are regulated as drugs[12].
This article comprehensively reviews the use of RYR in the management of hypercholesterolemia. We also reviewed the biological activity and mechanism of action regarding MK in the reduction process and clinical data supporting its use in mild to moderate dyslipidemia. Its anti-inflammatory and antibacterial properties are briefly mentioned.
RYR is a food substance that has been used since the Tang dynasty in 800 AD as a naturally occurring remedy for stomach pain[13]. It has also been used to manufacture alcoholic refreshments and several fermented foods in Korea and China[13,14]. Following the 18th century, RYR has been used to produce tofuyo (Okinawan-style fermented tofu) in Japan[15]. More recently, MK (lovastatin), which possesses lipid plunging effects, was discovered in some strains of Monascus fungi[16]. At present, it is ingested as an ordinary food supplement in a large number of Chinese meals. In North America, it is used as a supplement to reduce cholesterol and enhance cardiac well-being[16,17].
Monascus purpureus yeast is fermented over rice to yield RYR that exhibits a deep red color[18]. This process produces a product that contains several mevinic acids or monacolins, with lovastatin being one of many[18,19]. MK is the main active ingredient in RYR, which is the principal agent responsible for reducing blood cholesterol levels[18-20].
MK, found in RYR, is structurally identical to lovastatin[21]. It is a white, transparent, needle-shaped crystal under normal laboratory conditions with hydrophobic properties, making it dissolvable in organic substances like acetone and benzene[21,22]. MK possesses the molecular formula C24H36O5 with a weight of 404.55. At low pH states, it adopts either the acidic (active) or lactone (inactive) form[8,22,23]. The lactone form is structurally identical to lovastatin, while the hydroxyl acid is responsible for the inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoAR), the enzyme catalyzing the rate-limited step in cholesterol formation[22-24]. The proportion of the acid form varies between 5% and 100%, depending on the pH[25]. The lactone version prevails at low pHs, whereas at neutral and basic pHs, the hydroxy acid predominates[25,26].
Lovastatin, structurally similar to MK, is a prodrug hydrolyzed to the acidic form, which is the active metabolite that blocks HMG-CoAR and cholesterol synthesis[27]. The acidic version is the only form that can form bonds with amino acid residues in the binding pocket of HMG-CoAR, stabilizing the interaction between the two molecules[27-29]. As such, the acidic form is naturally present in RYR, resulting in differences in clinical profiles and bioavailability between lovastatin and RYR[30,31].
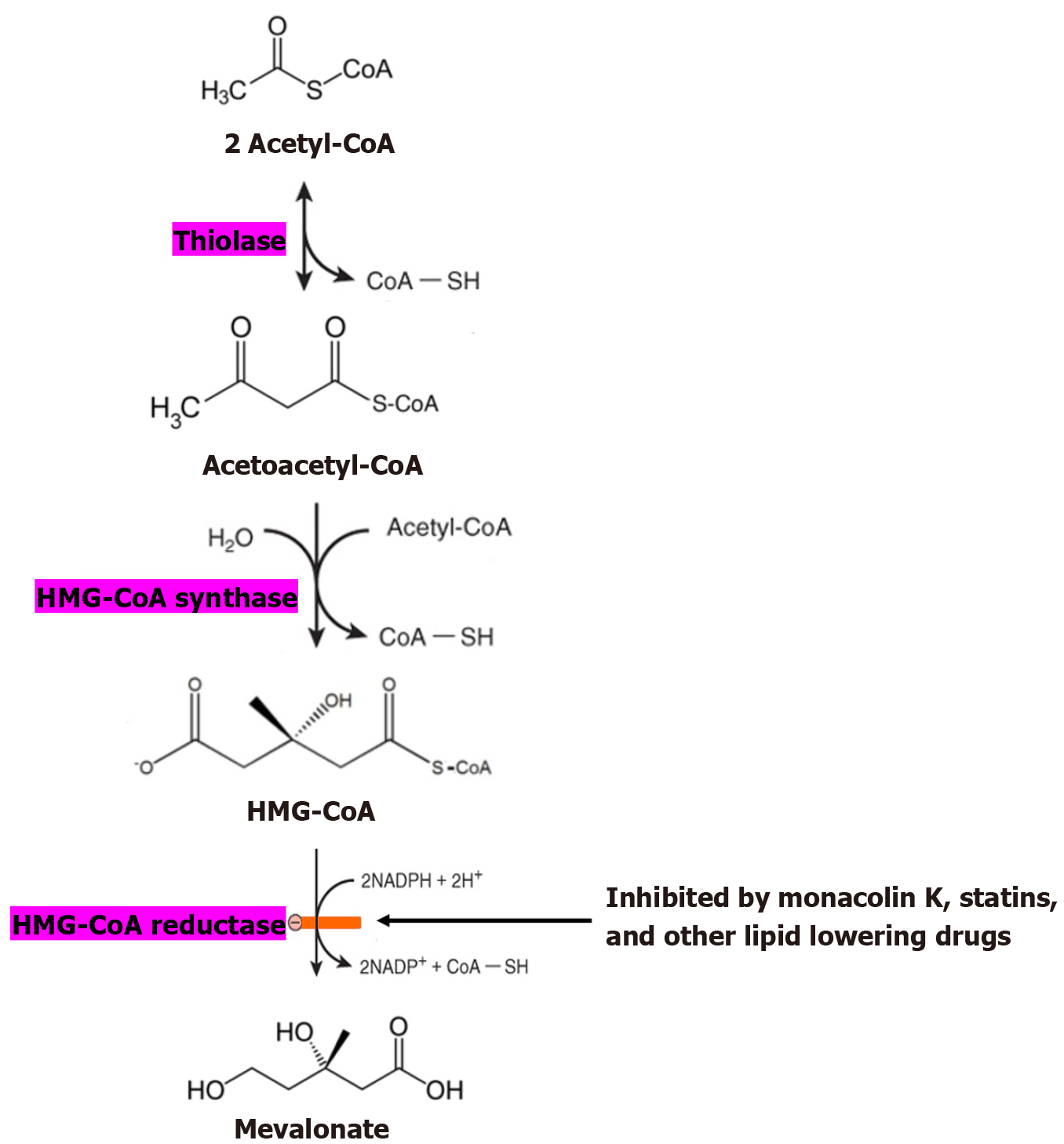
The mechanism through which MK facilitates cholesterol reduction is complicated, performing via a host of various avenues within the human body. It lowers lipid levels by decreasing internal lipid creation, decreasing digestion of exogenous lipids, and facilitating their dispatch and evacuation[13,32]. In the cholesterol synthesis passageway (Figure 2), acetyl-CoA synthesizes HMG-CoA, which then synthesizes mevalonate[33,34]. Cholesterol is then ultimately made via a sequence of biological reactions. HMG-CoA conversion to mevalonate occurs via HMG-CoAR, serving as the rate-limiting step in the biosynthetic pathway[33,34]. MK, the active ingredient in RYR, possesses a similar chemical structure to HMG-CoAR, owing to a high degree of competitive inhibition of the enzyme, resulting in the prevention of cholesterol synthesis and a decrease in blood lipid contents[35]. MK in RYR also works to decrease blood lipid levels through the LDL receptor[33-36]. LDL is an essential substance in controlling blood cholesterol. This key complex is responsible for circulating cholesterol to the peripheral tissues[37]. LDL is mostly dissipated and remodeled by the LDL receptors. MK inherently increases LDL receptor output, thereby decreasing LDL levels and lowering cholesterol within the blood[37,38]. Compared with purified lovastatin, RYR extract has a higher bioavailability, which makes it more effective in reducing cholesterol levels[39,40].
Hypercholesterolemia, especially elevated LDL-C, is a major adjustable risk factor for ASCVD[41]. Guidelines re
Several studies have confirmed the efficacy and safety of RYR in patients with hyperlipidemia[53-55]. Liu et al[56] performed a meta-analysis of randomized controlled trials (RCTs) to evaluate the effectiveness and safety of RYR preparations on lipid modification in primary hyperlipidemia. The study consisted of 93 trials, using a total of 9625 participants, in which three RYR formulations were tested. The integrated outcomes showed a drastic reduction of total cholesterol (TC) weighted mean difference (MD) -0.91 mmol/L, 95%CI: -1.12 to -0.71, LDL-C (-0.73 mmol/L, -1.02 to -0.043), and triglycerides (TG) (-0.41 mmol/L, -0.6 to -0.22) by RYR treatment compared with placebo. The study also showed an increase in high-density lipoprotein cholesterol (HDL-C) values (0.15 mmol/L, 0.09 to 0.22) vs placebo. Overall, the lipid-changing effects of RYR unfolded to be similar to simvastatin, pravastatin, atorvastatin, lovastatin, and fluvastatin. Contrasted with agents that are not statins, RYR formulations appeared superior to fish oils and nicotinate but equal to or less effective than gemfibrozil and fenofibrate. RYR preparations were associated with nonserious side effects such as gastrointestinal discomfort and dizziness.
Gerards et al[57] also conducted a meta-analysis of 20 RCTs to authenticate the safety and efficacy of RYR preparations in lowering LDL-C. These doses of RYR varied between 1200 mg and 4800 mg/day, containing a range from 4.8 to 24 mg of MK. The results showed that RYR decreased LDL-C by a mean of 1.02 mmol/L (39.4 mg/dL) after 2 to 24 months juxtaposed to placebo. These results signified that RYR extract could significantly reduce the LDL-C, which is comparable in effectiveness with statins. The study, therefore, concluded that RYR may be a safe and acceptable option for hyperlipidemia in patients with intolerance to statins to reduce the risk of ASCVD.
There have also been studies that confirmed the tolerability of RYR in patients with intolerance to statins[58-60]. Stefanutti et al[61] conducted a clinical study on 55 patients with heterozygous familial hypercholesterolemia who ceased statin therapy due to myalgia. They received a low-cholesterol diet that constituted 300 mg of RYR rice, equivalent to 10 mg of MK per day. Results showed a significant reduction in LDL-C after 6 months (16% for females and 17% for males, P < 0.005). These levels further declined to 27% and 24% in women and men at 12 months, respectively. There were no encounters of elevated C-reactive protein or aminotransferase.
Several other studies have been conducted that show that when RYR is used as a food addendum for patients with dyslipidemia, it dramatically reduces their symptoms[62,63]. Researchers have also collected clinical data from several other trials in patients who have used RYR as monotherapy for hypercholesterolemia[64-66]. These results showed that RYR can decrease cholesterol levels and often serve as a suitable alternative for patients who are intolerant to statins.
The combination of nutraceuticals with different mechanisms of action for patients with mild to moderate hypercholesterolemia who otherwise do not qualify for statin therapy based on ASCVD risk or intolerance is a valid alternative in preventing coronary artery disease (CAD)[67]. Certain interactions between RYR and natural commodities with different mechanisms of action may have harmonious effects[67,68]. For example, the combination of RYR, which inhibits HMG-CoAR, and berberine, which extends the half-life of LDL receptor messenger ribonucleic acid (mRNA), has been shown to reduce LDL-C comparable to those with prescription statin therapy[69]. Other nutraceuticals with lipid-lowering properties are listed in Table 1.
| Nutraceutical | Active ingredient(s) | Mechanism of action | Side effect(s) | Dosing |
| Bergamot (Citrus bergamia) | Flavonoids | Inhibits HMG-CoAR and ACAT | GI discomfort and muscle cramps | 500-1500 mg/day |
| Garlic extract | Allicin (diallyl thiosulfinate) | Inhibits HMG-CoAR, acetyl-CoA synthetase, and squalene-monooxygenase | GI upset, body odor, and increase risk of bleeding | 0.3-20 g/day |
| Artichoke leaf extract | Flavonoids, caffeic acid, volatile sesquiterpene, and mono- and dicaffeoylquinic acid | Flavonoids (luteolin) inhibits HMG-CoAR, SREBP, ACAT, and increase GI excretion of bile acids | Skin reactions, GI upset, and potential asthma exacerbation | 500-2800 mg/day |
| Green tea | Catechins | Inhibits inducible NO synthase and HMG-CoAR. Activates AMPK | GI discomfort, hypertension, and skin rashes. Rare adverse effects include hepatotoxicity, hypokalemia, and TTP | 100-500 mg/day |
| Oryza sativa | Gamma-Oryzanol | Inhibition of GI cholesterol absorption and increase fecal excretion of bile acids | Well tolerated | 100-300 mg/day |
| Olive extract | Phenols | Reduce lipid peroxidation, increases bile excretion, inhibits HMG-CoAR and ACAT activity | Well tolerated | 136.2 mg of oleuropein and 6.4 mg of hydroxytyrosol per day |
| Lupin protein | Lupin | Inhibits HMG-CoA and PCSK9 activity. Upregulated SREBP-2 | GI discomfort | ≤ 35 g/day |
| β-Glucan | β-Glucan | Decrease absorption from GI tract | Well tolerated | 3-5 g/day |
| Plantago seed | Psyllium | Increases hepatic LDL receptor expression | GI discomfort, allergic reactions, bowel and esophageal obstruction | 2-20 g/day |
| Amorphophallus konjac | Glucomannan | Inhibits HMG-CoAR | GI upset, esophageal, and bowel obstruction | 1-15 g/day |
| Guar (Cyamopsis tetragonoloba or Cyamopsis psoraloides) | Guar Gum | Prevent absorption of cholesterol in the GI tract and increase bile acid excretion | GI upset, esophageal, and bowel obstruction | 30-100 g/day |
| Nigella Sativa | Thymoquinone, flavonoids, and PUFA | Increase biliary excretion, reduce cholesterol synthesis, inhibit lipid oxidation, and upregulate LDL receptors | GI discomfort and transaminitis | 200 to 3000 mg for capsules, powders, and extracts. 1-2 mL for oil suspensions |
| Silymarin | Flavonolignans | Increase lipolysis and β-oxidation via the upregulation. Increase cholesterol efflux via the increased expression of ABCA1 | GI discomfort, headache, ureteric stones, and hemolytic anemia | 140-700 mg/day |
| Anthocyanins | Flavonoids | Downregulate the messenger RNA expression of SREBP-1c | Well tolerated | 100-450 mg/day |
| Spirulina | C-phycocyanin | Activates heme oxygenase-1 | GI discomfort, rashes, bleeding, cholestasis, and elevated transaminases | 1-10 g/day |
| Alpha lipoic acid | Alpha lipoic acid | Module fat synthesis, mitochondrial β-oxidation of fat, clearance of TG-rich lipoproteins in the liver, and adipose TG accumulation | GI discomfort, skin rashes, and rarely, insulin autoimmune syndrome | 300-1800 mg/day |
| Chitin | Chitosan | Interferes with GI absorption by binding to negatively charged fatty acids and bile acids and disrupting the emulsification of neutrally charged cholesterol | GI discomfort. Avoid use in patients with allergies to shellfish or crustaceans | 0.3-3 g/day |
| Pantothenic acid (B5) | Pantethine | Inhibits HMG-CoA reductase and acetyl-CoA carboxylase | GI upset | 600-1200 mg/day |
Natto, a fermented soybean product, has been used as a food supplement in Japan for many decades[70]. Nattokinase (NK), a potent fibrinolytic and antithrombotic compound, has been shown to have anti-atherosclerotic and cholesterol-lowering properties[70,71]. It is created by the bacterium Bacillus subtilis during the fermentation of soybeans to yield Natto[70-72]. Several RCTs have shown favorable outcomes of hypercholesterolemia in patients who combine RYR and NK[73-75].
An RCT was conducted by Yang et al[73] to evaluate the lipid-lowering effect of oral NK vs NK and the extract of RYR on lipids in patients with dyslipidemia. A total of 47 patients with hyperlipidemia were designated to one of three groups: NK mono formula (50 mg per capsule), the combined formula of NK with RYR (300 mg per capsule), and placebo. Compared to the mono group, which revealed no effects on blood lipids until 6 months, the conjoined formula improved all measured lipids at the end of month one. The combined formula also resulted in abatement in TG by 15%, TC by 25%, LDL-C by 41%, TC/HDL-C by 29.5%, and increases in HDL-C by 7.5%. After controlling for baseline levels, only the combined set revealed a noticeable difference (P < 0.0001) in LDL-C, TC, and TC/HDL-C ratio when compared with the placebo group.
Another RCT conducted by Liu et al[74] probed the influence of NK and RYR supplementations on cardiometabolic parameters in patients with stable CAD. One hundred seventy-eight patients with CAD were randomized into four groups: RYR, NK + RYR, RYR, and placebo. In comparison across groups, NK + RYR reduced TG (-0.39 mmol), TC (-0.66 mmol/L), and increased HDL-C (0.195 mmol/L) compared to all other groups (P < 0.01). Both NK + RYR and NK sets had noteworthy improved lactate dehydrogenase than the others (-29.1 U/L and -26.4 U/L). The NK + RYR group also showed more potent reductions in thromboxane B2 and elevations in antithrombin III compared to placebo (P < 0.01). This study revealed that combined NK and RYR are safe and more effective than their counterparts in reducing blood lipid levels.
Policosanol possesses a mixture of concentrated primary aliphatic alcohols withdrawn from sugar cane wax, widely respected as a lipid reduction agent, with some studies reporting its usefulness in treating hypertension[76]. Several studies have evaluated the safety and efficacy of combined RYR with policosanols. In an RCT by Stefanutti et al[61] 240 patients with an overall coronary risk of < 20% and moderate dyslipidemia were treated with RYR extract (200 mg = 3 mg on MK) combined with aliphatic alcohols (10 mg). Patients had a 26% reduction in HDL-C and a 29% reduction in LDL-C at their 4-month follow-up.
Cicero et al[77] administered octacasanols (10 mg) and RYR extract (340 mg containing 5 mg of MK) to 111 patients with moderate hypercholesterolemia and low risk for cardiovascular disease, defined by a Framingham Risk Score of < 20%. LDL-C was reduced by an average of 20% at the 2-month mark, a result that is comparable to patients taking pravastatin 20 mg per day.
Another randomized, multicenter investigation compared the metabolic effect of nutraceuticals plus diet vs diet alone on hypercholesterolemia[78]. RYR (200 mg = 3 mg of MK) combined with policosanol (10 mg) was evaluated in 743 elderly and 1665 adults. A 21% reduction in LDL-C and a 13% increase in HDL-C with no change in TG levels were seen at the 16-week follow-up.
Berberine is a Chinese herbal supplement used to treat heart failure and diabetes in China[79]. It has been shown to increase the hepatic expression of LDL receptors by extending the half-life of LDL-C mRNA, which serves as the primary mechanism by which it lowers cholesterol[80,81]. This effect is similar to the increased transcription of mRNA promoted by statins[82]. Berberine is well tolerated, apart from occasional GI upset in some patients[83]. When combined with RYR, it can achieve reductions in LDL-C comparable with prescription statins but without the associated adverse effects such as myopathy or hepatic damage[83,84].
The lipid-lowering characteristics regarding the combination of RYR (3 mg of MK), policosanols (10 mg), and berberine (500 mg) is one of the most studied associations for which several meta-analyses of RCTs are available. Millán et al[85] conducted a meta-analysis regarding the effect of the above combinations on lipid parameters. Data from 11 RCTs constituting 1970 nutraceutical combinations and 1954 control patients were included in the study. The results showed a 9.9% reduction in TC, a 13.7% decrease in LDL-C, a 7.0% reduction in TG, and a 3.7% increase in HDL-C.
Pirro et al[86] also conducted a meta-analysis of 14 RCTs involving 3159 patients to evaluate the cholesterol-lowering efficacy of RYR (3 mg of MK) compounded with policosanols (10 mg), and berberine (500 mg). Data showed that the RYR-policosanol-berberine combination enhanced LDL-C by 23.6 mg/dL, TG by 14.2 mg/dL, HDL-C by 2.71 mg/dL, and glucose by 2.52 mg/dL. These effects were maintained in the long term, and this mixture was found to be safe and well-received by the majority of adult and elderly patients who reported previous statin intolerance.
A double-blind, placebo-controlled RCT evaluated the effectiveness of the RYR-phytosterol combination in 90 patients with hypercholesterolemia[87]. Results showed a 19% and 27% reduction in apolipoprotein B and LDL-C, respectively.
Li et al[88] conducted a meta-analysis regarding the safety and efficacy of RYR in treating hyperlipidemia using 15 RCTs as determined by a Jadad scale of ≥ 4 points. A total of 1012 individuals participated in the study. Results showed that contrasted to statins, RYR was more successful in decreasing TG (MD: -19.90; 95%CI: -32.22 to -7.58; P = 0.002), less effective in lowering TC (MD: 12.24; 95%CI: 2.19 to 22.29; P = 0.02) and comparable in lowering LDL-C and elevating HDL-C. Compared with nutraceutical, RYR drastically lowered LDL-C (MD: -14.40; 95%CI: -22.71 to -6.09; P = 0.0007) and TC (MD: -17.80; 95%CI: -27.12 to -8.48; P = 0.0002), and elevated HDL-C (MD: 7.60; 95%CI: 4.33 to 10.87; P < 0.00001). RYR additionally effectively combined nutraceutical to further lower LDL-C (MD: -27.91; 95%CI: -36.58 to -19.24; P < 0.00001), TC (MD: -31.10; 95%CI: -38.83 to -23.36; P < 0.00001), and TG (MD: -26.32; 95%CI: -34.05 to -18.59; P < 0.00001). RYR also dramatically decreased apoB (MD: -27.98; 95%CI: -35.51 to -20.45; P < 0.00001) and was associated with no heightened risk of adverse events.
Another meta-analysis of 20 RCTs by Gerards et al[57] involving more than 6000 patients revealed that RYR (1200-4800 mg/day) was better than a placebo at decreasing TC (MD: -1.00 mmol/L; 95%CI: -1.23 to -0.77, P < 0.00001) and LDL (MD: -1.02 mmol/L; 95%CI: -1.20 to -0.83, P < 0.00001). The effect of RYR was homogenous to that of low-intensity statins on TC (MD: -0.05 mmol/L; 95%CI: -0.28 to 0.18, P = 0.67) and LDL (MD: 0.03 mmol/L; 95%CI: -0.36 to 0.41, P = 0.89).
Metabolic syndrome (MetS) is denoted by the coexistence of insulin resistance, dyslipidemia, hypertension, and obesity[89,90]. A meta-analysis was conducted by Yuan et al[91] to decipher whether RYR preparations enhance clinical endpoints and decrease risk factors for MetS. The primary outcome points were mortality and major adverse cardiac events (MACEs), and the alternative outcome estimates were blood glucose, blood lipids, and blood pressure. Of the 30 articles included in the analysis, RYR preparations showed drastic improvement in MetS compared to controls. RYR preparations lowered mortality and MACEs (RR = 0.62, 95%CI: 0.49-0.78; RR = 0.54, 95%CI: 0.43-0.66). Regarding glucose metabolism, fasting plasma glucose (MD: -0.46 mmol/L, 95%CI: -0.71 to -0.22), hemoglobin A1c (MD: -0.49, 95%CI: -0.71 to -0.26), and the homeostasis model assessment of insulin resistance (MD: -0.93, 95%CI: -1.64 to -0.21) were reduced. Mean arterial blood pressure (MD: -3.79 mmHg, 95%CI: -5.01 to -2.57) was decreased. With respect to blood lipids, TC (MD: -0.74 mmol/L, 95%CI: -1.02 to -0.46), TG (MD: -0.45 mmol/L, 95%CI: -0.70 to -0.21), and LDL-C (MD: -0.42 mmol/L, 95%CI: -0.78 to -0.06) were reduced, while HDL (MD: 0.14 mmol/L, 95%CI: 0.09-0.20) increased. The incidence of adverse events did not increase with RYR preparations (RR = 1.00, 95%CI: 0.69-1.43).
Sungthong et al[92] conducted a meta-analysis to assess the efficacy of RYR extract on cardiovascular outcomes in patients with myocardial infarction (MI) and borderline hypercholesterolemia. Seven investigations with 10699 MI patients with borderline hypercholesterolemia were included, with follow-up periods that ranged from 4 weeks to 4.5 years. RYR extract (1200 mg per day) decreased nonfatal MI (RR = 0.42, 95%CI: 0.34-0.52), sudden death (RR = 0.71, 95%CI: 0.53-0.94), and revascularization (RR = 0.58, 95%CI: 0.48-0.71). RYR extract also reduced LDL [weighted MD (WMD) = -20.70 mg/dL, 95%CI: -24.51 to -16.90], TG (WMD = -24.69 mg/dL, 95%CI/L -34.36 to -15.03), and TC (WMD = -26.61 mg/dL, 95%CI: -31.65 to -21.58), and increased HDL levels (WMD = 2.71 mg/dL, 95%CI: 1.24-4.17).
Another meta-analysis was conducted by Wang et al[93] to study the effects of RYR in carotid atherosclerosis. Carotid plaque score (SCORE), carotid plaque area (AREA), and intima-media thickness were set as the primary endpoints, while safety indicators and lipid profile were set as the secondary outcomes. The analysis performed with 20 RCTs including 2217 patients showed that compared to the control group, intima-media thickness [standardized MD (SMD) = -0.588, 95%CI: -0.792 to -0.384, P < 0.001], AREA (SMD = -0.855, 95%CI: -1.259 to -0.451, P < 0.001), LDL-C (SMD = -0.938, 95%CI: -1.375 to -0.502, P < 0.001), SCORE (SMD = -0.708, 95%CI: -1.135 to -0.282, P = 0.001), TG (SMD = -0.766, 95%CI: -0.980 to -0.551, P < 0.001), and TC (SMD = -0.858, 95%CI: -1.254 to -0.462, P < 0.001) were significantly lowered and HDL-C (SMD = 0.389, 95%CI: 0.044-0.733, P = 0.027) was significantly enhanced following RYR therapy. Based on these results, the study concluded that RYR supplementation showed significant efficacy in the treatment of carotid atherosclerosis in the Chinese population.
Song et al[94] discovered that fluconazole, in combination with lovastatin, has complementary effects on the repression of planktonic Candida albicans (C. albicans) cells in vitro and can induce gene expression in the prenylation and ergosterol passageways. Zhou et al[95] in 2018 substantially authenticated that combining itraconazole and lovastatin is an effective strategy against planktonic C. albicans cells and biofilms, even in itraconazole-resistant strains.
Strains of C. albicans with dysfunctional ERG3 and ERG11 genes are sensitive to lovastatin monotherapy but resistant to itraconazole[96,97]. This investigation revealed that the drug combination is effective in the treatment of resistant fungal pathogens and has an evident antibacterial effect against C. albicans when combined with other antibacterial agents.
RYR is generally safe for consumption, with most patients experiencing little to no symptoms[12,63]. However, in some cases, consuming RYR can lead to myalgias similar in nature to the side effects of statin-based therapies, which vary from mild muscle discomfort to severe pain or myopathy[98,99]. Additionally, MK in RYR can cause hepatic dysfunction[100]. In prospective controlled trials, RYR extract combinations have not been associated with clinically apparent liver injury. However, there have been several case reports of liver injury in patients who took RYR extracts[101-103]. Both my
Studies have shown that RYR significantly reduces TC, LDL-C, and TG and provides a new, novel, and supplemental approach to lowering cholesterol in patients with low ASCVD risk who otherwise would not qualify for statin therapy as well as those with statin intolerance or strong patient preference[110,111]. Although effective, it should not be customarily used in place of standard treatments (ezetimibe, statins, and PCKS9 inhibitors) for which higher quality long-term data exist[112-114]. However, in the specific situations listed above, RYR may be considered as an alternative in managing mild to moderate dyslipidemia. If the treating physician decides to use RYR as the lipid lower agent for their patients, most studies have shown that 600 mg, 2 to 4 times daily, for a total of 1200 to 2400 mg for 4 to 12 weeks has proven clinical benefit.
Despite the established causative connections between LDL reduction and decreased cardiovascular mortality, there are few data regarding the long-term use of RYR for lipid reduction. Furthermore, the quantity of MK in commercial RYR preparations varies by brand and is commonly unreported. A 2017 review analyzed 28 brands of RYR products from mainstream retailers in the United States, and none of the products included MK on the label[115]. MK was not detected in two brands, and the quantity significantly varied among the other 26 products. Given this significant variability based on the manufacturer, patients may be insufficiently treated. As such, careful research by providers is needed to choose the appropriate RYR preparation to facilitate cholesterol reduction. Physicians should consider the recommendations provided in this article for the appropriate patients based on the clinical context. However, oneness still lies with the medical professional in making the right clinical decision that is in the best interest of the patient and correlates well with evidence-based medicine.
RYR is a suitable option for decreasing LDL-C levels in patients with mild-to-moderate hypercholesterolemia who are unable to implement lifestyle modifications, intolerant to statins, and ineligible for pharmacologic treatment. Given the large amount of commercially available RYR nutraceutical products with its limited regulation, physicians should do their appropriate research to choose the best available RYR preparation that is high quality with a suitably low dose of MK, produced under good manufacturing practices. Choosing the best preparations of RYR may limit adverse effects and provide consistent MK dosing, which produces better clinical outcomes. Additional research, including larger clinical trials, is needed to further assess the safety and efficacy of RYR in the management of dyslipidemia.
| 1. | Balling M, Afzal S, Davey Smith G, Varbo A, Langsted A, Kamstrup PR, Nordestgaard BG. Elevated LDL Triglycerides and Atherosclerotic Risk. J Am Coll Cardiol. 2023;81:136-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Mortensen MB, Dzaye O, Bøtker HE, Jensen JM, Maeng M, Bentzon JF, Kanstrup H, Sørensen HT, Leipsic J, Blankstein R, Nasir K, Blaha MJ, Nørgaard BL. Low-Density Lipoprotein Cholesterol Is Predominantly Associated With Atherosclerotic Cardiovascular Disease Events in Patients With Evidence of Coronary Atherosclerosis: The Western Denmark Heart Registry. Circulation. 2023;147:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 3. | Muscella A, Stefàno E, Marsigliante S. The effects of exercise training on lipid metabolism and coronary heart disease. Am J Physiol Heart Circ Physiol. 2020;319:H76-H88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 4. | Shamim S, Al Badarin FJ, DiNicolantonio JJ, Lavie CJ, O'Keefe JH. Red yeast rice for dysipidemia. Mo Med. 2013;110:349-354. [PubMed] |
| 5. | Li Y, Jiang L, Jia Z, Xin W, Yang S, Yang Q, Wang L. A meta-analysis of red yeast rice: an effective and relatively safe alternative approach for dyslipidemia. PLoS One. 2014;9:e98611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Vrolijk MF, van de Koppel S, van Hunsel F. Red yeast rice (Monascus purpureus) supplements: Case series assessment of spontaneously reported cases to The Netherlands Pharmacovigilance Centre Lareb. Br J Clin Pharmacol. 2021;87:2146-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Gawborisut S, Muengkratok S. Red Yeast Rice and Optimal Fermentation Periods Improve the Quality of Esan Fermented Fish Sausage. Int J Food Sci. 2024;2024:4831279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Liasi E, Kantilafti M, Hadjimbei E, Chrysostomou S. Monacolin K supplementation in patients with hypercholesterolemia: A systematic review of clinical trials. Semergen. 2024;50:102156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Hachem R, Assemat G, Balayssac S, Martins-Froment N, Gilard V, Martino R, Malet-Martino M. Comparative Chemical Profiling and Monacolins Quantification in Red Yeast Rice Dietary Supplements by (1)H-NMR and UHPLC-DAD-MS. Molecules. 2020;25:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Jirasatid S, Nopharatana M, Kitsubun P, Tongta A. Degradation kinetics of monacolin K in red yeast rice powder using multiresponse modeling approach. J Food Eng. 2013;116:436-443. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Burke FM. Red yeast rice for the treatment of dyslipidemia. Curr Atheroscler Rep. 2015;17:495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert-Remy U, Kuhnle GG, Lambré C, Leblanc JC, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens-Berendsen I, Woutersen RA, Andrade RJ, Fortes C, Mosesso P, Restani P, Pizzo F, Smeraldi C, Wright M. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018;16:e05368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Zhu B, Qi F, Wu J, Yin G, Hua J, Zhang Q, Qin L. Red Yeast Rice: A Systematic Review of the Traditional Uses, Chemistry, Pharmacology, and Quality Control of an Important Chinese Folk Medicine. Front Pharmacol. 2019;10:1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Heber D, Lembertas A, Lu QY, Bowerman S, Go VL. An analysis of nine proprietary Chinese red yeast rice dietary supplements: implications of variability in chemical profile and contents. J Altern Complement Med. 2001;7:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Murata Y, Hemmi S, Akiya Y, Miyasato K, Kobayashi H, Maruyama T, Abe M. Certain Red Yeast Rice Supplements in Japan Cause Acute Tubulointerstitial Injury. Kidney Int Rep. 2024;9:2824-2828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 16. | Raguzzini A, Toti E, Palmery M, Abdel-Daim MM, Peluso I. Dietary Habits and Musculoskeletal Pain in Statin and Red Yeast Rice Users: A Pilot Study. Eur J Investig Health Psychol Educ. 2021;11:1156-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Chen G, Chen W, Xu J, Ma G, Hu X, Chen G. The current trend and challenges of developing red yeast rice-based food supplements for hypercholesterolemia. J Future Foods. 2023;3:312-329. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Klimek M, Wang S, Ogunkanmi A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. P T. 2009;34:313-327. [PubMed] |
| 19. | Prasad GV, Wong T, Meliton G, Bhaloo S. Rhabdomyolysis due to red yeast rice (Monascus purpureus) in a renal transplant recipient. Transplantation. 2002;74:1200-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Mazza A, Schiavon L, Rigatelli G, Torin G, Montanaro F, Lenti S. The short-term supplementation of monacolin K improves the lipid and metabolic patterns of hypertensive and hypercholesterolemic subjects at low cardiovascular risk. Food Funct. 2018;9:3845-3852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Monu M, Sehrawat KD, Singh A, Chaudhary G, Bamal D, Sehrawat AR. An overview on the therapeutic potential and anticancer mechanism of Monacolin K / Lovastatin. Pharmacol Res Mod Chin Med. 2022;5:100187. [DOI] [Full Text] |
| 22. | Xiong Z, Cao X, Wen Q, Chen Z, Cheng Z, Huang X, Zhang Y, Long C, Zhang Y, Huang Z. An overview of the bioactivity of monacolin K / lovastatin. Food Chem Toxicol. 2019;131:110585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Lee C, Wang J, Pan T. Synchronous Analysis Method for Detection of Citrinin and the Lactone and Acid Forms ofMonacolin K in Red Mold Rice. J AOAC Int. 2006;89:669-677. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Angelopoulos N, Paparodis RD, Androulakis I, Boniakos A, Argyrakopoulou G, Livadas S. Low Dose Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts Lowers LDL Cholesterol in Patients with Mild Dyslipidemia: A Multicenter, Randomized Controlled Trial. Nutrients. 2023;15:2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Vyas KP, Kari PH, Pitzenberger SM, Halpin RA, Ramjit HG, Arison B, Murphy JS, Hoffman WF, Schwartz MS, Ulm EH. Biotransformation of lovastatin. I. Structure elucidation of in vitro and in vivo metabolites in the rat and mouse. Drug Metab Dispos. 1990;18:203-211. [PubMed] |
| 26. | Klingelhöfer I, Morlock GE. Lovastatin in lactone and hydroxy acid forms and citrinin in red yeast rice powders analyzed by HPTLC-UV/FLD. Anal Bioanal Chem. 2019;411:6655-6665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Alberts AW. Discovery, biochemistry and biology of lovastatin. Am J Cardiol. 1988;62:10J-15J. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 239] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Walther U, Emmrich K, Ramer R, Mittag N, Hinz B. Lovastatin lactone elicits human lung cancer cell apoptosis via a COX-2/PPARγ-dependent pathway. Oncotarget. 2016;7:10345-10362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Steiner S, Gatlin CL, Lennon JJ, McGrath AM, Aponte AM, Makusky AJ, Rohrs MC, Anderson NL. Proteomics to display lovastatin-induced protein and pathway regulation in rat liver. Electrophoresis. 2000;21:2129-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Yang DJ, Hwang LS. Study on the conversion of three natural statins from lactone forms to their corresponding hydroxy acid forms and their determination in Pu-Erh tea. J Chromatogr A. 2006;1119:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Zhou J, Zhou D. Improvement of oral bioavailability of lovastatin by using nanostructured lipid carriers. Drug Des Devel Ther. 2015;9:5269-5275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Cicero AFG. [Red yeast rice, monacolin K, and pleiotropic effects.]. Recenti Prog Med. 2018;109:154e-157e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Cerqueira NM, Oliveira EF, Gesto DS, Santos-Martins D, Moreira C, Moorthy HN, Ramos MJ, Fernandes PA. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry. 2016;55:5483-5506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 34. | Russell DW. Cholesterol biosynthesis and metabolism. Cardiovasc Drugs Ther. 1992;6:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Lachenmeier DW, Monakhova YB, Kuballa T, Löbell-Behrends S, Maixner S, Kohl-Himmelseher M, Waldner A, Steffen C. NMR evaluation of total statin content and HMG-CoA reductase inhibition in red yeast rice (Monascus spp.) food supplements. Chin Med. 2012;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Gojkovic T, Vladimirov S, Kotur-Stevuljevic J, Bogavac-Stanojevic N, Zeljkovic A, Vekic J, Antonic T, Spasojevic-Kalimanovska V. Effects of monacolin K-containing nutraceutical on cholesterol homeostasis re-establishment and CVD risk reduction in hypercholesterolemic subjects. Eur Rev Med Pharmacol Sci. 2021;25:5261-5267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Islam MM, Hlushchenko I, Pfisterer SG. Low-Density Lipoprotein Internalization, Degradation and Receptor Recycling Along Membrane Contact Sites. Front Cell Dev Biol. 2022;10:826379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Heinz T, Schuchardt JP, Möller K, Hadji P, Hahn A. Low daily dose of 3 mg monacolin K from RYR reduces the concentration of LDL-C in a randomized, placebo-controlled intervention. Nutr Res. 2016;36:1162-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Chen CH, Yang JC, Uang YS, Lin CJ. Improved dissolution rate and oral bioavailability of lovastatin in red yeast rice products. Int J Pharm. 2013;444:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Righetti L, Dall'Asta C, Bruni R. Risk Assessment of RYR Food Supplements: Perception vs. Reality. Front Nutr. 2021;8:792529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Prasad K, Mishra M. Mechanism of Hypercholesterolemia-Induced Atherosclerosis. Rev Cardiovasc Med. 2022;23:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Watts GF, Gidding SS, Hegele RA, Raal FJ, Sturm AC, Jones LK, Sarkies MN, Al-Rasadi K, Blom DJ, Daccord M, de Ferranti SD, Folco E, Libby P, Mata P, Nawawi HM, Ramaswami U, Ray KK, Stefanutti C, Yamashita S, Pang J, Thompson GR, Santos RD. International Atherosclerosis Society guidance for implementing best practice in the care of familial hypercholesterolaemia. Nat Rev Cardiol. 2023;20:845-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 151] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 43. | Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2919] [Article Influence: 224.5] [Reference Citation Analysis (0)] |
| 44. | Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1331] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 45. | Pergolizzi JV Jr, Coluzzi F, Colucci RD, Olsson H, LeQuang JA, Al-Saadi J, Magnusson P. Statins and muscle pain. Expert Rev Clin Pharmacol. 2020;13:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Tournadre A. Statins, myalgia, and rhabdomyolysis. Joint Bone Spine. 2020;87:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Jacobson TA, Zimmerman FH. Fibrates in combination with statins in the management of dyslipidemia. J Clin Hypertens (Greenwich). 2006;8:35-41; quiz 42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Pradhan A, Bhandari M, Sethi R. Ezetimibe and Improving Cardiovascular Outcomes: Current Evidence and Perspectives. Cardiol Res Pract. 2020;2020:9815016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:597-613, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Hsueh TP, Lin WL, Hu WL, Hung YC. Red Yeast Rice and Statin Therapy in Patients with Hypercholesterolemia and the Comorbidities: A Retrospective Cohort Study on Lipid-Lowering Effects and Cardiovascular Outcomes. Am J Chin Med. 2024;52:417-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839, W147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 52. | Peng D, Fong A, Pelt AV. Original Research: The Effects of Red Yeast Rice Supplementation on Cholesterol Levels in Adults. Am J Nurs. 2017;117:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Lu Z, Kou W, Du B, Wu Y, Zhao S, Brusco OA, Morgan JM, Capuzzi DM; Chinese Coronary Secondary Prevention Study Group, Li S. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol. 2008;101:1689-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 54. | Lin CC, Li TC, Lai MM. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. Eur J Endocrinol. 2005;153:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Gheith O, Sheashaa H, Abdelsalam M, Shoeir Z, Sobh M. Efficacy and safety of Monascus purpureus Went rice in subjects with secondary hyperlipidemia. Clin Exp Nephrol. 2008;12:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fønnebø V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chin Med. 2006;1:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Gerards MC, Terlou RJ, Yu H, Koks CH, Gerdes VE. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain - a systematic review and meta-analysis. Atherosclerosis. 2015;240:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 58. | Venero CV, Venero JV, Wortham DC, Thompson PD. Lipid-lowering efficacy of red yeast rice in a population intolerant to statins. Am J Cardiol. 2010;105:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Halbert SC, French B, Gordon RY, Farrar JT, Schmitz K, Morris PB, Thompson PD, Rader DJ, Becker DJ. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 60. | Dujovne CA. Red Yeast Rice Preparations: Are They Suitable Substitutions for Statins? Am J Med. 2017;130:1148-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Stefanutti C, Mazza F, Mesce D, Morozzi C, Di Giacomo S, Vitale M, Pergolini M. Monascus purpureus for statin and ezetimibe intolerant heterozygous familial hypercholesterolaemia patients: A clinical study. Atheroscler Suppl. 2017;30:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 62. | Fogacci F, Giovannini M, Di Micoli V, Grandi E, Veronesi M, Borghi C, Cicero AFG. Evaluation of the effect of a dietary supplementation with a red yeast rice and fish oil-containing nutraceutical on lipid pattern, high sensitivity C-reactive protein, and endothelial function in moderately hypercholesterolaemic subjects: a double-blind, placebo-controlled, randomized clinical trial. Arch Med Sci Atheroscler Dis. 2023;8:e182-e189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 63. | Norata GD, Banach M. The Impact of Red Yeast Rice Extract Use on the Occurrence of Muscle Symptoms and Liver Dysfunction: An Update from the Adverse Event Reporting Systems and Available Meta-Analyses. Nutrients. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 64. | Ong YC, Aziz Z. Systematic review of red yeast rice compared with simvastatin in dyslipidaemia. J Clin Pharm Ther. 2016;41:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Minamizuka T, Koshizaka M, Shoji M, Yamaga M, Hayashi A, Ide K, Ide S, Kitamoto T, Sakamoto K, Hattori A, Ishikawa T, Kobayashi J, Maezawa Y, Kobayashi K, Takemoto M, Inagaki M, Endo A, Yokote K. Low dose red yeast rice with monacolin K lowers LDL cholesterol and blood pressure in Japanese with mild dyslipidemia: A multicenter, randomized trial. Asia Pac J Clin Nutr. 2021;30:424-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 66. | Zhao F, Chen L, Jiang Y, Guo Y, Lu L, Lu C, Xue X, Liu X, Jin X, Liu J, Chen K. Red yeast rice preparations for dyslipidemia: An overview of systematic reviews and network meta-analysis. J Funct Foods. 2023;104:105508. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 67. | Protic O, Bonfigli AR, Antonicelli R. Nutraceutical Combinations in Hypercholesterolemia: Evidence from Randomized, Placebo-Controlled Clinical Trials. Nutrients. 2021;13:3128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Santini A, Novellino E. Nutraceuticals in hypercholesterolaemia: an overview. Br J Pharmacol. 2017;174:1450-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 69. | McCarty MF, O'Keefe JH, DiNicolantonio JJ. Red Yeast Rice Plus Berberine: Practical Strategy for Promoting Vascular and Metabolic Health. Altern Ther Health Med. 2015;21 Suppl 2:40-45. [PubMed] |
| 70. | Wang C, Chen J, Tian W, Han Y, Xu X, Ren T, Tian C, Chen C. Natto: A medicinal and edible food with health function. Chin Herb Med. 2023;15:349-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 71. | Chen H, McGowan EM, Ren N, Lal S, Nassif N, Shad-Kaneez F, Qu X, Lin Y. Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark Insights. 2018;13:1177271918785130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 72. | Afzaal M, Saeed F, Islam F, Ateeq H, Asghar A, Shah YA, Ofoedu CE, Chacha JS. Nutritional Health Perspective of Natto: A Critical Review. Biochem Res Int. 2022;2022:5863887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | Yang NC, Chou CW, Chen CY, Hwang KL, Yang YC. Combined nattokinase with red yeast rice but not nattokinase alone has potent effects on blood lipids in human subjects with hyperlipidemia. Asia Pac J Clin Nutr. 2009;18:310-317. [PubMed] |
| 74. | Liu M, Xu Z, Wang Z, Wang D, Yang M, Li H, Zhang W, He R, Cheng H, Guo P, Li Z, Liang H. Lipid-lowering, antihypertensive, and antithrombotic effects of nattokinase combined with red yeast rice in patients with stable coronary artery disease: a randomized, double-blinded, placebo-controlled trial. Front Nutr. 2024;11:1380727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 75. | Liu X, Zeng X, Mahe J, Guo K, He P, Yang Q, Zhang Z, Li Z, Wang D, Zhang Z, Wang L, Jing L. The Effect of Nattokinase-Monascus Supplements on Dyslipidemia: A Four-Month Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2023;15:4239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 76. | Gouni-Berthold I, Berthold HK. Policosanol: clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am Heart J. 2002;143:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 77. | Cicero AF, Brancaleoni M, Laghi L, Donati F, Mino M. Antihyperlipidaemic effect of a Monascus purpureus brand dietary supplement on a large sample of subjects at low risk for cardiovascular disease: a pilot study. Complement Ther Med. 2005;13:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Cicero AFG, Benvenuti C; ARMoweb study Group. Efficacy of a red yeast rice based nutraceutical in large subgroups of hypercholesterolemic subjects in every day clinical practice. Med J Nutrition Metab. 2010;3:239-246. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Li Z, Wang Y, Xu Q, Ma J, Li X, Yan J, Tian Y, Wen Y, Chen T. Berberine and health outcomes: An umbrella review. Phytother Res. 2023;37:2051-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 80. | Abidi P, Zhou Y, Jiang JD, Liu J. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol. 2005;25:2170-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 81. | Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 959] [Cited by in RCA: 1068] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 82. | Garnett DJ, Greenhough TJ. Statins cause profound effects on gene expression in human cancer cells in vitro: the role of membrane microdomains. Gene Expr. 2012;15:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Chen C, Yu Z, Li Y, Fichna J, Storr M. Effects of berberine in the gastrointestinal tract - a review of actions and therapeutic implications. Am J Chin Med. 2014;42:1053-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 84. | Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan J, Sun G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;161:69-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 321] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 85. | Millán J, Cicero AF, Torres F, Anguera A. Effects of a nutraceutical combination containing berberine (BRB), policosanol, and red yeast rice (RYR), on lipid profile in hypercholesterolemic patients: A meta-analysis of randomised controlled trials. Clin Investig Arterioscler. 2016;28:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Pirro M, Mannarino MR, Bianconi V, Simental-Mendía LE, Bagaglia F, Mannarino E, Sahebkar A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016;110:76-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 87. | Cicero AFG, Fogacci F, Rosticci M, Parini A, Giovannini M, Veronesi M, D'Addato S, Borghi C. Effect of a short-term dietary supplementation with phytosterols, red yeast rice or both on lipid pattern in moderately hypercholesterolemic subjects: a three-arm, double-blind, randomized clinical trial. Nutr Metab (Lond). 2017;14:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Li P, Wang Q, Chen K, Zou S, Shu S, Lu C, Wang S, Jiang Y, Fan C, Luo Y. Red Yeast Rice for Hyperlipidemia: A Meta-Analysis of 15 High-Quality Randomized Controlled Trials. Front Pharmacol. 2021;12:819482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 89. | Hayden MR. Overview and New Insights into the Metabolic Syndrome: Risk Factors and Emerging Variables in the Development of Type 2 Diabetes and Cerebrocardiovascular Disease. Medicina (Kaunas). 2023;59:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 90. | Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 623] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 91. | Yuan R, Yuan Y, Wang L, Xin Q, Wang Y, Shi W, Miao Y, Leng SX, Chen K, Cong W; and BPNMI Consortium. Red Yeast Rice Preparations Reduce Mortality, Major Cardiovascular Adverse Events, and Risk Factors for Metabolic Syndrome: A Systematic Review and Meta-analysis. Front Pharmacol. 2022;13:744928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Sungthong B, Yoothaekool C, Promphamorn S, Phimarn W. Efficacy of red yeast rice extract on myocardial infarction patients with borderline hypercholesterolemia: A meta-analysis of randomized controlled trials. Sci Rep. 2020;10:2769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Wang S, Chen Y, Wang R, Ma B, Wang Z, Tang G, Wang S, He Y, Qu L. Effectiveness of red yeast rice on carotid atherosclerosis: A systematic review and meta-analysis. Front Pharmacol. 2022;13:937809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 94. | Song JL, Lyons CN, Holleman S, Oliver BG, White TC. Antifungal activity of fluconazole in combination with lovastatin and their effects on gene expression in the ergosterol and prenylation pathways in Candida albicans. Med Mycol. 2003;41:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Zhou Y, Yang H, Zhou X, Luo H, Tang F, Yang J, Alterovitz G, Cheng L, Ren B. Lovastatin synergizes with itraconazole against planktonic cells and biofilms of Candida albicans through the regulation on ergosterol biosynthesis pathway. Appl Microbiol Biotechnol. 2018;102:5255-5264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | Zhou Y, Liao M, Zhu C, Hu Y, Tong T, Peng X, Li M, Feng M, Cheng L, Ren B, Zhou X. ERG3 and ERG11 genes are critical for the pathogenesis of Candida albicans during the oral mucosal infection. Int J Oral Sci. 2018;10:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Henry KW, Nickels JT, Edlind TD. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob Agents Chemother. 2000;44:2693-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 169] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 98. | Lapi F, Gallo E, Bernasconi S, Vietri M, Menniti-Ippolito F, Raschetti R, Gori L, Firenzuoli F, Mugelli A, Vannacci A. Myopathies associated with red yeast rice and liquorice: spontaneous reports from the Italian Surveillance System of Natural Health Products. Br J Clin Pharmacol. 2008;66:572-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Becker DJ, French B, Morris PB, Silvent E, Gordon RY. Phytosterols, red yeast rice, and lifestyle changes instead of statins: a randomized, double-blinded, placebo-controlled trial. Am Heart J. 2013;166:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Likhitsup A, Chen VL, Fontana RJ. Estimated Exposure to 6 Potentially Hepatotoxic Botanicals in US Adults. JAMA Netw Open. 2024;7:e2425822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 101. | Kurnik M, Markelj T, Žgavc B, Hudournik B, Meznarič M, Podbregar M. Fulminant Red Yeast Rice-Associated Rhabdomyolysis with Acute Liver Injury and Hyperkalemia Treated with Extracorporeal Blood Purification Using CytoSorb. Drug Healthc Patient Saf. 2025;17:109-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 102. | García-García MD, Bellido Muñoz F, Cordero Ruiz P, Fernández Álvarez P, Carmona Soria MI, Caunedo Álvarez Á. Drug-induced liver injury associated to red yeast rice. Rev Esp Enferm Dig. 2024;116:384-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 103. | Santos S, Gomes S, Carvalho I, Bonito I, Carmo C. Rhabdomyolysis Related to Red Yeast Rice Ingestion. Cureus. 2023;15:e33532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Chen CH, Uang YS, Wang ST, Yang JC, Lin CJ. Interaction between Red Yeast Rice and CYP450 Enzymes/P-Glycoprotein and Its Implication for the Clinical Pharmacokinetics of Lovastatin. Evid Based Complement Alternat Med. 2012;2012:127043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Avula B, Cohen PA, Wang YH, Sagi S, Feng W, Wang M, Zweigenbaum J, Shuangcheng M, Khan IA. Chemical profiling and quantification of monacolins and citrinin in red yeast rice commercial raw materials and dietary supplements using liquid chromatography-accurate QToF mass spectrometry: Chemometrics application. J Pharm Biomed Anal. 2014;100:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 106. | Childress L, Gay A, Zargar A, Ito MK. Review of red yeast rice content and current Food and Drug Administration oversight. J Clin Lipidol. 2013;7:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | Doi T, Shimizu A, Morimoto E, Morii K, Okubo A, Mizuiri S, Nishizawa Y, Masaki T. A Case of Acute Kidney Injury and Fanconi Syndrome Caused by a Red Yeast Rice Supplement. Intern Med. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Yoshikawa Y, Anzai H, Odajima K, Asakawa S, Arai S, Yamazaki O, Tamura Y, Ohashi R, Shibata S, Fujigaki Y. Fanconi syndrome and renal tubular necrosis in patients following ingestion of potentially contaminated red yeast rice supplement: Two case reports. Physiol Rep. 2024;12:e70049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 109. | Kawai Y, Ozawa M, Isomura A, Mitsuhashi H, Yamaguchi S, Nagayama S, Tanaka S, Abe E, Saka S, Nagahama K, Iwamoto T, Tamura K. A case of Fanconi syndrome that developed following a year of consumption of a red yeast rice supplement. CEN Case Rep. 2025;14:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 110. | Bogsrud MP, Ose L, Langslet G, Ottestad I, Strøm EC, Hagve TA, Retterstøl K. HypoCol (red yeast rice) lowers plasma cholesterol - a randomized placebo controlled study. Scand Cardiovasc J. 2010;44:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 111. | Verhoeven V, Van der Auwera A, Van Gaal L, Remmen R, Apers S, Stalpaert M, Wens J, Hermans N. Can red yeast rice and olive extract improve lipid profile and cardiovascular risk in metabolic syndrome?: A double blind, placebo controlled randomized trial. BMC Complement Altern Med. 2015;15:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 112. | Katsiki N, Theocharidou E, Karagiannis A, Athyros VG, Mikhailidis DP. Ezetimibe therapy for dyslipidemia: an update. Curr Pharm Des. 2013;19:3107-3114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 113. | Azemawah V, Movahed MR, Centuori P, Penaflor R, Riel PL, Situ S, Shadmehr M, Hashemzadeh M. State of the Art Comprehensive Review of Individual Statins, Their Differences, Pharmacology, and Clinical Implications. Cardiovasc Drugs Ther. 2019;33:625-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 114. | Coppinger C, Movahed MR, Azemawah V, Peyton L, Gregory J, Hashemzadeh M. A Comprehensive Review of PCSK9 Inhibitors. J Cardiovasc Pharmacol Ther. 2022;27:10742484221100107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 115. | Cohen PA, Avula B, Khan IA. Variability in strength of red yeast rice supplements purchased from mainstream retailers. Eur J Prev Cardiol. 2017;24:1431-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/