Published online Sep 16, 2024. doi: 10.12998/wjcc.v12.i26.5901
Revised: July 9, 2024
Accepted: July 12, 2024
Published online: September 16, 2024
Processing time: 67 Days and 18 Hours
Being too light at birth can increase the risk of various diseases during infancy.
To explore the effect of perinatal factors on term low-birth-weight (LBW) infants and build a predictive model. This model aims to guide the clinical management of pregnant women’s healthcare during pregnancy and support the healthy growth of newborns.
A retrospective analysis was conducted on data from 1794 single full-term pregnant women who gave birth. Newborns were grouped based on birth weight: Those with birth weight < 2.5 kg were classified as the low-weight group, and those with birth weight between 2.5 kg and 4 kg were included in the normal group. Multiple logistic regression analysis was used to identify the factors in
Among the 1794 pregnant women, there were 62 cases of neonatal weight < 2.5 kg, resulting in an LBW incidence rate of 3.46%. The factors influencing full-term LBW included low maternal education level [odds ratio (OR) = 1.416], fewer prenatal examinations (OR = 2.907), insufficient weight gain during pregnancy (OR = 3.695), irregular calcium supplementation during pregnancy (OR = 1.756), and pregnancy hypertension syndrome (OR = 2.192). The prediction model equation was obtained as follows: Logit (P) = 0.348 × maternal education level + 1.067 × number of prenatal examinations + 1.307 × insufficient weight gain during pregnancy + 0.563 × irregular calcium supplementation during pregnancy + 0.785 × pregnancy hypertension syndrome − 29.164. The area under the ROC curve for this model was 0.853, with a sensitivity of 0.852 and a specificity of 0.821. The Hosmer–Leme show test yielded χ2 = 2.185, P = 0.449, indicating a good fit. The overall accuracy of the clinical validation model was 81.67%.
The occurrence of full-term LBW is related to maternal education, the number of prenatal examinations, weight gain during pregnancy, calcium supplementation during pregnancy, and pregnancy-induced hypertension. The constructed predictive model can effectively predict the risk of full-term LBW.
Core Tip: Being too light at birth can increase the risk of various diseases during infancy. While premature birth is a significant factor causing low-birth-weight (LBW) infants, it involves many uncontrollable factors. Our research innovation lies in excluding premature infants and analyzing only the factors influencing LBW in full-term infants, thereby revealing the impact of other characteristics on LBW. We discovered that full-term LBW is related to maternal education level, frequency of prenatal examinations, weight gain during pregnancy, calcium supplementation during pregnancy, and factors associated with preeclampsia. Based on these findings, we constructed a risk prediction model that can effectively predict the risk of full-term LBW.
- Citation: Xu L, Sheng XJ, Gu LP, Yang ZM, Feng ZT, Gu DF, Gao L. Influence of perinatal factors on full-term low-birth-weight infants and construction of a predictive model. World J Clin Cases 2024; 12(26): 5901-5907
- URL: https://www.wjgnet.com/2307-8960/full/v12/i26/5901.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i26.5901
The birth weight of a newborn reflects fetal growth and development in utero and is an important indicator of maternal and child health in a community. Low-birth-weight (LBW) refers to a birth weight of less than 2.5 kg[1,2]. Babies who are underweight at birth are at an increased risk of various diseases and even death during infancy[3]. LBW is also closely related to physical and mental development during adolescence and chronic conditions such as cardiovascular disease and diabetes in adulthood[4-6]. Thus, newborn birth weight is crucial for their future health. The causes of LBW are complex, as any factor affecting the rate of fetal growth and development in the uterus can lead to being underweight at birth[7]. Premature birth has been recognized as a significant cause of LBW, but it involves many uncontrollable factors, such as multiple births and low placental position. This study excludes premature infants, focusing only on the influencing factors of LBW in full-term infants. By doing so, we aim to reveal the impact of other characteristics on LBW and develop a corresponding risk prediction model. This model will provide valuable insights for the clinical management of pregnant women’s healthcare during pregnancy and promote the healthy growth of newborns.
This study retrospectively analyzed data from 1794 women with singleton full-term pregnancies who delivered at Suzhou Ninth People’s Hospital from January 2019 to December 2021. The newborns were grouped based on birth weight: Those weighing less than 2.5 kg were classified as the low-weight group, and those weighing between 2.5 kg and 4 kg were classified as the normal group. The perinatal data from both groups were analyzed to identify the factors influencing full-term LBW and to construct a prediction model. Additionally, maternal data from 300 women with singleton full-term pregnancies who delivered at Suzhou Ninth People’s Hospital from January 2022 to December 2023 were used for model validation. The maternal data from both periods met the following conditions: (1) Pregnant women aged 18 years or older; (2) Gestational age at delivery between 37 and 42 weeks, and (3) No history of anemia or smoking. Exclusion criteria included the following: (1) The presence of a malignant tumor; (2) Pregnancy through assisted reproductive technology; (3) Multiple pregnancies; and (4) Macrosomia (newborn weight greater than 4 kg).
Data were collected from the hospital information system on maternal age, education level, number of prenatal examinations, weight gain during pregnancy, calcium supplementation during pregnancy, pregnancy complications (e.g., pregnancy hypertension syndrome, gestational diabetes mellitus, and pregnancy-associated thyroid disease), fetal distress, premature rupture of membranes, amniotic fluid volume, gestational age at delivery, and the newborn’s weight and sex within 1 hour of birth.
Pregnancy weight gain (gestational weight gain) was calculated as weight at delivery (kg) minus the weight before pregnancy (kg). According to the guidelines for maternal weight gain during pregnancy, weight gain ranges are classified as follows[8]: 12.5–18.0 kg for underweight before pregnancy, 11.5–16.0 kg for normal weight before pregnancy, and 7.0–11.5 kg for overweight before pregnancy. Weight gain within these ranges is considered appropriate, below the lower limit is classified as insufficient, and above the upper limit is considered excessive.
Calcium supplementation during pregnancy was defined according to the “Dietary Guidelines for Pregnant Women”[9]. Regular calcium supplementation is considered an intake of ≥ 1000 mg/day from early pregnancy.
Less amniotic fluid was determined using B-ultrasound, with a maximum vertical depth of the amniotic fluid dark area ≤ 2 cm or an amniotic fluid index ≤ 5 cm[10].
The Statistical Package for the Social Sciences 19.0 software was used for data analysis. Measurement data are expressed as mean ± SD and were compared between the two groups using the t-test. Categorical variables are expressed as frequencies and were compared using the χ² test. Multivariate logistic regression was employed to analyze the factors influencing LBW in full-term infants, and a prediction model formula was constructed based on these factors. The Hosmer–Leme show test and receiver operating characteristic (ROC) curve analysis were used to evaluate the performance of the model. Maternal data from different time points were included to test the accuracy of the model’s predictions. Statistical significance was set at P < 0.05.
Among 1794 neonates born to singleton full-term pregnant women, 62 cases had a birth weight of less than 2.5 kg (low-weight group), resulting in an incidence rate of 3.46%. The remaining 1732 neonates had a birth weight between 2.5 kg and 4.0 kg (normal group). Statistically significant differences (P < 0.05) were found between the low-weight and normal groups regarding maternal education level, number of prenatal examinations, weight gain during pregnancy, regular calcium supplementation during pregnancy, and pregnancy hypertension syndrome (Table 1).
| Factor | Low-weight group | Normal group | t/χ2 value | P value |
| Maternal age (years) (mean ± SD) | 28.24 ± 4.09 | 28.14 ± 4.39 | 0.685 | 0.495 |
| Maternal education level | 14.628 | < 0.001 | ||
| Junior high school and below | 27 (43.55) | 392 (22.63) | ||
| High school and above | 35 (56.45) | 1340 (77.37) | ||
| Frequency of prenatal examination (times) (mean ± SD) | 10.01 ± 0.35 | 10.57 ± 0.39 | 8.278 | < 0.001 |
| Weight gain during pregnancy | ||||
| Insufficient | 37 (59.68) | 119 (6.87) | 210.361 | < 0.001 |
| Suitable | 21 (33.87) | 1412 (81.52) | ||
| Overweight | 4 (6.45) | 201 (11.61) | ||
| Calcium supplementation during pregnancy | 15.232 | < 0.001 | ||
| Rule | 13 (20.97) | 798 (46.07) | ||
| Irregularity | 49 (79.03) | 934 (53.93) | ||
| Pregnancy hypertension syndrome | 12.162 | < 0.001 | ||
| Yes | 9 (14.52) | 81 (4.68) | ||
| No | 53 (85.48) | 1651 (95.32) | ||
| Gestational diabetes | 0.548 | 0.459 | ||
| Yes | 7 (11.29) | 254 (14.67) | ||
| No | 55 (88.71) | 1478 (85.33) | ||
| Pregnancy complicated with thyroid disease | 0.254 | 0.614 | ||
| Yes | 4 (6.45) | 87 (5.02) | ||
| No | 58 (93.55) | 1645 (94.98) | ||
| Fetal distress | 1.386 | 0.239 | ||
| Yes | 8 (12.90) | 149 (8.60) | ||
| No | 54 (87.10) | 1583 (91.40) | ||
| Premature rupture of membranes | 2.547 | 0.110 | ||
| Yes | 6 (9.68) | 88 (5.08) | ||
| No | 56 (90.32) | 1644 (94.92) | ||
| Oligohydramnios | 2.968 | 0.085 | ||
| Yes | 5 (8.06) | 65 (3.75) | ||
| No | 57 (91.94) | 1667 (96.25) | ||
| Gestational weeks at delivery (weeks) (mean ± SD) | 38.43 ± 0.96 | 38.72 ± 1.13 | 1.515 | 0.132 |
| Neonatal sex | 2.882 | 0.090 | ||
| Male | 22 (35.48) | 804 (46.42) | ||
| Female | 40 (64.52) | 928 (53.58) |
The dependent variable was whether full-term newborns were LBW (0 = no, 1 = yes). Independent variables included the statistically significant indicators from the single-factor analysis (Table 1). Multivariate logistic regression analysis showed that low maternal education level, fewer prenatal examinations, insufficient weight gain during pregnancy, irregular calcium supplementation during pregnancy, and pregnancy hypertension syndrome were all significant influencing factors of full-term LBW (P < 0.05) (Table 2).
| Variable | β | SE | χ2 | P value | OR (95%CI) |
| Low level of maternal education | 0.348 | 0.134 | 6.744 | 0.012 | 1.416 (1.058–2.975) |
| Low number of prenatal examinations | 1.067 | 0.282 | 14.316 | < 0.001 | 2.907 (1.893–6.278) |
| Insufficient weight gain during pregnancy | 1.307 | 0.329 | 15.782 | < 0.001 | 3.695 (2.092–7.455) |
| Irregular calcium supplementation during pregnancy | 0.563 | 0.204 | 7.617 | 0.009 | 1.756 (1.146–3.984) |
| Pregnancy hypertension syndrome | 0.785 | 0.226 | 12.065 | < 0.001 | 2.192 (1.423–5.159) |
| Constant | −29.164 | 6.574 | 19.680 | < 0.001 | - |
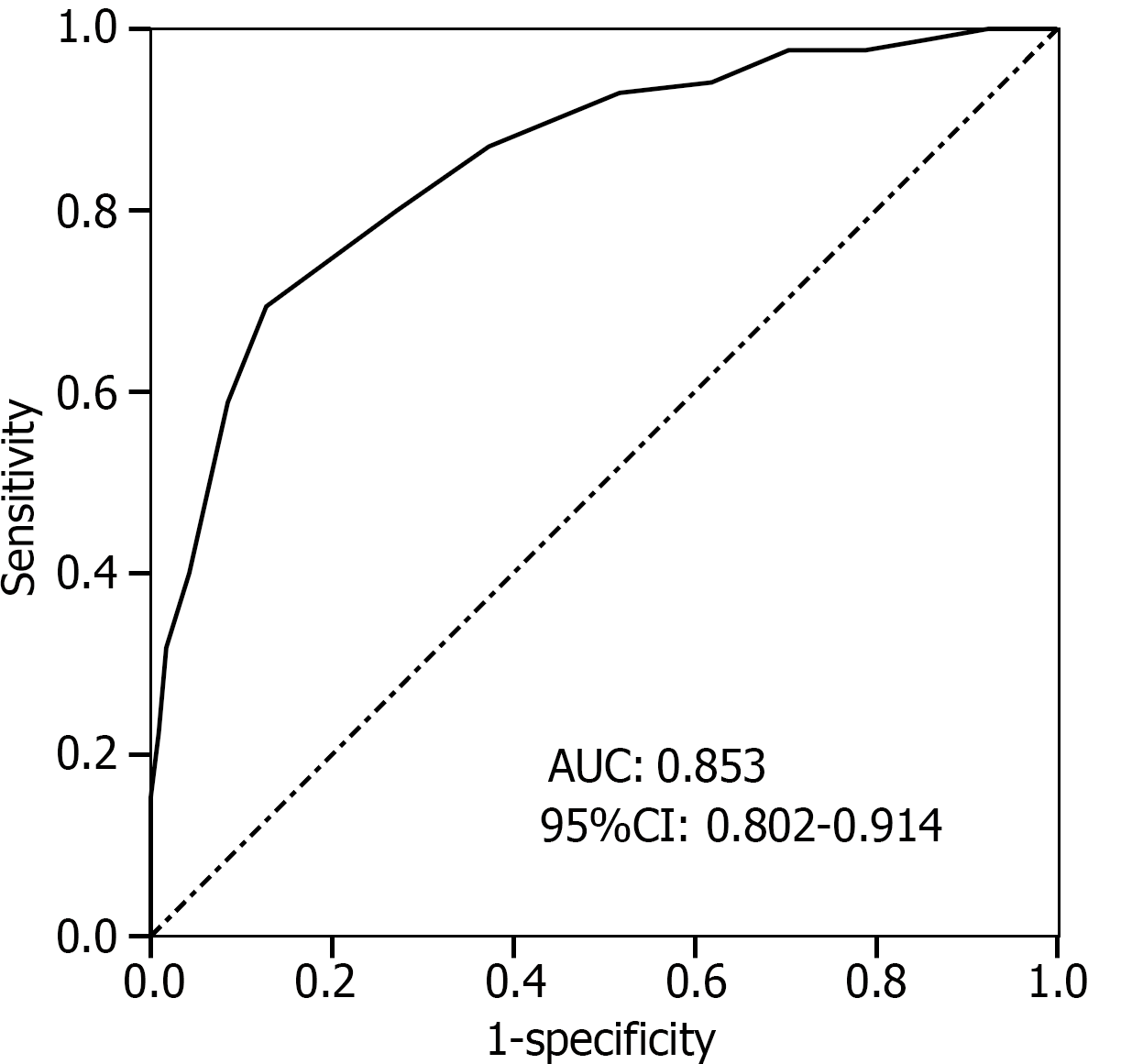
The predictive model was constructed based on the regression coefficients and constant terms of the influencing factors identified through multivariate logistic regression analysis (Table 2). The risk prediction model equation is as follows: Logit (P) = 0.348 × maternal education level (0 = high school and above; 1 = junior high school and below) + 1.067 × number of prenatal examinations (actual value) + 1.307 × insufficient weight gain during pregnancy (0 = no; 1 = yes) + 0.563 × calcium supplementation during pregnancy (0 = regular; 1 = irregular) + 0.785 × pregnancy hypertension syndrome (0 = no; 1 = yes) − 29.164. The area under the ROC curve of the risk prediction model was 0.853 (95%CI: 0.802–0.914), with a sensitivity of 0.852 and a specificity of 0.821 (Figure 1). The Hosmer–Leme show goodness-of-fit test showed that the predicted value of the model and the actual value were χ2 = 2.185, P = 0.449, demonstrating a good fit.
The model was validated using data from 300 full-term deliveries at Suzhou Ninth People’s Hospital from January 2022 to December 2023. The sensitivity of the model in predicting the risk of full-term LBW was 83.33% (10/12), the specificity was 81.59% (235/288), and the accuracy rate was 81.67% (245/300) (Table 3).
| Model prediction results | Actual results | Total | Sensitivity | Specificity | Accuracy | |
| LBW infants | Normal-birth-weight infants | |||||
| LBW infants | 10 | 53 | 63 | |||
| Normal-birth-weight infants | 2 | 235 | 237 | |||
| Total | 12 | 288 | 300 | 83.33 | 81.59 | 81.67 |
The occurrence of full-term LBW is complex and likely results from the interaction of multiple factors. Previous research by Chinese scholars, who surveyed 103678 newborns in 39 hospitals across 14 provinces, found an LBW incidence of 7.21%[11]. In contrast, our study found the incidence of full-term LBW to be 3.46% (62/1794), which is significantly lower. This discrepancy may be due to the exclusion of premature births and multiple pregnancies in this study, as well as regional economic differences that impact LBW rates.
There is a correlation between an individual’s educational level and their professional environment and living standards. Kundu et al[12] reported that low education levels in pregnant women, combined with a low family wealth index, have a cumulative effect on LBW, which is consistent with our findings. Pregnant women with higher education levels are more likely to have better knowledge of pregnancy care and maternal and child health, which promotes fetal development and growth. Conversely, those with lower education levels often have poorer economic conditions[13,14], leading to limited access to nutrition and healthcare[15]. This can result in neglect of nutrition and healthcare during pregnancy, adversely affecting fetal development and increasing the risk of LBW.
Prenatal examination is crucial for the timely detection of abnormal characteristics in pregnant women, such as gestational hypertension, umbilical cord abnormalities, and placental abnormalities. Bellizzi et al[16] demonstrated that inadequate prenatal care consultations are associated with LBW. Nagamine et al[17] similarly found that fewer prenatal examinations during pregnancy increased the risk of fetal LBW, aligning with the findings of this study. Some pregnant women may not undergo frequent prenatal examinations, which hinders doctors from timely monitoring the health status of both the pregnant woman and fetal development. This situation is particularly critical for pregnant women with abnormal conditions, potentially leading to missed treatment opportunities that are essential for normal fetal deve
Calcium is essential for the body and crucial for the health of pregnant women and normal fetal development[20,21]. China’s dietary guidelines recommend a daily calcium supplement of 1000–1200 mg from early pregnancy[9]. Cormick et al[22] found that over 50% of pregnant women in China have insufficient calcium intake during pregnancy. This study identified irregular calcium supplementation during pregnancy as a contributing factor to full-term LBW. Insufficient calcium supplementation may result from increased maternal blood volume during fetal growth, leading to decreased maternal blood calcium levels. Relying solely on daily meals may not meet the calcium needs of both the mother and fetus, potentially impairing fetal development and increasing LBW risk.
Pregnancy-induced hypertension is characterized by elevated blood pressure[23]. Getaneh et al[24] demonstrated that gestational hypertension significantly impacts newborn birth weight, consistent with our findings. The increase in blood pressure can induce continuous fluctuations and shear forces that damage vascular walls, leading to vascular endothelial injury and spasmodic contractions in uterine placental arteries[25,26]. This process results in placental and uterine ischemia and hypoxia, which in turn disrupts normal fetal development and increases the risk of LBW.
The occurrence of full-term LBW is primarily due to the lack of risk assessment for pregnant women during pregnancy, leading to insufficient early prevention. The logistic regression model, a generalized linear regression analysis model, is often used in clinical practice for disease diagnosis or to explore the influencing factors of diseases. This method can effectively demonstrate the relationship between independent variables and dependent variables. In this study, a predictive model was constructed based on the factors influencing the occurrence of full-term LBW. After validation, the model’s overall accuracy was 81.67%, indicating that it is highly effective in predicting the risk of full-term LBW. This model can help identify high-risk populations in clinical practice. By incorporating factors such as the education level of pregnant women, frequency of prenatal examinations, weight gain during pregnancy, regular calcium supplementation during pregnancy, and the presence of preeclampsia, the model can calculate the potential risk of LBW for full-term fetuses. This enables the development of personalized management measures based on the probability of risk. For high-risk pregnant women, it is essential to strengthen pregnancy health education, guide them to adhere to standardized prenatal examinations, maintain a reasonable diet and nutrition, actively prevent pregnancy complications, and ultimately reduce the incidence of LBW.
This study is limited by its single-center retrospective design. Therefore, the clinical practicality and generalizability of the predictive model need to be further validated and optimized through prospective studies with larger sample sizes to improve the predictive performance of the model.
In summary, the occurrence of full-term LBW is related to low educational levels in pregnant women, fewer prenatal examinations, insufficient weight gain during pregnancy, irregular calcium supplementation during pregnancy, and pregnancy-induced hypertension. Based on these factors, the predictive model for full-term LBW risk demonstrates good discrimination.
| 1. | Tchamo ME, Prista A, Leandro CG. Low birth weight, very low birth weight and extremely low birth weight in African children aged between 0 and 5 years old: a systematic review. J Dev Orig Health Dis. 2016;7:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Chung YH, Hwang IS, Jung G, Ko HS. Advanced parental age is an independent risk factor for term low birth weight and macrosomia. Medicine (Baltimore). 2022;101:e29846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Gupta S, Adhisivam B, Bhat BV, Plakkal N, Amala R. Short Term Outcome and Predictors of Mortality Among Very Low Birth Weight Infants - A Descriptive Study. Indian J Pediatr. 2021;88:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Durá-Travé T, San Martín-García I, Gallinas-Victoriano F, Chueca Guindulain MJ, Berrade-Zubiri S. [Catch-up growth and associated factors in very low birth weight infants]. An Pediatr (Engl Ed). 2020;93:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Yadav DK, Shukla GS, Gupta N, Shrestha N, Singh A, Kaphle HP. Maternal and Obstetric Factors Associated with Low Birth Weight. J Nepal Health Res Counc. 2020;17:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Nakano Y. Adult-Onset Diseases in Low Birth Weight Infants: Association with Adipose Tissue Maldevelopment. J Atheroscler Thromb. 2020;27:397-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Bonnar K, Fraser D. Extrauterine Growth Restriction in Low Birth Weight Infants. Neonatal Netw. 2019;38:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Rasmussen KM, Yaktine AL, editors. Washington (DC): National Academies Press (US); 2009. [PubMed] |
| 9. | Hovdenak N, Haram K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2012;164:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Zilberman Sharon N, Pekar-Zlotin M, Kugler N, Accart Z, Nimrodi M, Melcer Y, Cuckle H, Maymon R. Oligohydramnios: how severe is severe? J Matern Fetal Neonatal Med. 2022;35:5754-5760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Dong BQ, You JP, Liang QY, Lyu W, Ma JF, Wei HW, Li H. [Study on the distribution and related factors of birth weight of live births in Guangxi Zhuang Autonomous Region of China from 2016 to 2018]. Zhong hua Yu Fang Yi Xue Za Zhi. 2019;53:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Kundu RN, Ghosh A, Chhetri B, Saha I, Hossain MG, Bharati P. Regional with urban-rural variation in low birth weight and its determinants of Indian children: findings from National Family Health Survey 5 data. BMC Pregnancy Childbirth. 2023;23:616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Altenhöner T, Köhler M, Philippi M. The Relevance of Maternal Socioeconomic Characteristics for Low Birth Weight - a Case-Control Study. Geburtshilfe Frauenheilkd. 2016;76:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Khatun S, Rahman M. Quality of antenatal care and its dose-response relationship with birth weight in a maternal and child health training institute in Bangladesh. J Biosoc Sci. 2008;40:321-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Sebayang SK, Dibley MJ, Kelly PJ, Shankar AV, Shankar AH; SUMMIT Study Group. Determinants of low birthweight, small-for-gestational-age and preterm birth in Lombok, Indonesia: analyses of the birthweight cohort of the SUMMIT trial. Trop Med Int Health. 2012;17:938-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Bellizzi S, Padrini S. Quality utilization of antenatal care and low birth weight: evidence from 18 demographic health surveys. East Mediterr Health J. 2020;26:1381-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Nagamine M, Matsumura K, Tsuchida A, Inadera H; Japan Environment and Children’s Study (JECS) Group. Relationship between prenatal checkup status and low birth weight: a nationwide birth cohort-the Japan Environment and Children's Study. Ann Epidemiol. 2023;83:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Dalfra' MG, Burlina S, Lapolla A. Weight gain during pregnancy: A narrative review on the recent evidences. Diabetes Res Clin Pract. 2022;188:109913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Sun Y, Shen Z, Zhan Y, Wang Y, Ma S, Zhang S, Liu J, Wu S, Feng Y, Chen Y, Cai S, Shi Y, Ma L, Jiang Y. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth. 2020;20:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 20. | Oh C, Keats EC, Bhutta ZA. Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients. 2020;12:491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 21. | Farias PM, Marcelino G, Santana LF, de Almeida EB, Guimarães RCA, Pott A, Hiane PA, Freitas KC. Minerals in Pregnancy and Their Impact on Child Growth and Development. Molecules. 2020;25:5630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Cormick G, Betrán AP, Romero IB, Lombardo CF, Gülmezoglu AM, Ciapponi A, Belizán JM. Global inequities in dietary calcium intake during pregnancy: a systematic review and meta-analysis. BJOG. 2019;126:444-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Reddy S, Jim B. Hypertension and Pregnancy: Management and Future Risks. Adv Chronic Kidney Dis. 2019;26:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Getaneh T, Negesse A, Dessie G, Desta M. The impact of pregnancy induced hypertension on low birth weight in Ethiopia: systematic review and meta-analysis. Ital J Pediatr. 2020;46:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Wang L, Cheng L, Zhang S, Su M, Jin Y, Luo D. Mediation effect of pregnancy-induced hypertension on the association between assisted reproductive technology and adverse neonatal outcomes: a population-based study. BMC Pregnancy Childbirth. 2023;23:385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Liu Y, Li N, An H, Li Z, Zhang L, Li H, Zhang Y, Ye R. Impact of gestational hypertension and preeclampsia on low birthweight and small-for-gestational-age infants in China: A large prospective cohort study. J Clin Hypertens (Greenwich). 2021;23:835-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/