Published online Oct 26, 2024. doi: 10.12998/wjcc.v12.i30.6374
Revised: July 14, 2024
Accepted: August 16, 2024
Published online: October 26, 2024
Processing time: 178 Days and 20.1 Hours
Visceral leishmaniasis (VL) is a systemic protozoan infection caused by Leishmania donovani (L. donovani) and transmitted by sand flies, causing macrophage invasion in the liver, spleen, and bone marrow. Diagnosis of VL is currently based on clinical signs, symptoms, and specific in-vitro markers and bone marrow investigations. However, VL's specific hematological and bone marrow manifestation in Sudanese pediatric patients is not well studied.
To examine the blood and bone marrow characteristics in pediatric patients from Sudan who have VL.
This is a retrospective hospital-based study with a sample of 107 consecutive Sudanese pediatric patients. The data focused on hematological and bone marrow results. We included only the completed records of the pediatric patients with VL in the Tropical Disease Teaching Hospital in Khartoum, Sudan from the period of 2016 to 2020.
The majority of pediatric patients included in this study are below 5-years-old (n = 59, 55.2%). Moreover, anemia, thrombocytopenia, and leukopenia were among the prevalent characteristics in the population under study. To further analyze the data, we developed a machine learning model using boosted forest algorithms to predict L. donovani parasites load, with a mean accuracy of 0.88 for the training dataset and an accuracy of 0.46, 0.50, and 0.74 for mild, moderate, and severe L. donovani parasite load in the validation dataset.
This study shows that the most common bone marrow change among Sudanese VL children was increased chronic inflammatory cells (n = 88, 82.2%) with present macrophage hemophagocytes (n = 103, 96.3%). While anemia and thrombocytopenia were the most common hematological changes. These results will hopefully lead to an early diagnosis and hence better management for Sudanese pediatric patients with suspected VL.
Core Tip: Visceral leishmaniasis (VL) is a neglected tropical disease; although many parts of Sudan are hyperendemic, many centers have very limited diagnostic capabilities and data reporting. This is one of the first studies from Sudan to investigate both hematological and bone marrow changes associated with pediatric VL and Leishmania donovani load from bone marrow biopsies. The data were collected from tertiary and specialized centers for treating VL in the country and modern analytical methods were used to describe the result for the global audience and highlight the importance of these new findings derived from a country that is hyperendemic to VL.
- Citation: Elnoor ZIA, Abdelmajeed O, Mustafa A, Gasim T, Musa SAM, Abdelmoneim AH, Omer IIA, Fadl HAO. Hematological picture of pediatric Sudanese patients with visceral leishmaniasis and prediction of leishmania donovani parasite load. World J Clin Cases 2024; 12(30): 6374-6382
- URL: https://www.wjgnet.com/2307-8960/full/v12/i30/6374.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i30.6374
Visceral leishmaniasis (VL) or Kala-azar caused by Leishmania donovani (L. donovani) is a tropical neglected disease[1] and a major health problem in Sudan, particularly among newborns and young adults with an estimated pooled prevalence of 18% according to the meta-analysis done by Ahmed et al[2] in Sudan. The disease is endemic mainly in the eastern and central parts of the country, which lies between four states (white Nile State in the west, Gadarif State in the east, Blue Nile State in the south, and Kassala State in the northeast)[3]. Globally, according to World Health Organization, an estimated 50000 to 90000 new cases of VL occur worldwide annually mostly in Brazil, India, and East Africa including Sudan[4].
Due to parasite replication in the reticuloendothelial system, L. donovani can accumulate in the spleen, liver, and bone marrow[5], this causes hemolysis, splenic sequestration, and bone marrow suppression resulting in severe anemia. Also, edema, hypoalbuminemia, and severe cachexia are linked to advanced kala-azar[6], while ascites, jaundice, and hepatic dysfunction might appear late in the course of the illness, moreover, hemorrhagic complications can result from thrombocytopenia and hepatic dysfunction[5]. Rarely, chronic diarrhea and malabsorption can occur because of parasitic invasion of the intestine[5].
In VL, peripheral hematological changes are well studied[7,8]. However, bone marrow changes have been less reported in the literature[9-11]. The alterations in peripheral hematological parameters were reported from different pediatric populations around the world[10,11]. Currently, only a few outdated studies assess and predict bone marrow cytology and histopathological biopsies in children with VL[12-14] emphasizing the need for updating the evidence. Sudan is a hyperendemic region for VL, yet there are limited data on bone marrow changes associated with the disease, largely due to limited clinical resources in much of the country. Unlike traditional hematological changes investigated by complete blood counts, this study analyzed bone marrow histopathological biopsies associated with VL using novel machine-learning methods. Additionally, this study aims to identify fewer common changes in the bone marrow, including bone marrow aplasia, granuloma, hemophagocytosis, histiocytosis, and co-infections associated with VL. These findings could contribute to developing improved management plans for VL patients.
This is a retrospective study adopted STROBE guideline and conducted as a collaboration between the Tropical Diseases Teaching Hospital (Pediatrics department) and the hematology and laboratory department in Mohamed El-Amin Hamid Pediatric Teaching Hospital [both are centers for leishmaniasis diagnosis and management) for children which is located in Khartoum state Omdurman, Sudan. At Tropical Diseases Teaching Hospital which is a national referral and spe
In regions where VL is hyperendemic like Sudan, positive VL antibody test results may be observed among asymptomatic individuals with subclinical infection or recovered VL patients. These antibody tests have false positive results[15]. In these cases, bone marrow biopsies are necessary for accurate diagnosis and treatment. These biopsies are taken by skilled medical professionals to Mohamed El-Amin Hamid Hospital for analysis and examination. The later hospital specializes in conducting detailed examinations of bone marrow samples, utilizing advanced laboratory techniques and equipment. By collaborating with Mohamed El-Amin Hamid Pediatric Teaching Hospital, we ensured that patients received accurate and reliable results from their bone marrow biopsies, enabling a clear diagnosis and effective treatment, management, and follow-up.
This study was conducted among all the consecutive pediatric VL patients who have complete hematological and bone marrow reports and were diagnosed at the Tropical Disease Teaching Hospital from 2016 to 2020.
A bone marrow aspiration and trephine biopsy were performed according to the local sterile procedure guidelines. Here, the skin around the posterior iliac crest was cleaned, and 2-5 mL of 2% lidocaine was injected into the skin, subcutaneous tissue, and periosteum as a local anesthetic. After waiting 2-3 minutes for anesthesia to take effect, we selected a stainless-steel aspiration needle, size 16G or 18G, depending on the patient's age and size. For the trephine biopsy, we used a 16G needle. The patient was positioned laterally with the right knee flexed. The needle was inserted perpendicularly into the cavity of the ilium at the center of the oval posterior superior iliac spine. Once the bone was penetrated, the stiletto was removed, and marrow contents were aspirated for film preparation. Using the same opening, a trephine biopsy was obtained, with the sample size not exceeding 2 cm for pediatric patients. In terms of data collection, demographic, hematological data, and laboratory records reports (CBC and bone marrow) of patients diagnosed with VL at Tropical Disease Teaching Hospital were collected during the study period according to the following criteria:
Inclusion and exclusion criteria: Patients less than 18-years-old, who were diagnosed and confirmed with VL at the Tropical Disease Teaching Hospital laboratory unit during the study period, although patients who have incomplete forms were excluded from the study.
Ethical clearance: We obtained written ethical approval from the Tropical Disease Teaching Hospital administrative authority (TDTH/B/51/1 data: January 1, 2021). The information acquired was kept confidential and only utilized in the context of this study.
Age, sex, traveling history and L. donovani load are independent variables. Peripheral hematological alterations (hemoglobin level, platelets, and white blood cell count) and bone marrow aspirate changes (cellularity, hemophagocytosis, chronic inflammatory cells, and megaloblastic changes) were dependent variables. For quantitative data, we used descriptive statistics in the form of frequency tables with percentages and graphs. The χ2 test and Cochran-Armitage test were used to examine the associations between bone marrow alterations due to VL and the other relevant factors. Any P value ≤ 0.05 was judged statistically significant.
Moreover, we developed an artificial intelligence algorithm to predict the L. donovani load from our dataset. Here, we used boosted forest algorithms with training data composed of 75% and validation data accounting for 25%. We used an early regularization technique to avoid model overfitting, and the optimal numbers of iteration and sampled splits were implemented by the algorithm. We visualized the data using the receiver operating characteristic curve (ROC), and lift curve to examine model performance compared to the baseline random classifier. In the L. donovani load model, entropy R Square is 0.26, -0.004 and RMSE is 0.56 and 0.66 for training and validation data, respectively. This indicates weak moderate predictability[16] for severe cases but not for mild and moderate parasitic loads. We analyzed the data using a combination of statistical software including SPSS 26, SAS JMP 14, and NCSS12.
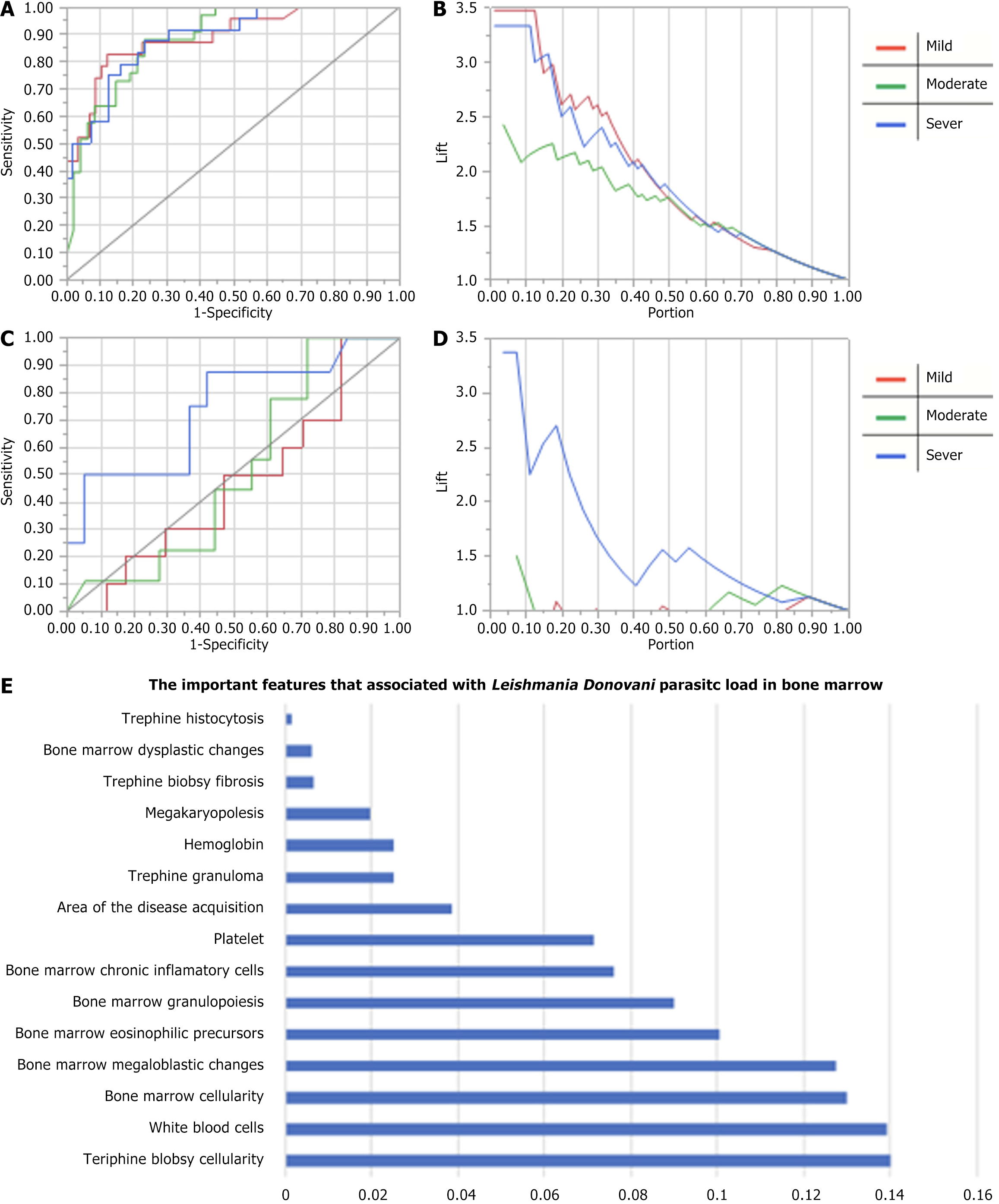
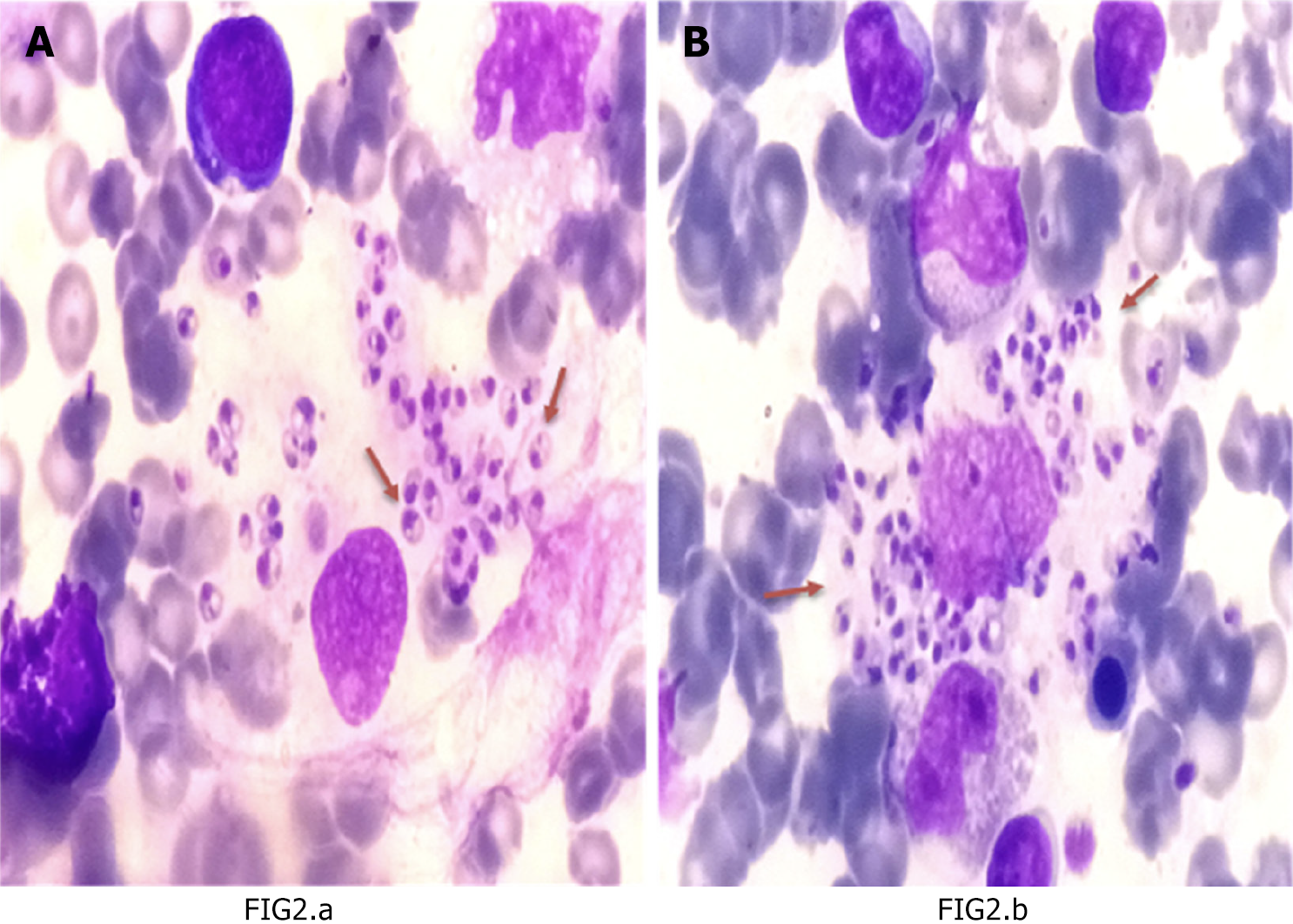
The majority of data analyzed in this study indicate that the major part of the samples studied are male patients and are under 5-years-old, as illustrated in Table 1. Furthermore, anemia, thrombocytopenia, and leukopenia were prevalent in the study population. Additional parameters are detailed in Table 1. Notable results from the study indicate that most trephine biopsy samples showed normoplasia 57.0% (n = 61) and a high prevalence of histiocytosis 92.5% (n = 99). Additionally, bone marrow analysis revealed hypercellularity in 40.2% (n = 43) of cases, with 46.2% (n = 49) exhibiting active megakaryopoiesis. Hemophagocytosis was also highly prevalent, observed in 96.3% (n = 103) of bone marrow samples. Increased inflammatory cells in bone marrow were observed among 82.2% (n = 88) (Table 2). Moreover, a statistical model was designed and validated to further analyze the data, as depicted in Figure 1. A bone marrow biopsy sample is shown in Figure 2.
| Characteristic | Subcategory | Count | % |
| Background characteristics | |||
| Age group | Birth to 1 year | 11 | 10.3 |
| 1 to 5 years | 48 | 44.9 | |
| 5 to 10 years | 23 | 21.5 | |
| 10 to 18 years | 25 | 23.4 | |
| Sex | Male | 76 | 71.0 |
| Female | 31 | 29.0 | |
| Area of the disease acquisition | Traveling to an endemic area | 17 | 15.9 |
| Residence in endemic area | 90 | 84.1 | |
| Peripheral hematological parameter | |||
| Peripheral hematological changes hemoglobin | Less than 5 mg/dL | 30 | 28.0 |
| 5 to 10 mg/dL | 68 | 63.6 | |
| More than 10 mg/dL | 9 | 8.4 | |
| Peripheral hematological changes platelet | Less than 50 × 103/µL | 42 | 39.3 |
| 50 to 100 × 103/µL | 57 | 53.3 | |
| 100 to 150 × 103/µL | 7 | 6.5 | |
| More than 150 × 103/µL | 1 | 0.9 | |
| Peripheral hematological changes white blood cells | Less than 2 × 103/µL | 26 | 24.3 |
| 2 to 4 × 103/µL | 64 | 59.8 | |
| 4 to 10 × 103/µL | 15 | 14.0 | |
| More than 10 × 103/µL | 2 | 1.9 | |
| Findings | Subcategory | Count | % |
| Trephine biopsy findings | |||
| Trephine biopsy cellularity | Hypoplasia | 15 | 14.0 |
| Normoplasia | 61 | 57.0 | |
| Hyperplasia | 31 | 29.0 | |
| Trephine biopsy fibrosis | No | 100 | 93.5 |
| Yes | 7 | 6.5 | |
| Trephine histiocytosis | No | 8 | 7.5 |
| Yes | 99 | 92.5 | |
| Trephine granuloma | No | 96 | 89.7 |
| Yes | 11 | 10.3 | |
| Trephine parasite load | Mild | 33 | 30.8 |
| Moderate | 42 | 39.3 | |
| Severe | 32 | 29.9 | |
| Bone marrow findings | |||
| Bone marrow cellularity | Hypocellular | 14 | 13.1 |
| Normocellular | 50 | 46.7 | |
| Hypercellular | 43 | 40.2 | |
| Megakaryopoiesis | Adequate | 15 | 14.2 |
| Decreased | 42 | 39.6 | |
| Active | 49 | 46.2 | |
| Bone marrow dysplastic changes | No | 12 | 11.2 |
| Yes | 95 | 88.8 | |
| Bone marrow megaloblastic changes | No | 16 | 15.0 |
| Yes | 91 | 85.0 | |
| Bone marrow granulopoiesis | Orderly maturation | 6 | 5.6 |
| Arrest maturation | 73 | 68.2 | |
| Hyperactive | 28 | 26.2 | |
| Bone marrow eosinophilic precursors | Depressed | 46 | 43.0 |
| Prominent | 61 | 57.0 | |
| Bone marrow hemophagocytosis | No | 4 | 3.7 |
| Yes | 103 | 96.3 | |
| Bone marrow chronic inflammatory cells | Depressed | 19 | 17.8 |
| Increased | 88 | 82.2 | |
In this retrospective study, we analyzed 107 VL pediatric patient records from 2016-2020 from a tropical disease hospital. Most of the patients were male 71% (n = 76), 55.2% (n = 59) of pediatric patients with VL were below the age of five, and only 23.4% (n = 25) were between 10 years and 18 years (Table 1). This is like studies conducted in Iraq which report that most of the cases were below 5 years[17]. In this study, most of the patients live in endemic regions to VL in Sudan 84.1% (n = 90), and only 15.9% (n = 17) acquired VL through traveling.
In the current study, most of the VL patients presented with pancytopenia, as 91.6% (n = 98) had hemoglobins less than 10 mg/dL, 92.6% (n = 99) had platelet counts less than 100 × 103/µL, and 84.1% (n = 90) had white blood cell counts less than 4 × 103/µL (Table 1). These findings which describe the peripheral blood changes are common hematological findings in VL patients[17]. The high level of pancytopenia in this patient group is probably because of the longer course of VL and splenomegaly before presentation to the hospital and increased peripheral blood destruction[18]. The leading causes of anemia among VL patients are sequestration and destruction of RBCs in an enlarged spleen and alteration in RBC membrane permeability. Moreover, we report that only one VL patient has a platelet count of more than 150 × 103/µL like Daneshbod et al[19] who found that thrombocytopenia occurred in 80%-90% of the VL patients. It is postulated that the thrombocytopenia and leukopenia observed in the peripheral blood of VL are due to hemophagocytosis and partly because of poor platelet formation[20].
Previous studies found that there is an association between VL and bone marrow change in patients[21]. In the current study, the trephine biopsy data showed that 29% (n = 31) of VL patients have hyperplastic bone marrow activities, whereas 93.5% (n = 100), and 89.7% (n = 96) of them do not have trephine fibrosis and trephine granuloma, respectively. However, 92.5℅ (n = 99) have trephine histiocytosis and this is because of bone marrow hyperactivity and hepatosplenomegaly. Moreover, only 30.8% (n = 33) of VL patients have a mild L. donovani load and the remaining 69.2% (n = 74) (Table 2) have moderate to high L. donovani load. This rise in parasitic load can be explained by several immune evading mechanisms of the organism.
Moreover, among VL patients, 88.8% (n = 95), 85% (n = 91), 96.3% (n = 103), and 82.2% (n = 88) have bone marrow dysplastic, megaloblastic, hemophagocytosis activities and presence of chronic inflammatory cells, respectively. The bone marrow data showed hypercellularity and granulopoiesis hyperactivity in 40.2% (n = 43), and 26.2% (n = 28), respectively, (Table 2). Children below 5 years are more frequently affected to have trephine biopsy changes, with an increase in L. donovani parasite load ranging from moderate to severe[22,23].
Moreover, a higher L. donovani load is associated with a decrease in megaloblastic maturation activities, eosinophilic precursors, and chronic inflammatory cells P value < 0.05. This inverse relation has been noted before in previous studies in both humans and animals, especially in mice and dogs. The increased eosinophils affect the parasite count by either directly phagocytizing the parasite or improving the antimicrobial activity of the macrophage[24]. Figure 1A-D illustrate the accuracy matrix for predicting the L. donovani load from the study variables. Figure 1A and B show the ROC, and fit curves for the training data, respectively, with a mean of 0.88 AUC for all L. donovani load categories, however, the area under the curve (AUC) for the validation set varies considerably as the AUC is 0.46, 0.50 and 0.74 for mild, moderate, and severe L. donovani load in the validation set. This variation indicates that the model can predict severe L. donovani load better than mild or moderate load, Figure 1C shows that the validation prediction for mild and moderate parasite load is weak, and below the random classifier, as shown in Figure 1C and D. Only severe cases were predicted better than the random classifier. The variables and features that are associated with L. donovani load prediction include bone marrow cellularity, white blood cells, and megaloblastic changes (Figure 1E). However, a high misclassification rate is present in the training dataset 29% and 62% in the validation dataset, despite using a robust algorithm. It is hard to predict the parasitic load with good accuracy indicating the need of more samples to tune the algorithm again for better generalization and predictability. There are several limitations to this study. To begin with, this study was a retrospective single-center study. Single-center studies may have significant bias due to insufficient data heterogeneity[25]. To further verify the accuracy and clinical applicability of these prognostic models, more patients from other centers are required. In this case, we emphasize the need for international collaborative cohort studies[26] from all the VL treating centers in the world. This approach can generate generalizable results that can inform better diagnostic models and enhance the VL research evidence.
This study shows most patients with VL are living in the disease’s endemic regions in Sudan. In addition to peripheral pancytopenia, bone marrow normoplasia and histiocytosis are common. Additionally, common bone marrow changes associated with VL include hypercellularity, megakaryopoiesis, hemophagocytosis, and increased inflammatory cells and eosinophilic precursors. The mean accuracy for predicting the L. donovani load is 0.88 for the training dataset, and accuracy of 0.46, 0.50, and 0.74 for mild, moderate, and severe parasite load in the validation dataset.
We would like to express our deepest gratitude to Ms. Paula Greenspan for her invaluable support in reviewing the English language of this work. We are immensely grateful for her dedication and assistance.
| 1. | Casulli A, Antinori S, Bartoloni A, D'Amelio S, Gabrielli AF, Gazzoli G, Rinaldi L, Bruschi F; IN-NTD network. Neglected Tropical Diseases in Italy: introducing IN-NTD, the Italian network for NTDs. Parasitology. 2023;150:1082-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Ahmed M, Abdulslam Abdullah A, Bello I, Hamad S, Bashir A. Prevalence of human leishmaniasis in Sudan: A systematic review and meta-analysis. World J Methodol. 2022;12:305-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 3. | Dereure J, El-Safi SH, Bucheton B, Boni M, Kheir MM, Davoust B, Pratlong F, Feugier E, Lambert M, Dessein A, Dedet JP. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes Infect. 2003;5:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | WHO. Leishmaniasis. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. |
| 5. | Mueller YK, Nackers F, Ahmed KA, Boelaert M, Djoumessi JC, Eltigani R, Gorashi HA, Hammam O, Ritmeijer K, Salih N, Worku D, Etard JF, Chappuis F. Burden of visceral leishmaniasis in villages of eastern Gedaref State, Sudan: an exhaustive cross-sectional survey. PLoS Negl Trop Dis. 2012;6:e1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Costa CHN, Chang KP, Costa DL, Cunha FVM. From Infection to Death: An Overview of the Pathogenesis of Visceral Leishmaniasis. Pathogens. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 7. | Pippard MJ, Moir D, Weatherall DJ, Lenicker HM. Mechanism of anaemia in resistant visceral leishmaniasis. Ann Trop Med Parasitol. 1986;80:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Marwaha N, Sarode R, Gupta RK, Garewal G, Dash S. Clinico-hematological characteristics in patients with kala azar. A study from north-west India. Trop Geogr Med. 1991;43:357-362. [PubMed] |
| 9. | Idris M, Farid J, Gul N. Morphology of Bone Marrow In Visceral Leishmaniasis. J Ayub Med Coll Abbottabad. 2018;30:342-344. [PubMed] |
| 10. | Al-Ghazaly J, Al-Dubai W, Abdullah M, Al-Gharasi L. Hematological Characteristics of Yemeni Adults and Children with Visceral Leishmaniasis. Could Eosinopenia be a Suspicion Index? Mediterr J Hematol Infect Dis. 2017;9:e2017056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Solmaz S, Boğa C, Kozanoğlu İ, Asma S, Turunç T, Demiroğlu YZ. A rare hematological complication of visceral leishmaniasis: hemophagocytic syndrome. Cukurova Med J. 2016;41:161. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Al-Sohaibani MO. Bone marrow histopathological changes in visceral leishmaniasis. Ann Saudi Med. 1996;16:304-307. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Rohtagi A, Agarwal SK, Bose M, Chattopadhya D, Saha K. Blood, bone marrow and splenic lymphocyte subset profiles in Indian visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1996;90:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kumar PV, Vasei M, Sadeghipour A, Sadeghi E, Soleimanpour H, Mousavi A, Tabatabaei AH, Rizvi MM. Visceral leishmaniasis: bone marrow biopsy findings. J Pediatr Hematol Oncol. 2007;29:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Bern C, Chowdhury R, Breiman RF, Wahed MA, Wagatsuma Y, Secor WE, Maguire JH, Ali M, Vaz L, Amann J, Kurkjian KM, Williamson J, Haque R. The epidemiology of visceral leishmaniasis and asymptomatic leishmanial infection in a highly endemic bangladeshi village. The American Journal of Tropical Medicine and Hygiene. 2007;76:909-914. [RCA] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Chai T, Draxler RR. Root mean square error (RMSE) or mean absolute error (MAE)? – Arguments against avoiding RMSE in the literature. Geosci Model Dev. 2014;7:1247-1250. [DOI] [Full Text] |
| 17. | Majeed B, Sobel J, Nawar A, Badri S, Muslim H. The persisting burden of visceral leishmaniasis in Iraq: data of the National Surveillance System, 1990-2009. Epidemiol Infect. 2013;141:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Debash H, Bisetegn H, Nigatie M, Abeje G, Feleke DG. Epidemiological, clinical and hematological profiles of visceral leishmaniasis among patients visiting Tefera Hailu Memorial Hospital, Northeast Ethiopia: a 4 year retrospective study. Sci Rep. 2023;13:931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 19. | Daneshbod Y, Dehghani SJ, Daneshbod K. Bone marrow aspiration findings in kala-azar. Acta Cytol. 2010;54:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Kumar V, Agarwal P, Marwah S, Nigam AS, Tiwari A. Spectrum of clinicohematological profile and its correlation with average parasite density in visceral leishmaniasis. Cytojournal. 2018;15:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Sarkari B, Naraki T, Ghatee MA, Abdolahi Khabisi S, Davami MH. Visceral Leishmaniasis in Southwestern Iran: A Retrospective Clinico-Hematological Analysis of 380 Consecutive Hospitalized Cases (1999-2014). PLoS One. 2016;11:e0150406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Elnaiem DE. Ecology and control of the sand fly vectors of Leishmania donovani in East Africa, with special emphasis on Phlebotomus orientalis. J Vector Ecol. 2011;36 Suppl 1:S23-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | el-Hassan AM, Zijlstra EE. Leishmaniasis in Sudan. Mucosal leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95 Suppl 1:S19-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Rodríguez NE, Wilson ME. Eosinophils and mast cells in leishmaniasis. Immunol Res. 2014;59:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Kotani Y, Turi S, Ortalda A, Baiardo Redaelli M, Marchetti C, Landoni G, Bellomo R. Positive single-center randomized trials and subsequent multicenter randomized trials in critically ill patients: a systematic review. Crit Care. 2023;27:465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1203] [Cited by in RCA: 1244] [Article Influence: 207.3] [Reference Citation Analysis (4)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/