Published online Mar 6, 2024. doi: 10.12998/wjcc.v12.i7.1326
Peer-review started: November 11, 2023
First decision: December 26, 2023
Revised: January 6, 2024
Accepted: January 25, 2024
Article in press: January 25, 2024
Published online: March 6, 2024
Processing time: 110 Days and 22.6 Hours
Cellular myofibroma is a rare subtype of myofibroma that was first described in 2017. Its diagnosis is often challenging because of its relative rarity, lack of known genetic abnormalities, and expression of muscle markers that can be confused with sarcomas that have myogenic differentiation. Currently, scholars have limited knowledge of this disease, and published cases are few. Further accumulation of diagnostic and treatment experiences is required.
A 16-year-old girl experienced left upper limb swelling for 3 years. She sought medical attention at a local hospital 10 months ago, where magnetic resonance imaging revealed a 5-cm soft tissue mass. Needle biopsy performed at a local hospital resulted in the diagnosis of a spindle cell soft tissue sarcoma. The patient was referred to our hospital for limb salvage surgery with endoprosthetic replacement. She was initially diagnosed with a synovial sarcoma. Consequently, clinical management with chemotherapy was continued for the malignant sarcoma. Our pathology department also performed fluorescence in situ hybridization for result validation, which returned negative for SS18 gene breaks, indicating that it was not a synovial sarcoma. Next-generation sequencing was used to identify the SRF-RELA rearrangement. The final pathological diagnosis was a cellular/myofibroblastic neoplasm with an SRF-RELA gene fusion. The patient had initially received two courses of chemotherapy; however, chemother
This case was misdiagnosed because of its rare occurrence, benign biological behavior, and pathological similarity to soft tissue sarcoma.
Core Tip: Cellular myofibroma (CMF) is a rare subtype of myofibroma that exhibits a broad age distribution, and manifests in diverse anatomical locations, including deep soft tissues and skeletal muscles. Owing to the absence of distinct features, CMF is frequently misdiagnosed. Microscopic examination revealed active mitotic figures (non-pathological), focal areas of necrosis, and occasional infiltrative growth. Next-generation sequencing aids in the accurate pathological diagnosis of CMF by detecting the SRF-RELA gene fusion.
- Citation: Zhou Y, Sun YW, Liu XY, Shen DH. Misdiagnosis of synovial sarcoma - cellular myofibroma with SRF-RELA gene fusion: A case report. World J Clin Cases 2024; 12(7): 1326-1332
- URL: https://www.wjgnet.com/2307-8960/full/v12/i7/1326.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i7.1326
Myofibromas are common benign mesenchymal tumors that predominantly affect infants and young children. This disease was first reported by Williams and Sohm in 1951, and was initially referred to as congenital fibrosarcoma. In 1954, Stout renamed juvenile fibromatosis as congenital generalized fibromatosis. However, in 1981, Chung and Enzinger demonstrated that this tumor is actually a myofibroblastic proliferation, leading to its reclassification as infantile myofibromatosis[1]. The 2002 World Health Organization (WHO) classification categorizes it as one of the most common fibroblastic and myofibroblastic proliferations in infants and children. Moreover, morphologically, myofibromas share some continuity with myopericytomas; thus, they were included in the category of myopericytic tumors in the 2013 WHO classification.
In 2017, a subset of cellular variants of myofibroma and myopericytoma with a smooth muscle-like immunophenotype, harboring a recurrent SRF-RELA gene fusion was reported. Diagnosing cellular myofibroma (CMF) presents certain challenges as it is rare, and its histological features resemble those of other sarcomas[2]. Furthermore, CMF is characterized by high cell density and visible nuclear divisions. Some CMF tumors exhibit a distinct fascicular growth pattern that morphologically resembles that of spindle cell sarcomas such as rhabdomyosarcoma, leiomyosarcoma, and synovial sarcoma. this type of tumor lacks specific immunohistochemical markers and exhibits significant overlap with low-grade myofibroblastic sarcomas and fibroblastic tumors within the immunophenotypic spectrum. This necessitates the use of next-generation sequencing (NGS) to detect characteristic genetic aberrations. Therefore, the differential diagnosis of CMF requires consideration of other sarcomas with similar morphologies or similar expression of immunohistochemical markers. Additionally, CMF is even rarer in young or adult patients and is more likely misdiagnosed as "sarcoma" in older patients.
Herein, we report a case of CMF and discuss its clinical, pathological, and molecular features to elucidate the differential diagnosis of this rare tumor subtype.
The patient had a palpable lump in the left arm for > 3 years and numbness in the fingers for > 1 month.
A 16-year-old girl was admitted to the Bone Tumor Department of our hospital with left upper limb pain that had started 1 wk prior to presentation. The patient first noticed symptoms of left upper limb pain 3 years prior when she accidentally discovered a painless "walnut-sized" lump while bathing. However, she did not seek medical attention at that time. Over time, the lump size gradually increased. She had recently started to experience numbness in her fingers and decided to seek medical care at a local hospital. Magnetic resonance imaging (MRI) of the left humerus revealed a 5-cm soft tissue mass surrounding the bone. A fine-needle aspiration biopsy was performed at the local hospital, and the pathological report indicated the possibility of a malignant soft tissue tumor.
The patient had been previously healthy.
The patient was transferred to our hospital for expert consultation and further treatment on June 23, 2023. Considering the immunohistochemical staining results from the original institution, along with the imaging findings, the diagnosis was consistent with spindle cell mesenchymal sarcoma. The differential diagnoses included synovial sarcoma, malignant peripheral nerve sheath tumor, and other tumors with similar presentations.
No evident abnormality was identified in the personal and family history.
A firm lump was palpated on the anterolateral aspect of the middle segment of the left upper arm. It was immobile and approximately 5 cm × 3 cm in size. Mild tenderness and scarring from the previous punch biopsy were observed.
No evident abnormality in the serum tumor markers was detected.
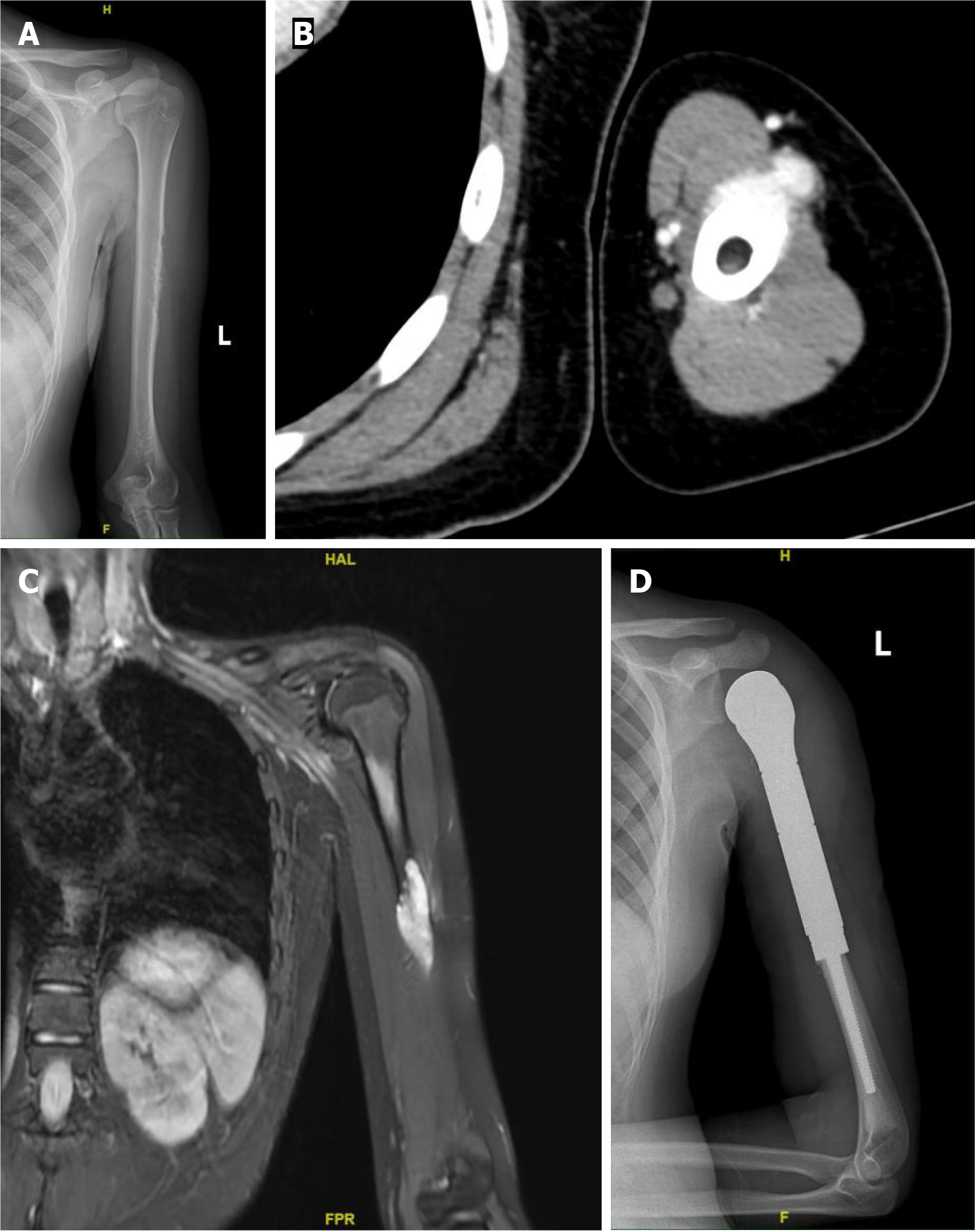
Left humerus radiography (Figure 1A), computed tomography (Figure 1B), and MRI (Figure 1C) examinations revealed bone destruction in the middle segment of the left humerus, accompanied with the formation of a soft tissue mass.
On July 7, 2023, the patient underwent segmental resection of the left humerus with prosthetic replacement (Figure 1D).
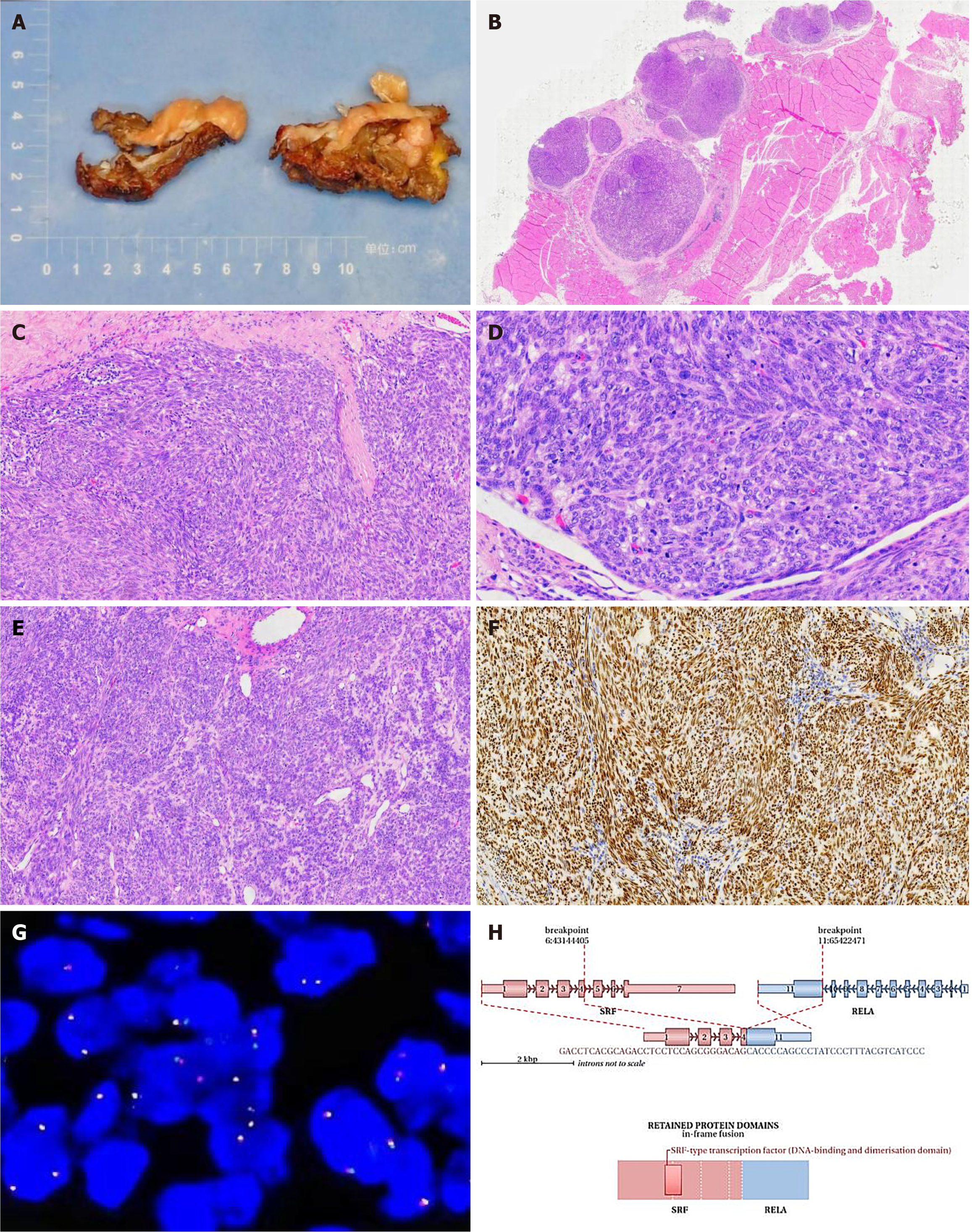
Macroscopic examination: The specimen consisted of muscle and adipose tissues with visible tumors. The tumor appeared as multiple nodules with a total size of 5 cm × 4.5 cm × 2 cm. The diameter of each nodule ranged from 1.5 to 2 cm. The cut surface of the tumor was solid, slightly tough in texture, and grayish-yellow in color. No clear areas of bleeding were observed (Figure 2A).
Microscopic examination: Nodular tumors were observed in the fibromuscular tissue (Figure 2B). The cells were oval- or spindle-shaped (Figure 2C), densely arranged in bundles or swirling patterns. Focal necrosis and epithelioid cell-like regions were observed (Figure 2D). The average mitotic count was approximately 10/10 high-power fields (HPF), with visible focal mitosis (approximately 20/10 HPF) (Figure 2E).
Immunohistochemical staining: SATB2 (+), INI1 (+), S-100 (partially +), Sox-10 (−), ALK (−), CD99 (focally +), TLE1 (diffusely strong +) (Figure 2F), Desmin (focally +), SMA (+), Myogenin (−), Bcl-2 (focal +), CD34 (−), Ki-67 (30%+), H3K27Me3 (+), EMA (−), CD56 (+), CK19 (−), MUC4 (−), and Calretinin (−).
The preliminary pathological diagnosis was considered as synovial sarcoma (spindle cell type).
The final pathological diagnosis was a cellular fibroma with SRF-RELA fusion.
The patient received two cycles of chemotherapy with pegylated liposomal doxorubicin 70 mg/d, combined with ifosfamide 9 g/d postoperatively. Chemotherapy was discontinued based on the final diagnosis.
The patient was followed-up for 3 months, and no recurrence was noted.
Cellular fibromas, also known as atypical fibromas, are benign with no metastasis and limited growth[3,4]. Surgical removal typically results in a good prognosis, with a local recurrence rate of approximately 5%. However, CMF may be misdiagnosed owing to its histological similarity to soft tissue sarcomas[5]. Limited research has been conducted on CMF, domestically and internationally, and pathologists have not paid sufficient attention to diagnosing and distinguishing spindle cell tumors of soft tissues. Previous case reports have highlighted the misdiagnosis of CMF as leiomyomas, low-grade malignant fibrous histiocytomas, fibrosarcomas, and rhabdomyosarcomas. However, subsequent confirmation through NGS revealed the presence of the SRF-RELA gene fusion[6,7]. Positive outcomes were observed during the follow-up.
Furthermore, CMFs can occur in children, adolescents, and adults of varying ages and manifests in different anato
The diagnostic journey of the patient described in this article was intricate and demanding. The patient was initially diagnosed with a leiomyosarcoma based on a biopsy conducted at a local hospital. However, upon referral to our hospital for consultation, malignant mesenchymal tumors were considered. The patient received surgical intervention and postoperative chemotherapy based on the suspicion of a malignant mesenchymal tumor. Immunohistochemical staining of the postoperative specimen revealed a strong and diffuse expression of TLE1 in the tumor cells, suggesting a preliminary diagnosis of synovial sarcoma. However, further examination using the SS18 gene probe dual-color break-apart FISH yielded a negative result. Nevertheless, NGS gene testing identified the SRF-RELA gene fusion. Based on the onset location, histological morphology, and immunohistochemical findings, a final diagnosis of cellular fibroma was established.
In this case, the diagnostic challenge arose from the unusual location, age of onset, presence of active mitotic figures, abundant cellular content, and diffusely strong expression of the TLE-1 protein in tumor cells. After reviewing the histopathological slides and existing literature, we identified several clinical and pathological features of CMF. Generally, CMFs have well-defined borders that distinguish them from classic sarcomas[11]. They do not exhibit the typical "fish-flesh" appearance upon cross-section, and extensive areas of necrosis or hemorrhage are rarely observed under microscopic examination. Additionally, CMFs tend to grow at a slower pace than classic sarcomas and do not undergo sudden enlargement[12]. In this particular case, the tumor had a long duration and demonstrated slow growth with focal infiltrative growth or limited stromal wedge-shaped infiltration, all of which are not characteristic of malignancy[13]. Upon reviewing the literature, we identified that this is the first reported case of CMF expressing TLE-1, which further complicated the diagnosis. Moreover, TLE-1 is commonly observed in synovial sarcoma tumor cells; however, fibromas have not been reported to express TLE-1. Both tumors typically present as well-defined nodules and share similarities in tissue morphology, such as a bundled cell arrangement, abundant cellularity, and short spindle-shaped cytology. This poses significant diagnostic challenges. This case highlights the need for future studies to differentiate between synovial sarcoma and fibroma, and to enhance our understanding of this rare tumor subtype. Although active mitotic figures were present in this case, the absence of pathological or atypical mitotic figures did not support the diagnosis[14]. Our literature review did not reveal any relevant reports on TLE-1 expression in fibromas or cellular fibromas. Through a retrospective analysis, we observed that cellular or atypical fibromas may exist. However, owing to the limited number of evidence-based cases of CMF, the biological behavior of this tumor remains unknown, necessitating close follow-up.
The 5th edition of the WHO classification of soft tissue and bone tumors in 2020 identified the SRF-RELA rearrangement as an aid in the pathological diagnosis of cellular/atypical fibroma[9]. Additionally, SRF is a protein-coding gene that stimulates cell proliferation and differentiation. Similarly, RELA (RELA proto-oncogene, NF-κB subunit) is another protein-coding gene[15]. In this case, the SRF-RELA gene rearrangement was consistent with previous findings. The fusion gene retained the 5′ end of the SRF gene promoter up to exon 4 and the 3′ end of the RELA gene from exon 11 to the termination codon, without any predicted frameshift mutations.
With the rapid development of NGS technology, breakthroughs in the classification of classic tumors have been achieved. Currently, with the application of this technology, various tumors can be diagnosed more accurately, including those that were previously misdiagnosed as low-grade sarcomas or synovial sarcomas. Using second-generation sequencing technology, we discovered that these tumors were benign. This article describes a rare case of cellular fibroma with the SRF-RELA gene fusion. The tumor exhibits atypical and intriguing cytological features that may be mistaken for malignant sarcomas with smooth or striated muscle differentiation. The current follow-up results demonstrate a good prognosis for the patient. This case further suggests that the accuracy of pathological diagnosis relies on the assistance of new technologies, and that diagnosis based on emerging technologies must be mutually complementary with traditional pathology
| 1. | Granter SR, Badizadegan K, Fletcher CD. Myofibromatosis in adults, glomangiopericytoma, and myopericytoma: a spectrum of tumors showing perivascular myoid differentiation. Am J Surg Pathol. 1998;22:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 253] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Antonescu CR, Sung YS, Zhang L, Agaram NP, Fletcher CD. Recurrent SRF-RELA Fusions Define a Novel Subset of Cellular Myofibroma/Myopericytoma: A Potential Diagnostic Pitfall With Sarcomas With Myogenic Differentiation. Am J Surg Pathol. 2017;41:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Papa B, Nguyen MA, Kumar A, Song L, Dorwal P, Cheah AL. Cellular myofibromas with SRF fusions: clinicopathological and molecular study of 3 cases of a rare entity and a potential mimic of sarcoma. Hum Pathol. 2023;138:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Jin L, Xu S, Wang J. [Adult myofibroma: a clinicopathological analysis of 15 cases]. Zhonghua Bing Li Xue Za Zhi. 2021;50:1335-1340. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Linos K, Carter JM, Gardner JM, Folpe AL, Weiss SW, Edgar MA. Myofibromas with atypical features: expanding the morphologic spectrum of a benign entity. Am J Surg Pathol. 2014;38:1649-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Mentzel T, Dry S, Katenkamp D, Fletcher CD. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol. 1998;22:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 195] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 7. | Nihous H, Macagno N, Baud-Massière J, Haffner A, Jouve JL, Gentet JC, Touzery C, Le Loarer F, Bouvier C. Genetic variant of SRF-rearranged myofibroma with a misleading nuclear expression of STAT6 and STAT6 involvement as 3' fusion partner. Virchows Arch. 2021;478:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Li Y, Huang D, Bi R, Yao Q, Ge L, Yu L, Zhou X, Yang W. Uterine tumours with myogenic differentiation harbouring SRF::RELA fusions. Histopathology. 2022;81:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Oudijk L, den Bakker MA, Hop WC, Cohen M, Charles AK, Alaggio R, Coffin CM, de Krijger RR. Solitary, multifocal and generalized myofibromas: clinicopathological and immunohistochemical features of 114 cases. Histopathology. 2012;60:E1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Calonje E, Fletcher CD. Myoid differentiation in dermatofibrosarcoma protuberans and its fibrosarcomatous variant: clinicopathologic analysis of 5 cases. J Cutan Pathol. 1996;23:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Fukasawa Y, Ishikura H, Takada A, Yokoyama S, Imamura M, Yoshiki T, Sato H. Massive apoptosis in infantile myofibromatosis. A putative mechanism of tumor regression. Am J Pathol. 1994;144:480-485. [PubMed] |
| 12. | Foss RD, Ellis GL. Myofibromas and myofibromatosis of the oral region: A clinicopathologic analysis of 79 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Chung EB, Enzinger FM. Infantile myofibromatosis. Cancer. 1981;48:1807-1818. [PubMed] [DOI] [Full Text] |
| 14. | Beck JC, Devaney KO, Weatherly RA, Koopmann CF Jr, Lesperance MM. Pediatric myofibromatosis of the head and neck. Arch Otolaryngol Head Neck Surg. 1999;125:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Pagès M, Pajtler KW, Puget S, Castel D, Boddaert N, Tauziède-Espariat A, Picot S, Debily MA, Kool M, Capper D, Sainte-Rose C, Chrétien F, Pfister SM, Pietsch T, Grill J, Varlet P, Andreiuolo F. Diagnostics of pediatric supratentorial RELA ependymomas: integration of information from histopathology, genetics, DNA methylation and imaging. Brain Pathol. 2019;29:325-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hrgovic Z, Germany; van Kessel A, The Netherlands S-Editor: Zhang H L-Editor: A P-Editor: Zhao S