Published online Sep 26, 2025. doi: 10.12998/wjcc.v13.i27.104916
Revised: March 25, 2025
Accepted: July 2, 2025
Published online: September 26, 2025
Processing time: 200 Days and 9.9 Hours
Papillary thyroid cancer (PTC) often recurs following surgical excision, necessitating reliable long-term screening techniques after initial management. Ultrasound scans have a poor predictive value and biopsy and genetic testing have a low sensitivity. Biomarker detection, including thyroglobulin, has reduced accuracy as residual thyroid tissue remains following surgery. Serum/tissue microRNA detection offers a promising alternative to screen for thyroid malignancy. Based on our previous systematic review, miR-146, miR-221 and miR-222 appear most strongly associated with PTC.
To perform a systematic review and meta-analysis, evaluating the use of circulating miR-146, miRNA-221 and miR-222 in PTC diagnosis and staging.
A systematic literature search of MEDLINE, Scopus and the EMBASE library was performed. Human participants of any age, sex or geographical distribution were considered. Original studies assessing the diagnostic and prognostic accuracy of circulating serum miRNAs in histologically-confirmed PTC were included. Proportion and regression meta-analyses (logit-transformed) were conducted. PRISMA guidelines were followed throughout the process.
Among the 1530 studies screened, 6 met the inclusion criteria, reporting non-overlapping populations. For the diagnosis of PTC vs benign nodules (BN), the pooled sensitivity of miR-146 was 80.7% (95%CI: 65.2%-90.4%), specificity was 66.9% (95%CI: 55.5%-76.6%), and false positive rate was 33.1% (95%CI: 23.4%-44.5%). Pooled sensitivity, specificity and false positive rate of miR-222 for diagnosis of PTC vs BN was 64.3% (95%CI: 50.3%-76.2%), 88.8% (95%CI: 82.4%-93%) and 11.2% (95%CI: 7%-17.6%) respectively. Pooled sensitivity, specificity and false positive rate of miR-221 in this population demonstrated reduced accuracy. Pooled sensitivity and specificity of PTC vs healthy controls for total serum miRNAs were 82% (95%CI: 77%-86%) and 84% (95%CI: 76%-90%) respectively. The summary area under receiver operating characteristic curve value for the same analysis was 0.89 (95%CI: 0.86-0.92).
miRNA-146 and miRNA-222 were most sensitive, validating their efficacy in PTC diagnosis. Larger studies are needed for confident population generalisability. Use of two-MRNA types in conjunction needs to be assessed.
Core Tip: While miRNA levels are strongly associated with papillary thyroid cancer (PTC), no consensus has been reached on their diagnostic accuracy for the condition. This is the first systematic review and meta-analysis assessing statistical strength of miRNA in the diagnosis and staging of PTC. We found that miRNA-146 and miRNA-222 were most sensitive, validating their efficacy in PTC diagnosis.
- Citation: Dean B, Geropoulos G, Richardson-Jones T, Fornasiero M, Papapanou M, Konstantinidis C, Madouros N, Spinos D, Koimtzis G, Giannis D, Athanasiou C, Psarras K. Diagnostic accuracy of circulating miR-146, miR-221 and miR-222 in papillary thyroid cancer: A systematic review and meta-analysis. World J Clin Cases 2025; 13(27): 104916
- URL: https://www.wjgnet.com/2307-8960/full/v13/i27/104916.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i27.104916
It has been well established that papillary thyroid cancer (PTC) is the most common cancer of the thyroid gland with an incidence increase of 174% since the early 1990’s[1-3]. Whilst its prognosis is good, a large proportion of patients are likely to experience recurrence emphasizing the need to develop alternative screening methods[4]. Ultrasound scans are routinely used as a first-line technique for screening and diagnosis of PTC, however their predictive value remains relatively inconsistent when used in conjunction with clinical symptoms alone; ranging from 30% to 48%[5]. The gold standard investigation for definitive diagnosis is a fine needle aspiration (FNA) biopsy of the suspicious thyroid nodule(s); with intermediate and non-diagnostic findings reported as high as 30%[6].
It has been demonstrated that molecular DNA and RNA testing of known mutations can enhance the diagnostic accuracy of thyroid cancer in addition to the cytological evaluation of FNA. Most PTCs and over 80% of patients with recurrent metastatic PTC have kinase-activating mutations in the V-Raf murine sarcoma viral oncogene homolog B1 (BRAF) and telomerase reverse transcriptase (TERT) promoter[7,8]. Together, BRAF V600E and TERT promoter mutations seem to have a potent combined effect on PTC aggression[9]. Despite their diagnostic value, the sensitivity of these mutations in helping to differentiate malignant from benign disease in the first instance is limited.
Growing research exploring the prognostic utility of serum and/or tissue biomarker levels for the assessment of persistent or recurrent PTC has been undertaken; the main currently being serum thyroglobulin[10,11]. The specificity of thyroglobulin as a surrogate is, however, dependent on patients having undergone a total thyroidectomy with absence of anti-thyroglobulin autoantibodies. Given the recent decline of total thyroidectomy in the treatment of thyroid cancer, many patients are left with residual thyroid tissue which is problematic in restricting accuracy of this test[12,13].
In light of this, more recent research into microRNAs (miRNAs) has shown them to be a potentially promising alternative. These small, non-coding RNAs regulate gene expression and control various cellular processes, including differentiation, cell cycle progression and apoptosis[14]. An established role in the context of malignancy has shown them to be relevant in not only oncogenesis but defining specific subtypes, patient survival and treatment response[15]. Their implication in thyroid cancer specifically has been consistently observed with increases in serum/tissue levels when compared to those with benign disease and/or healthy controls (HC)[16,17].
In our previous systematic review, we identified 108 circulating serum miRNAs with miR-146, miRNA-221 and miR-222 being distinguished amongst the literature as showing the strongest affiliation with PTC. With some studies reporting specificity of miRNA-221-3p being as high as 100% and sensitivity of miRNA-146b being 94.3%, we proposed that miRNA assay of the identified miRNAs poses a promising minimally invasive diagnostic alternative to the current aforementioned standards[18]. In this meta-analysis we set out to provide a definitive answer to the use of miR-146, miRNA-221 and miR-222 in the diagnosis and staging of PTC.
This systematic review was performed according to the PRISMA guidelines[19] after review and approval of the protocol by all authors. A literature search, (last conducted: 1st July, 2024) was performed by two researchers (Michail Papapanou and Christos Konstantinidis) in PubMed (MEDLINE), Scopus and EMBASE with variations of the following search term: (plasma OR biomarker OR serum OR sera OR blood OR peripheral) AND (miRNAs OR miR OR microRNA OR exosomal OR exosomes) AND (thyroid OR ptc) AND (146 OR 221 OR 222). A manual search was also performed according to the “snowball” methodology to identify any relevant studies in the references’ list of the included articles. The articles that included met the following criteria: (1) They involved human samples; (2) They assessed the diagnostic and prognostic accuracy of circulating serum miRNAs for the detection of PTC comparing to the levels of the same type of miRNAs in the blood of patients with a benign nodule(s) (BN) and/or HC; (3) They used the results of the histopathological reports to confirm the diagnosis; (4) They were original studies; (5) They reported the sensitivity and specificity of the miRNAs that were studied; and (6) There were no limitations for sex, age, or geographical distribution.
The exclusion criteria were the following: (1) No full articles; (2) Case reports, errata, comments, perspectives, letters to the editor, editorials that did not provide any primary patient data; (3) In vitro or animal studies; (4) Repeated research publications/duplication; (5) The expression of miRNAs was detected within tumor tissues or other body fluids; (6) Literature with insufficient and overlap data; (7) In vitro and in vivo studies; and (8) Non-English articles.
Two researchers (Georgios Geropoulos and Nikolaos Madouros) independently screened the literature and extracted the data according to the inclusion and exclusion criteria described above[19]. They extracted primary data from the studies, including first author, publication year, region, number of patients with PTC, BN and HC, patients’ age, patients’ gender and total miRNAs tested in the particular study. In addition, the characteristics of the thyroid tumor, such as its size, its multifocality, lymph node metastasis, its extrathyroidal extension and its metastases, as well as the recurrency were recorded. Moreover, the performance indices of miR-146, miR-221 and miR-222 were also extracted from each included article, including each miRNAs’ diagnostic sensitivity, specificity and false positive rate, comparing these parameters between PTC, BN and HC. In the present meta-analysis, if an article studied more than one miRNAs tests, we considered each miRNA test to be an independent study.
The included studies were assessed by the standardized Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool[20]. Patient selection, index test, reference standard and flow of the patients of each study were assessed for risk of bias and applicability concerns as indicated by the QUADAS-2 questionnaire. Histopathological examination was considered as the index test and circulating miRNA was used as the reference standard. In the domain of flow and timing, we defined as the confirmation of PTC presence when histopathological examination took place after the measuring circulating miRNAs. The QUADAS-2 tool was applied on each study by two independent reviewers (Michail Papapanou and Christos Konstantinidis). Discrepancies between the reviewers (Michail Papapanou and Christos Konstantinidis) were discussed and resolved, while a third reviewer (Georgios Geropoulos or Dimitrios Giannis) was consulted if needed[20].
The data were extracted from the included studies and then used to construct a series of 2 × 2 tables (including true positives, false positives, false negatives, and true negatives). First, the summary sensitivity and specificity, the false positive rates were calculated to examine the pooled diagnostic accuracy of circulating serum miRNAs in PTC detection. In addition, the data from different studies included in our analysis were simultaneously used to construct the summary area under receiver operating characteristic curve. Statistical analysis was performed using Stata 15.0 (STATA, College Station, TX, United States)[21].
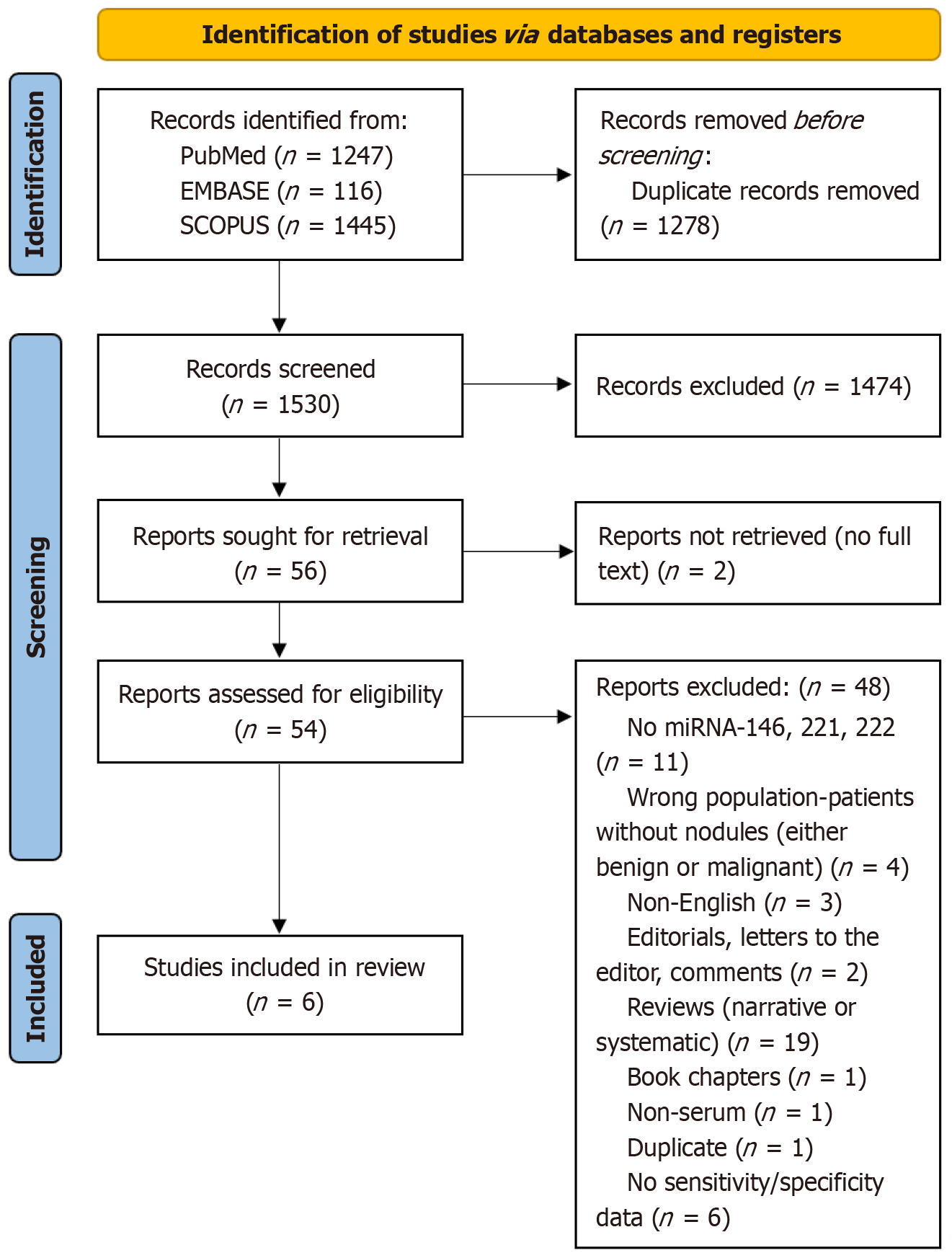
Our initial literature search yielded 166 unique articles, of which 54 articles were considered to be relevant and underwent full-text assessment. Following removal of the irrelevant, and non-eligible studies, six studies were included in this systematic review and meta-analysis project (Figure 1, Table 1)[22-27].
| Ref. | Year | Country | Total PTC/BN/HC cases (n) | Male/females (n) | Age | miRNA tested |
| Lee et al[26] | 2014 | Korea | 70/19/0 | 15/74 | BN: 49.79 ± 12.49, PTC (no nodal involvement) 51.4 ± 12.66, PTC (with nodal involvement) 51.51 ± 15.34 | miR-146b, miR-221, miR-222, miR-155 |
| Zhang et al[24] | 2017 | China | 85/35/40 | 50/110 | PTC-49 were < 45 years, 36 were ≥ 45 years. BN: 21 were < 45 years and 19 were ≥ 45 years | miR-146b, miR-221, miR-222, miR-16 |
| Zhang et al[27] | 2018 | China | 58/35/40 | NG | PTMC: 26 were < 45 years, 32 were ≥ 45 years. PTC: 31 were < 45 years, 16 were ≥ 45 years | miR-146b, miR-221, miR-222, miR-16, miR-21 |
| Rosignolo et al[23] | 2017 | Italy | 44/19/20 | NG | NG | mi-146a-5p, miR-221-3p, miR-222-3p, miR-146b-5p, miR-191-5p, miR-103a-3p, miR-28-3p |
| Jiang et al[25] | 2020 | China | 64/0/0 | 14/50 | PTC without LNM: 42.2 ± 13. PTC with LNM: 41.0 ± 12.7 | miR-21-52, miR-146b-5p, miR-204-5p, miR-221-3p, miR-222-3p, miR-451a, miR-7-5p, miR-30a-3p, miR-138-5p, miR-199a-3p |
| Yu et al[22] | 2012 | China/Canada | 106/95/44 | 61/184 | PTC: 65 were < 45 years and 41 were ≥ 41 years. BN: 60 were < 45 years and 35 were ≥ 45 years | let-7e, miR-181b, miR-1975, miR-144, miR-100, miR-151-5p, miR-222, miR-543, miR-127-3p, miR-16 |
A total of 6 studies investigated circulating miR-146, miR-221 and miR-222 for discriminating between PTC patients, those with benign thyroid nodules and HC (Table 2). The majority of the studies investigating Asian populations and only one[23] study originates from Europe. In this study a total of 426 PTC, 203 BN and 144 HC patients. All studies investigated a variety of circulating miRNAs, however this study is focused on the most common ones: MiR-146, miR-221 and miR-222.
| Ref. | Lymph node metastasis (n) | Distant metastasis | Extrathyroid extension (n) | Multifocality (n) | PTC recurrence | Tumor size |
| Lee et al[26] | 45 | 0 | 40 | NG | NG | Benign: 29.48 ± 11.25 mm. PTC (node negative): 10.50 ± 7.67 mm. PTC (node negative): 13.71 ± 8.29 mm |
| Zhang et al[24] | 59 | 29 | 17 | 20 | 12 | PTC (newly diagnosed)-64 were ≤ 2 cm and 21 were > 2 cm |
| Zhang et al[27] | 61 | 40 | 18 | 34 | NG | NG |
| Rosignolo et al[23] | 32 | 3 | 24 | 12 | NG | Screening cohort median size and range in mm: 11 (5-33). Validation cohort median and range in mm: 10 (1-60) |
| Yu et al[22] | 60 | 20 | NG | 36 | NG | PTC: 67 were ≤ 2 cm and 39 were > 2 cm |
| Jiang et al[25] | 49 | 8 | NG | 20 | 3 | PTC without LNM (mm): 10.6 ± 4.2 of which 2 were > 20 mm and 13 were ≤ 20 mm. PTC with LNM (mm): 13.5 ± 5.5 of which 8 were > 20 mm and 41 were ≤ 20 mm |
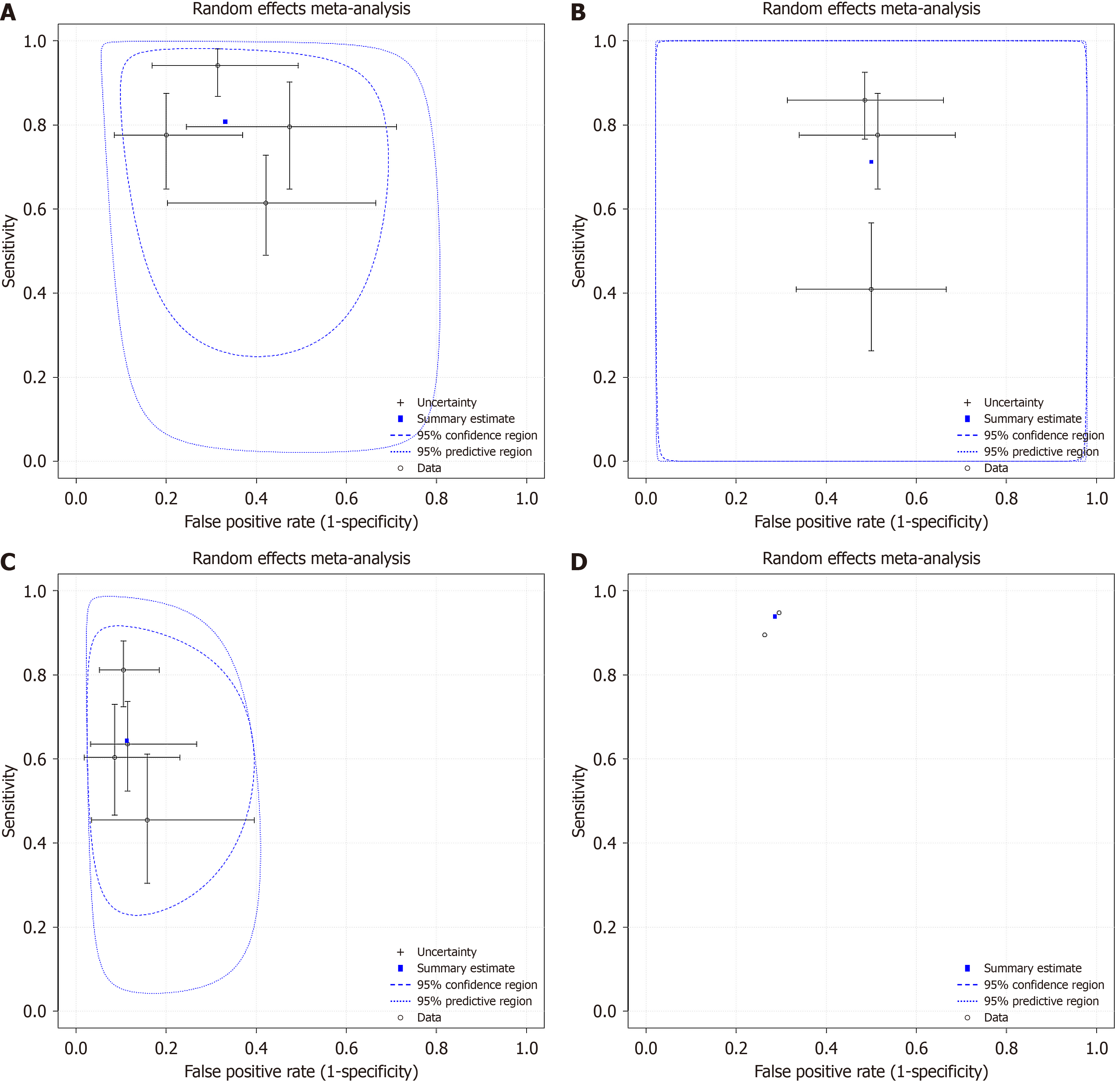
Four studies[23,24,26,27] investigated the diagnostic sensitivity and specificity of miR 146 between PTC and BN with a pooled sensitivity 80.7% (95%CI: 65.2%-90.4%), specificity 66.9% (95%CI: 55.5%-76.6%) and false positive rate 33.1% (95%CI: 23.4%-44.5%) (Figure 2A). When comparing the diagnostic sensitivity and specificity of miR 221 in the same groups of patients (PTC vs BN), three studies[23,24,27] showed a pooled sensitivity 71.3% (95%CI: 46.2%-87.7%), specificity 50% (95%CI: 40.7%-59.3%) and false positive rate 50% (95%CI: 40.7-59.3) (Figure 2B). Another four studies[22-24,27] investigated the sensitivity and specificity of miR 222, in PTC vs BN, resulting in pooled sensitivity 64.3% (95%CI: 50.3%-76.2%), specificity 88.8% (95%CI: 82.4%-93%) and false positive rate 11.2% (95%CI: 7%-17.6%) (Figure 2C). As far as diagnosis between BN and healthy volunteers was concerned, two studies[22,23] compared sensitivity and specificity of circulating miR 222 in BN and HC. The pooled sensitivity and specificity, as well as the false positive rate were the following: 93.9% (95%CI: 87.7%-97%), 71.4% (95%CI: 59.1%-81.2%) and 28.6% (95%CI: 18.8%-40.9%), respectively (Figure 2D).
All studies were considered as low risk of bias in reference standard and flow/timing domains. All studies were of unclear risk in the index test domain given the heterogeneity among the included studies in quantification and qualification method of circulating miRNAs. Finally, two studies were considered of high risk in patient selection domain, in view of high probability of overlapping population (Table 3).
| Ref. | Risk of bias | Applicability concerns | |||||
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Lee et al[26] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Low risk |
| Zhang et al[24] | High risk | Unclear risk | Low risk | Low risk | High risk | Unclear risk | Low risk |
| Zhang et al[27] | High risk | Unclear risk | Low risk | Low risk | High risk | Unclear risk | Low risk |
| Rosignolo et al[23] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Low risk |
| Jiang et al[25] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Low risk |
| Yu et al[22] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Low risk |
In our meta-analysis we set out to review the current literature in order to further validate the utility of miRNA-146, miRNA-221 and miRNA-222 in the diagnosis and staging of PTC. We included a total of 13 studies with 913 PTC patients. Regarding the differentiation of PTC from BN, the miRNA with the highest pooled sensitivity from our analysis was miRNA-146 at 80.7%. This finding is consistent with a previous meta-analysis by Liu et al[28], which reported a pooled sensitivity of 82% for miR-146 across nine studies involving over 2100 PTC cases. The close alignment between our pooled results and those of prior work further supports the diagnostic utility of miR-146 in differentiating malignant from benign thyroid nodules[28]. When used for the same diagnostic distinction, miRNA-222 was shown to have the highest pooled specificity: 88.8%. We also found miRNA-222 to be a sensitive marker for distinguishing BN from HC with a pooled sensitivity of 93.9%.
The sensitivity of miRNA-146 for identifying patients with PTC from those with BN established in our study is consistent with results published in other recent literature. For example, in their 2021 meta-analysis comprising 9 studies and 2114 cases of PTC, Liu et al[28] concluded a sensitivity of 82%. Our results are directly comparable, as the meta-analysis by Liu et al[28] also used a control group comprising only individuals with benign thyroid nodules, thereby excluding HC from the sensitivity analysis. This methodological similarity enhances the comparability of the pooled sensitivity for miR-146 between our study and theirs.
Furthermore, our value for the pooled specificity of miR-222 mentioned above is also congruent with other available analysis. The same 2021 meta-analysis from Liu et al[28] elicited a specificity of 90% and, more recently, a 2022 study published by Chen et al[29] determined exactly the same result: Specificity 90%. Their 2022 paper included 6 studies with 463 PTC patients, approximately half the number obtained and analyzed in our meta-analysis. Moreover, we have isolated each miRNA in our analysis to better demonstrate their respective sensitivities and specificities thus reducing the opportunity for bias. In addition, our research included more comprehensive analysis showcasing miR-222 as a useful marker for discerning patients with benign disease from HC.
The implication of micro-RNAs in the oncogenesis of various different human malignancies has been well established due to their role in gene expression associated with fundamental cellular processes including proliferation, differentiation and apoptosis[30]. Dysregulated expression, commonly due to genetic and epigenetic modifications, is paramount in the initiation and progression of malignancy as they may act as both oncogenes and tumour suppressors.
In 2002, Calin et al[31] identified deletions and down-regulations in the miRNA-15/16 cluster on chromosome 13q14 to be associated with chronic lymphocytic leukaemia; the first link between miRNAs and human malignancy. This paved the way for a vast new area of research surrounding miRNA and implications for various types of cancer. For example, subsequent studies have shown miRNA-15/16 to be involved in acute myeloid leukaemia, neuroblastoma, lung adenocarcinomas/squamous cell carcinomas, and breast cancer[32-35]. Soon after the Calin et al[31] 2002 paper, Michael et al[36] isolated the first cancer-specific miRNAs in colorectal cancer (miRNA-143 and miRNA-145) and since the discovery of miRNAs, 1917 have been identified in humans[36,37].
This study set out to further elucidate the role of miRNA-146, miRNA-221 and miRNA-222 in PTC amongst an already established association with a plethora of different hormone-related cancers[38-40]. In prostate cancer for example, elevated levels of 146a/b have been shown to be present in normal prostate tissues[38]. When prostate cancer cells were transfected with miRNA-146a, there was a significant decrease in cancer cell invasion, proliferation and bone marrow metastases indicating miRNA-146a functioned as a tumour suppressor in these cells[38,39]. MiRNA-221 and miRNA-222 have also been independently shown to have a tumour-suppressor role in prostate cancer. Researchers have found that the miRNA-221, miRNA-222, miRNA-30a and miRNA-30d can bind to the 3’-UTR of Bmi-1 in prostate tissue samples. In contrast to the elevated Bmi-1 expression, it has been shown that the expression of miRNA-221 is much lower in tissue samples from prostate cancer patients[40].
All three of our studied miRNAs have been implicated in the pathogenesis of breast cancer. By means of mechanisms that remain largely unknown, cancer cells frequently develop a constitutively active nuclear factor-κB (NF-κB) program to support metastatic potential and cell proliferation. Bhaumik et al[41] demonstrated through immunologic testing that high levels of miRNA-146a/b down-regulates NF-κB activity and impairs invasion and migration of the cancerous cells. In contrast, miRNA-221 and miRNA-222 have negative implications in treating breast cancer. In 2008, Miller et al[42] produced the first paper demonstrating that oestrogen positive primary breast cancers are largely resistant to tamoxifen therapy if miRNA-221/222 are over-expressed in the tissues. Further studies also have shown that reducing levels of miRNA-221/222 in MDA-MB-468 cells increased their sensitivity to tamoxifen by restoring oestrogen receptor α expression[43].
Notably, miRNA-221 and miRNA-222have also been identified as having a significant role in pancreatic cancer. Increased expression of miRNA-221/222 has been shown to reduce apoptosis, increase cell invasion and proliferation in pancreatic cancer cells[44]. Cell invasion is increased by miRNA-221/222 binding TIMP-2 which upregulates MMP-2 and MMP-9 and cell proliferation is promoted by these miRNAs increasing G1-phase of cancer cell division[25,45].
The role of miRNA-146,221 and 222 in PTC is mediated via a variety of mechanisms. MiRNA-146 suppresses ZNRF3 in PTC which results in enhancement of Wnt/β-catenin signalling and subsequent stimulation of cell invasion and migration[26]. In addition, miRNA-146 increases the rate of cell proliferation in PTC due to disruption of cell signalling via its role in downregulating SMAD4 which interferes with TGF-β[27]. The expression of the tumour-suppressor gene thyroid hormone receptor β declines in the presence of miRNA-146a and miRNA-221 in PTC[21,22]. It is well established that miRNA-222 is also elevated in patients with PTC and causes oncogenesis via a variety of different mechanisms[23]. For example, miRNA-222 plays a role in oncogene activation by targeting p27, p57 and PUMA[16,21,24,46,47]. It has also been shown to increase tumour aggression by targeting the AKT signalling pathway and protein phosphatase 2 regulatory subunit alpha which potentiates tumour invasion[21,48].
Studies have shown that micro-RNAs are packaged and selectively secreted extracellularly in the form of either encapsulated exosomes (up to 83%-99%) or micro-vesicles[49]. The possibility for miRNAs to exist in the blood plasma/serum without a membrane-bound vesicle was then observed when associated solely with one of the four Argonaute (AGO) proteins which, during their intracellular synthesis, mature miRNAs bind to[50]. The protection provided by either a membrane vesicle and/or the stable AGO protein with which the miRNA is complexed (notably AGO2) allows for an increased resistance to degradation by various nucleic acid metabolising enzymes and thus an improved stability within the blood[51]. During tumorigenesis, the rate of tumour cell derived exosome secretion is at least 10-fold more than normal increasing the potential for their uptake by recipient cells[52]. The pathological significance of miRNA upregulation at the tumour microenvironment in conjunction with their enhanced stability is the opportunity for their action at distant organ sites in preparing for tumour colonization[53]. Conversely, it is this high level of stability in various biological fluids which allows miRNAs to serve as good biomarkers and aid novel investigative and therapeutic advancements in the diagnosis and treatment of cancer.
The established stability of miRNAs and their role as a relatively non-invasive marker for cancers leaves the question of effective methods of detection. The ever-growing number of identified miRNAs in human pathology has been largely owed to the development of techniques which are specific and sensitive enough to be able to distinguish between the highly homologous and small sequence molecules[54-56]. The main methods of identifying miRNAs at present are northern blotting, microarray analysis, qPCR-based methods and next-generation sequencing[57]. Whilst these methods have proven effective, they pose challenges when being used in clinical practice with regards to their cost, complexity and time taken to complete. Although NGS may be considered a lower-cost and higher-throughput option than the other methods, its accessibility and feasibility remain currently limited for most clinical settings. To determine the utility of these tests for the diagnosis of PTC, further research needs to take place to compare their cost-effectiveness with current diagnostic standards.
Our systematic review on diagnostic test accuracy only included published full-text studies with non-overlapping populations providing the sensitivity and specificity of the miRNAs of interest (only 146, 221, and 222 as potentially among the most applicable to clinical practice in the future) and using the results of histopathological reports to confirm the diagnosis. Therefore, we identified few studies fulfilling these strict criteria. In doing so, we hoped to eliminate the heterogeneous reporting of these miRNAs’ efficacy and generate more interest in the field so that future research will focus on the circulating forms of these miRNAs. Even though we did not apply any restrictions regarding geographical regions, most studies currently fulfilling our review’s criteria derive from Asian populations, while only one is from Europe. By generating scientific interest in this field, more studies including different populations may emerge allowing more powerful and clinically meaningful subgroup analyses in future iterations of our review. One important limitation of the current literature is the heterogeneity in the quantification methods of the circulating miRNAs. The variety of these methods has been highlighted before and may involve both sample-related and experiment-related factors[58]. The most important sample-related factors include the sample source (i.e., serum or plasma), the sample’s potential contamination with platelets or other cells, its collection time across the participant’s circadian cycle, and the participant’s sex, diet, and exercise[58]. On the other hand, experimental factors may include the sample’s processing (e.g., the presence of heparin or citrate), storage time and conditions, the RNA extraction and measurement platforms, and the data analysis methods[58]. Addressing some of these factors (such as adding heparinase) may further increase the time and cost. Therefore, future studies should use more standardized populations and methods to enable more robust conclusions to be drawn on the clinical utility of these miRNAs.
In comparison to previously published meta-analyses, our study focused on the diagnostic utility of miRNA-146, miRNA-221 and miRNA-222 for differentiating PTC from benign thyroid nodules. From our analysis, miRNA-146 has the highest sensitivity (80.7%), whereas miRNA-222 has the highest specificity (88.8%). Whilst these results validate their already established isolated efficacy in PTC diagnosis and mainly Asian populations, larger studies using more standardized sample processing and RNA quantification methods are needed to improve diagnostic accuracy and reproducibility across populations, as variability in these steps can significantly affect circulating miRNA measurements[30]. Employing standardized sample processing protocols are essential to minimize technical variability and enhance the reproducibility of miRNA biomarker research. This necessity is underscored by best practice recommendations that highlight the impact of pre-analytical variables—such as sample collection timing, processing methods, and storage conditions—on the accuracy and consistency of miRNA detection. When such studies become available, future iterations of the present work can conduct more powerful and clinically meaningful subgroup analyses by geographical region. Furthermore, at present, we could not find any literature investigating the use of these two miRNAs in conjunction with one another to serve as a more potent diagnostic tool. Our results suggest this is an opportune area for further research. Regarding clinical practice, further studies are needed to determine how their diagnostic accurate, may be improved when paired with commonly used techniques; namely ultrasound and FNA. Cost-effectiveness studies are also necessary to ensure that the complexity, time, and cost of these methods add benefit to current standard practices.
| 1. | Bann DV, Goyal N, Camacho F, Goldenberg D. Increasing incidence of thyroid cancer in the Commonwealth of Pennsylvania. JAMA Otolaryngol Head Neck Surg. 2014;140:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Kitahara CM, Sosa JA. Understanding the ever-changing incidence of thyroid cancer. Nat Rev Endocrinol. 2020;16:617-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 3. | United Kingdom Cancer Research. Thyroid Cancer Statsitics 2023. [cited 23 June, 2025]. Available from: https://www.cancerresearchuk.org/health-professional/cancerstatistics/statistics-by-cancer-type/thyroid-cancer. |
| 4. | Yoruker EE, Terzioglu D, Teksoz S, Uslu FE, Gezer U, Dalay N. MicroRNA Expression Profiles in Papillary Thyroid Carcinoma, Benign Thyroid Nodules and Healthy Controls. J Cancer. 2016;7:803-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 5. | Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore FD Jr, Larsen PR, Marqusee E, Alexander EK. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411-3417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 422] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 6. | Pallante P, Visone R, Ferracin M, Ferraro A, Berlingieri MT, Troncone G, Chiappetta G, Liu CG, Santoro M, Negrini M, Croce CM, Fusco A. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer. 2006;13:497-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 381] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 7. | Roth MY, Witt RL, Steward DL. Molecular testing for thyroid nodules: Review and current state. Cancer. 2018;124:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (2)] |
| 8. | Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943-3949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 387] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. 2016;23:R143-R155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 316] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 10. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 10297] [Article Influence: 1029.7] [Reference Citation Analysis (1)] |
| 11. | Pacini F, Molinaro E, Lippi F, Castagna MG, Agate L, Ceccarelli C, Taddei D, Elisei R, Capezzone M, Pinchera A. Prediction of disease status by recombinant human TSH-stimulated serum Tg in the postsurgical follow-up of differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:5686-5690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Durante C, Tognini S, Montesano T, Orlandi F, Torlontano M, Puxeddu E, Attard M, Costante G, Tumino S, Meringolo D, Bruno R, Trulli F, Toteda M, Redler A, Ronga G, Filetti S, Monzani F; PTC Study Group. Clinical aggressiveness and long-term outcome in patients with papillary thyroid cancer and circulating anti-thyroglobulin autoantibodies. Thyroid. 2014;24:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Spencer C, Petrovic I, Fatemi S, LoPresti J. Serum thyroglobulin (Tg) monitoring of patients with differentiated thyroid cancer using sensitive (second-generation) immunometric assays can be disrupted by false-negative and false-positive serum thyroglobulin autoantibody misclassifications. J Clin Endocrinol Metab. 2014;99:4589-4599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Cannell IG, Kong YW, Bushell M. How do microRNAs regulate gene expression? Biochem Soc Trans. 2008;36:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 293] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1316] [Cited by in RCA: 1278] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 16. | He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, Kloos RT, Croce CM, de la Chapelle A. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2005;102:19075-19080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 931] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 17. | Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93:1600-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 467] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 18. | Geropoulos G, Psarras K, Papaioannou M, Giannis D, Meitanidou M, Kapriniotis K, Symeonidis N, Pavlidis ET, Pavlidis TE, Sapalidis K, Ahmed NM, Abdel-Aziz TE, Eddama MMR. Circulating microRNAs and Clinicopathological Findings of Papillary Thyroid Cancer: A Systematic Review. In Vivo. 2022;36:1551-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 52110] [Article Influence: 10422.0] [Reference Citation Analysis (2)] |
| 20. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 10415] [Article Influence: 694.3] [Reference Citation Analysis (3)] |
| 21. | Nyaga VN, Arbyn M. Metadta: a Stata command for meta-analysis and meta-regression of diagnostic test accuracy data - a tutorial. Arch Public Health. 2022;80:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 22. | Yu S, Liu Y, Wang J, Guo Z, Zhang Q, Yu F, Zhang Y, Huang K, Li Y, Song E, Zheng XL, Xiao H. Circulating microRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97:2084-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Rosignolo F, Sponziello M, Giacomelli L, Russo D, Pecce V, Biffoni M, Bellantone R, Lombardi CP, Lamartina L, Grani G, Durante C, Filetti S, Verrienti A. Identification of Thyroid-Associated Serum microRNA Profiles and Their Potential Use in Thyroid Cancer Follow-Up. J Endocr Soc. 2017;1:3-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Xu D, Pan J, Yang Z, Chen M, Han J, Zhang S, Sun L, Qiao H. Dynamic monitoring of circulating microRNAs as a predictive biomarker for the diagnosis and recurrence of papillary thyroid carcinoma. Oncol Lett. 2017;13:4252-4266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Jiang K, Li G, Chen W, Song L, Wei T, Li Z, Gong R, Lei J, Shi H, Zhu J. Plasma Exosomal miR-146b-5p and miR-222-3p are Potential Biomarkers for Lymph Node Metastasis in Papillary Thyroid Carcinomas. Onco Targets Ther. 2020;13:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Lee YS, Lim YS, Lee JC, Wang SG, Park HY, Kim SY, Lee BJ. Differential expression levels of plasma-derived miR-146b and miR-155 in papillary thyroid cancer. Oral Oncol. 2015;51:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Pan J, Xu D, Yang Z, Sun J, Sun L, Wu Y, Qiao H. Combination of serum microRNAs and ultrasound profile as predictive biomarkers of diagnosis and prognosis for papillary thyroid microcarcinoma. Oncol Rep. 2018;40:3611-3624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Geng H, Liu X, Cao M, Zhang X. A meta-analysis of circulating microRNAs in the diagnosis of papillary thyroid carcinoma. PLoS One. 2021;16:e0251676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Chen Y, Dong B, Huang L, Huang H. Serum microRNAs as biomarkers for the diagnosis of papillary thyroid carcinoma: a meta-analysis. Bosn J Basic Med Sci. 2022;22:862-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Khan AQ, Ahmed EI, Elareer NR, Junejo K, Steinhoff M, Uddin S. Role of miRNA-Regulated Cancer Stem Cells in the Pathogenesis of Human Malignancies. Cells. 2019;8:840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 31. | Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524-15529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3675] [Cited by in RCA: 3834] [Article Influence: 159.8] [Reference Citation Analysis (0)] |
| 32. | Lovat F, Nigita G, Distefano R, Nakamura T, Gasparini P, Tomasello L, Fadda P, Ibrahimova N, Catricalà S, Palamarchuk A, Caligiuri MA, Gallì A, Malcovati L, Minden MD, Croce CM. Combined loss of function of two different loci of miR-15/16 drives the pathogenesis of acute myeloid leukemia. Proc Natl Acad Sci U S A. 2020;117:12332-12340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Chava S, Reynolds CP, Pathania AS, Gorantla S, Poluektova LY, Coulter DW, Gupta SC, Pandey MK, Challagundla KB. miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol Oncol. 2020;14:180-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (9)] |
| 34. | Bandi N, Zbinden S, Gugger M, Arnold M, Kocher V, Hasan L, Kappeler A, Brunner T, Vassella E. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 2009;69:5553-5559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 35. | Janaki Ramaiah M, Lavanya A, Honarpisheh M, Zarea M, Bhadra U, Bhadra MP. MiR-15/16 complex targets p70S6 kinase 1 and controls cell proliferation in MDA-MB-231 breast cancer cells. Gene. 2014;552:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Michael MZ, O' Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882-891. [PubMed] |
| 37. | miRBase. miRBase: the microRNA databasevthe archive for microRNA sequences and annotationsv. [cited 23 June, 2025]. Available from: https://www.mirbase.org/browse/results/?organism=hsa. |
| 38. | Etikala DM, Liu R, Wang L. FOXP3-microRNA-146-NF-κB as oncotarget. Oncoscience. 2015;2:839-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Leite KR, Tomiyama A, Reis ST, Sousa-Canavez JM, Sañudo A, Camara-Lopes LH, Srougi M. MicroRNA expression profiles in the progression of prostate cancer--from high-grade prostate intraepithelial neoplasia to metastasis. Urol Oncol. 2013;31:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Xuan H, Xue W, Pan J, Sha J, Dong B, Huang Y. Downregulation of miR-221, -30d, and -15a contributes to pathogenesis of prostate cancer by targeting Bmi-1. Biochemistry (Mosc). 2015;80:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643-5647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 527] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 42. | Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283:29897-29903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 609] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 43. | Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, Coppola D, Cheng JQ. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008;283:31079-31086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 44. | Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 699] [Cited by in RCA: 709] [Article Influence: 37.3] [Reference Citation Analysis (9)] |
| 45. | Xu Q, Li P, Chen X, Zong L, Jiang Z, Nan L, Lei J, Duan W, Zhang D, Li X, Sha H, Wu Z, Ma Q, Wang Z. miR-221/222 induces pancreatic cancer progression through the regulation of matrix metalloproteinases. Oncotarget. 2015;6:14153-14164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Vriens MR, Weng J, Suh I, Huynh N, Guerrero MA, Shen WT, Duh QY, Clark OH, Kebebew E. MicroRNA expression profiling is a potential diagnostic tool for thyroid cancer. Cancer. 2012;118:3426-3432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han L, Jia ZF, Yang WD, Wang GX, Jiang T, You YP, Pu PY, Cheng JQ, Kang CS. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Cancer. 2010;9:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 48. | Huang Y, Yu S, Cao S, Yin Y, Hong S, Guan H, Li Y, Xiao H. MicroRNA-222 Promotes Invasion and Metastasis of Papillary Thyroid Cancer Through Targeting Protein Phosphatase 2 Regulatory Subunit B Alpha Expression. Thyroid. 2018;28:1162-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 49. | Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 879] [Article Influence: 62.8] [Reference Citation Analysis (22)] |
| 50. | Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci. 2012;37:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 51. | Wu Q, Li L, Jia Y, Xu T, Zhou X. Advances in studies of circulating microRNAs: origination, transportation, and distal target regulation. J Cell Commun Signal. 2023;17:445-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 627] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 53. | Salido-Guadarrama I, Romero-Cordoba S, Peralta-Zaragoza O, Hidalgo-Miranda A, Rodríguez-Dorantes M. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther. 2014;7:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 54. | Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 1132] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 55. | Hagiwara S, Kantharidis P, Cooper ME. MicroRNA as biomarkers and regulator of cardiovascular development and disease. Curr Pharm Des. 2014;20:2347-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Wang J, Chen J, Sen S. MicroRNA as Biomarkers and Diagnostics. J Cell Physiol. 2016;231:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 600] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 57. | Costa MC, Leitão AL, Gabriel AF, Enguita FJ. Wet-lab methods for miRNA analysis. MicroRNA in Human Malignancies. Portugal: Academic Press, 2022. [DOI] [Full Text] |
| 58. | Lee I, Baxter D, Lee MY, Scherler K, Wang K. The Importance of Standardization on Analyzing Circulating RNA. Mol Diagn Ther. 2017;21:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/