Published online Sep 16, 2024. doi: 10.12998/wjcc.v12.i26.5908
Revised: June 19, 2024
Accepted: July 3, 2024
Published online: September 16, 2024
Processing time: 57 Days and 15.7 Hours
Preoperative risk stratification is significant for the management of endometrial cancer (EC) patients. Radiomics based on magnetic resonance imaging (MRI) in combination with clinical features may be useful to predict the risk grade of EC.
To construct machine learning models to predict preoperative risk stratification of patients with EC based on radiomics features extracted from MRI.
The study comprised 112 EC patients. The participants were randomly separated into training and validation groups with a 7:3 ratio. Logistic regression analysis was applied to uncover independent clinical predictors. These predictors were then used to create a clinical nomogram. Extracted radiomics features from the T2-weighted imaging and diffusion weighted imaging sequences of MRI images, the Mann-Whitney U test, Pearson test, and least absolute shrinkage and selection operator analysis were employed to evaluate the relevant radiomic features, which were subsequently utilized to generate a radiomic signature. Seven ma
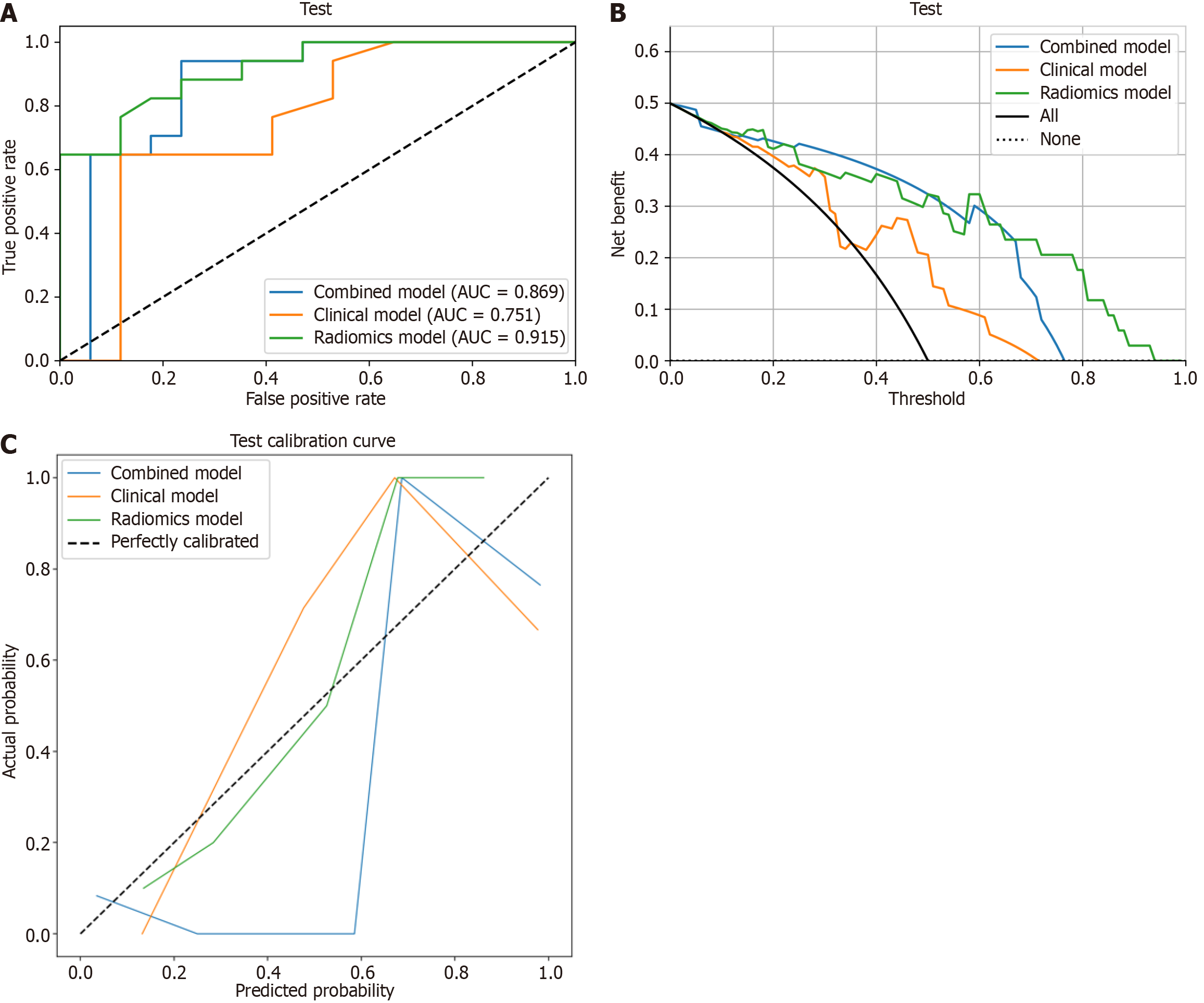
Having an accuracy of 0.82 along with an area under the curve (AUC) of 0.915 [95% confidence interval (CI): 0.806-0.986], the random forest method trained on radiomics characteristics performed better than expected. The predictive accuracy of radiomics prediction models surpassed that of both the clinical nomogram (AUC: 0.75, 95%CI: 0.611-0.899) and the combined nomogram (AUC: 0.869, 95%CI: 0.702-0.986) that integrated clinical parameters and radiomic signature.
The MRI-based radiomics model may be an effective tool for preoperative risk grade prediction in EC patients.
Core Tip: Our research focused on the utilization of clinical features and radiomics derived from magnetic resonance imaging (MRI) in order to predict the risk grade of endometrial cancer (EC). In our studies, we constructed a predictive model capable of predicting preoperative EC risk. The MRI-based radiomics model put forth in this research showed strong predictive ability and great potential value for assessing the level of EC risk. The integration of predictive models into clinical practice would greatly enhance the preoperative selection of customized therapy.
- Citation: Wei ZY, Zhang Z, Zhao DL, Zhao WM, Meng YG. Magnetic resonance imaging-based radiomics model for preoperative assessment of risk stratification in endometrial cancer. World J Clin Cases 2024; 12(26): 5908-5921
- URL: https://www.wjgnet.com/2307-8960/full/v12/i26/5908.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i26.5908
Endometrial cancer (EC), characterized by the abnormal growth of cells in the lining of the uterus, is a prevalent gynecologic malignancy with a significant global health impact for females[1]. The early detection rate of EC is normally high, with approximately 80% of cases being diagnosed in stage I, with almost 95% 5-year survival rates[2]. However, if a disease has spread regionally or far away, the 5-year survival rate is substantially lower (68% and 17%, respectively)[3].
According to the European Society for Medical Oncology, the European Society for Radiotherapy and Oncology, and the European Society of Gynaecological Oncology Consensus Conference non-molecular risk classification of EC from 2020[4], patients who are at a heightened risk demonstrate the presence of at least one of the subsequent histologic characteristics: Deep infiltration into the myometrium (DMI); a tumor of high grade; a histological subtype that is non-endometrioid; lymph-vascular spaces invasion (LVSI); spread beyond the uterus; or involvement of lymph nodes.
Bilateral salpingo-oophorectomy and total abdominal hysterectomy are the standard treatments for low-risk EC. Although numerous clinical trials have sought to demonstrate the benefits of adjuvant therapy and lymphadenectomy (LA) for EC in its early stages, whether it truly meets the needs of patients remains controversial[5]. Approximately 7%-10% of patients undergoing lymph node dissection have postoperative lymphatic fistula, and roughly 23% of people are said to have lower extremity lymphedema on average[6]. According to the guidelines, individuals diagnosed with low-risk endometrioid carcinoma exhibit a diminished likelihood of lymph node involvement, hence LA is not advised for these patients. However, high-risk patients who had previously had partial surgery who needed LA to complete staging may be a good candidate for this procedure. For patients with intermediate-risk and high-risk endometrial carcinoma, surgical tumor debulking including enlarged lymph nodes and adjuvant treatment is recommended.
Therefore, we merged intermediate and high-risk groups as the high-risk group. Hence, identifying an effective approach for accurately evaluating risk stratification in EC cancer before treatment is crucial to ensure precise decision-making. Precise evaluation of the primary tumor, lymph nodes, and distant metastases is crucial for predicting the risk stratification of EC, particularly in the context of DMI.
Transvaginal ultrasonography is inferior to magnetic resonance imaging (MRI), which provides higher diagnostic performance[7,8]. Preoperative evaluation of EC staging using combined MRI T2-weighted imaging (T2WI) sequences is considered the best way for accurate assessment of DMI, particularly in cases where there is disappearance of the junctional zone (commonly seen in postmenopausal patients) and poor tumor-myometrial contrast[9]. Nevertheless, there is often an overestimation of the stage in instances where the tumor affects the uterine horns, when the tumor extends extensively into the uterine wall causing thinning of the myometrium, or in the event it occurs in a leiomyomatous uterus marked due to the varied signal strength observed in the myometrium[10,11].
The utilization of radiomics within clinical decision systems has witnessed a notable surge in recent years, leading to enhanced precision in the realms of diagnosis, prognosis, and prediction[12,13]. The primary objective of radiomics is transforming medical pictures into digital information that encompasses physiological and fundamental descriptive variables, such as contrast enhancement, diffusion properties, and tracer pickup. Additionally, it encompasses funda
In the assessment of the predictive capacity of three risk stratification models for lymph node transfer in endometrioid EC, Korkmaz et al[17] found that the model that exhibited the highest performance attained an area under the curve (AUC) value of 0.780. Liu et al[18] concluded that the utilization of texture analysis exhibited promising prospects in its capacity to function as imaging indicators for evaluating preoperative risk. However, additional study is required to examine the evaluation of MRI-based radiomic analysis in conjunction with clinical factors for the purpose of pre
The objective of the present research was to inquire into the utilization of clinical features and radiomics derived from MRI in order to predict the risk grade of EC.
The retrospective inquiry in question was exempt from the requirement of informed consent as it had obtained approval from the institutional review board. We reviewed 315 patients with EC that had postoperative pathological results between January 2018 and January 2020 in our hospital. Based on the following criteria, 112 cases were included in the study. Patients were first stratified according to the European Society of Gynaecological Oncology, the European Society for Medical Oncology, and the European Society of Pathology 2020 guidelines and then divided into two groups: Low-risk (including patients belonging to the low-risk class according to the 2020 guidelines classification); and high-risk (including intermediate, intermediate-high, and high-risk classes).
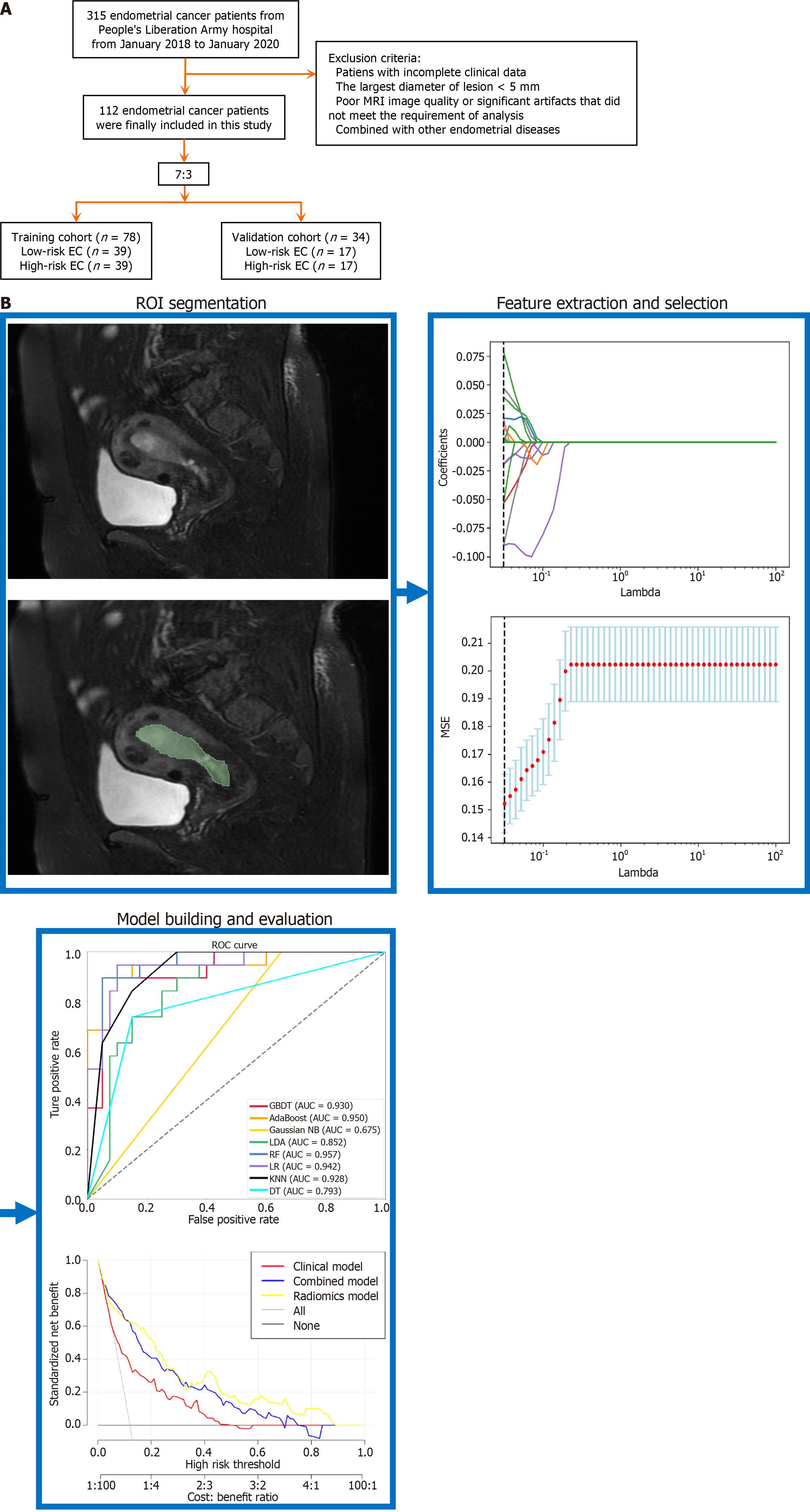
The inclusion criteria were: (1) Patients with pathologically confirmed EC who received an MRI exam 2 wk before surgery; and (2) Individuals who had not received any therapeutic interventions, including surgical procedures, biopsies, radiation therapy, chemotherapy, or hormone therapy, prior to their MRI. The exclusion criteria were: (1) Patients with missing clinical data; (2) Poor MRI image quality or significant artifacts that did not match the requirement of analysis; and (3) Lesions with a diameter of less than 5 mm. A random assignment method was used to allocate patients in a 7:3 ratio between the training group (n = 78) and the validation cohort (n = 34). The research flowchart is portrayed in Figure 1.
Clinical and histopathological traits of all selected patients, such as age, histological grade and subtype, International Federation of Gynaecology and Obstetrics staging, body mass index, hypertension, hyperlipidemia, diabetes mellitus, menstruation, menopause status, fertility history, serum cancer antigen (CA) 125 level, serum CA19-9 level, and drinking and smoking history, were collected. These data were obtained through consultation of the medical record system. MRI pictures of all patients in digital imaging and communications in the medicine type were exported.
All MRI examinations were performed using 3.0 T system MRI scanners (Magnetom Aera; Siemens Healthcare, Germany). The standard scanning protocol comprised axial fast spin-echo (FSE) T1-weighted images, axial FSE T2WI, axial fat suppression FSE T2WI, and sagittal FSE T2WI. The b values utilized in diffusion weighted imaging (DWI) encompassed the values of 0 and 1000 s/mm2. In the study, uterus-sagittal position T2WI were acquired for lesion seg
| Sequence | TR/TE in ms | FOV in mm × mm | Matrix | Slice gap in mm | Slice thickness in mm |
| Axial T1WI | 500/8.6 | 310 × 310 | 224 × 320 | 1 | 6 |
| Axial T2WI | 6200/95 | 310 × 310 | 307 × 384 | 1 | 6 |
| Axial (FS)-T2WI | 4000/93 | 310 × 310 | 256 × 320 | 1 | 5 |
| Sagittal (FS)-T2WI | 6000/86 | 240 × 240 | 205 × 256 | 1 | 6 |
| Axial DWI | 5000/69 | 327 × 245 | 115 × 192 | 1 | 4 |
The open-source software three-dimensional (3D) slicer (version 5.2.1), which may be accessed at https://www.slicer.org, was employed for the purpose of image segmentation. Segmentation was performed independently and manually by two experienced doctors who were unaware of the pathological result of the patient. The entire tumor was included in the 3D volume of interest (VOI), which the medical professionals defined, divided, and fused for a layer after layer screen. In the event of a disagreement, the two doctors talked it out until an agreement was reached.
The VOIs underwent resampling to a voxel size of 3 mm × 3 mm × 3 mm before extracting features to produce isotropic voxels; to ensure that the gray-level values of all photos were dispersed over the same range, image normalization was carried out. The VOI of each patient was utilized to extract radiomics features using the free and publicly accessible python program pyradiomics (https://pypi.org/project/pyradiomics/). The following categories were created from the extracted features: First-order features; two-dimensional features; gray-level cooccurrence matrix (GLCM); gray-level dependence matrix; gray-level size-zone matrix; gray-level run-length matrix; and neighboring gray tone difference matrix[19]. In total, 1037 radiomics characteristics were retrieved.
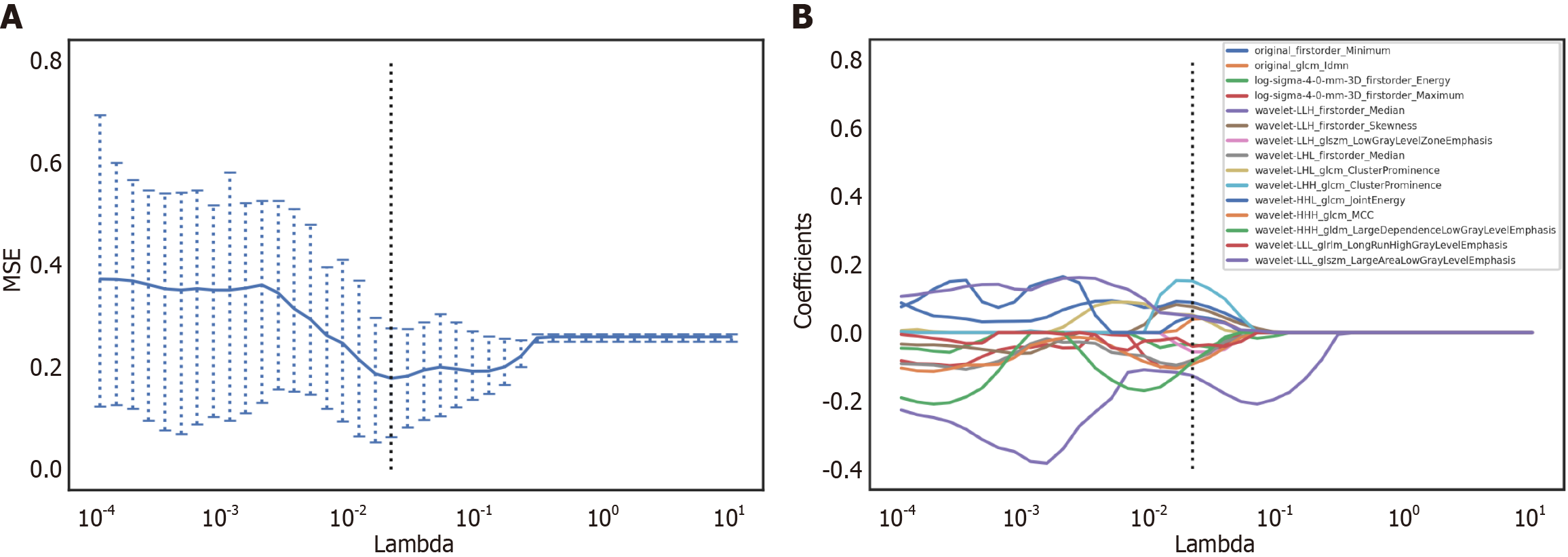
To mitigate interference across the feature dimensions, the features underwent standardization making use of the Z-score method, which involves deducting the average value and dividing by the standard deviation. Initially, to assess what features could individually separate the low-risk group from the high-risk group, we conducted the Mann-Whitney U test. Only features exhibiting a P value of 0.05 were retained. Subsequently, to eliminate highly correlated features, we removed the features with P > 0.9. The utilization of the least absolute shrinkage and selection operator (Lasso) in data analysis allows for the reduction of coefficients associated with variables that are not relevant to survival to zero while retaining features that have coefficients that are not equal to zero[20,21]. In this study, the Lasso method was employed to identify radiomics properties with the highest impact, and a 10-fold cross-validation approach was implemented within the training cohort. The minimum criteria (minimum lambda) were used to identify the ideal tuning parameter.
The radiomic signature (radscore) was calculated by adding up all of the filtered eigen values and multiplying the total by the appropriate coefficients. Radscore = β1X1 + β2X2 + …βnXn, with the radiomic signature denoted as radscore, βn was the coefficient, and Xn was the eigenvalue[22]. The assessment of the consistency between radscore in the training and testing sets was conducted by employing the Wilcoxon rank sum Mann-Whitney U test.
By applying univariate and multivariate regression analysis, the clinical independent predictors were discovered. Applying the independent predictors, a clinical nomogram was then produced. To categorize the EC based on their radiomics features because we needed a model to solve a binary classification problem and we did not have a very large sample size, seven popular binary machine learning algorithms [logistic regression (LR), K-nearest neighbor (KNN), decision tree (DT), random forest (RF), gradient boosting (GB), eXtreme GB (XGBoost), and GBDT] with good explanations were used. A combined prediction multivariate logistic regression model was developed by including the radiomic signature (radscore) and clinically significant predictors that demonstrated statistical significance in a multivariate regression analysis.
The building of the ideal radiomics model was accomplished by employing a machine learning technique that demon
Statistical tests suitable for the data type were employed to compare the baseline data between the training and validation cohorts. Specifically, the χ2 test or the Fisher exact test were used for categorical variables, while the continuous variables were assessed using either the two-sample t-test or the Mann-Whitney U test. Statistical significance was attributed to data sets with P values lower than 0.05. Statistical analyses were performed utilizing the statistical product and service solutions (version 26.0, international business machines corporation, https://www.ibm.com/spss), R software (version 3.5.1, https://www.r-project.org/), and Python (version 3.5.6, https://www.python.org/).
A sample size of 112 individuals, aged between 31 years and 84 years (average age of 58.35 ± 11.75 years), was partitioned into two separate sections: Low-risk (n = 56) and high-risk (n = 56; including 42 intermediate risk cases and 14 high-risk cases). The participants were randomized to the training and validation subgroups in a random manner, following a 7:3 ratio. In the training subgroup, there were 39 patients classified as low-risk and an equal number of 39 patients classified as high-risk. Similarly, in the validation cohort, there were 17 patients classified as low-risk and an equal number of 17 patients classified as high-risk. The training and validation cohorts did not significantly differ in either clinical or histological characteristics. Table 2 displays the fundamental characteristics of the individuals.
| Characteristics | Training cohort | Validation cohort | P value |
| Number | 78 | 34 | |
| Age in yr | 58.04 ± 11.54 | 59.06 ± 12.04 | 0.367 |
| Histological grade1 | 0.465 | ||
| G1/G2 | 58 | 23 | |
| G3 | 20 | 11 | |
| Risk classes | 1.000 | ||
| Low-risk | 39 | 17 | |
| High-risk | 39 | 17 | |
| Histological subtype1 | 0.018 | ||
| Endometrioid | 66 | 22 | |
| Serous | 6 | 5 | |
| Clear cell | 4 | 4 | |
| Carcinosarcoma | 2 | 3 | |
| FIGO staging | 0.765 | ||
| I | 50 | 21 | |
| II | 11 | 7 | |
| III | 9 | 4 | |
| IV | 8 | 2 | |
| BMI | |||
| > 24 | 44 | 19 | 0.959 |
| ≤ 24 | 34 | 15 | |
| Hypertension | 0.812 | ||
| Positive | 20 | 8 | |
| Negative | 58 | 26 | |
| DM | 0.605 | ||
| Positive | 15 | 8 | |
| Negative | 63 | 26 | |
| Hyperlipidemia | 0.338 | ||
| Positive | 5 | 4 | |
| Negative | 73 | 30 | |
| Menstruation | 0.395 | ||
| Regular | 48 | 18 | |
| Irregular | 30 | 16 | |
| Menopausal status | |||
| Premenopausal | 57 | 27 | 0.477 |
| Postmenopausal | 21 | 7 | |
| Fertility | 0.669 | ||
| Fertility | 69 | 31 | |
| Nonfertility | 9 | 3 | |
| CA125 | 0.617 | ||
| < 35 U/mL | 61 | 28 | |
| ≥ 35 U/mL | 17 | 6 | |
| CA19-9 | 0.631 | ||
| < 27 U/mL | 67 | 28 | |
| ≥ 27 U/mL | 11 | 6 | |
| Family history of cancer | 0.292 | ||
| Positive | 10 | 7 | |
| Negative | 68 | 27 | |
| Drinking history | 0.915 | ||
| Positive | 5 | 2 | |
| Negative | 73 | 32 | |
| Smoking history | 0.606 | ||
| Positive | 4 | 1 | |
| Negative | 74 | 33 |
The 3D Slicer software was utilized to extract a total of 1037 quantitative imaging feature parameters for each patient. Following deredundancy processing, 788 features were kept, and 15 feature parameters were chosen using the Lasso dimensionality reduction approach (Figure 2). The radiomics score for each patient was computed via a linear equation using the following formula:
Radscore = (0.085 × original_firstorder_Minimum) + (0.046 × original_glcm_ldmn) + [(-0.039) × log-sigma-4-4-mm-3D_firstorder_Energy] + [(-0.075) × log-sigma-4-4-mm-3D_firstorder_Maximum] + [(-0.133) × wavelet-LLH_fir
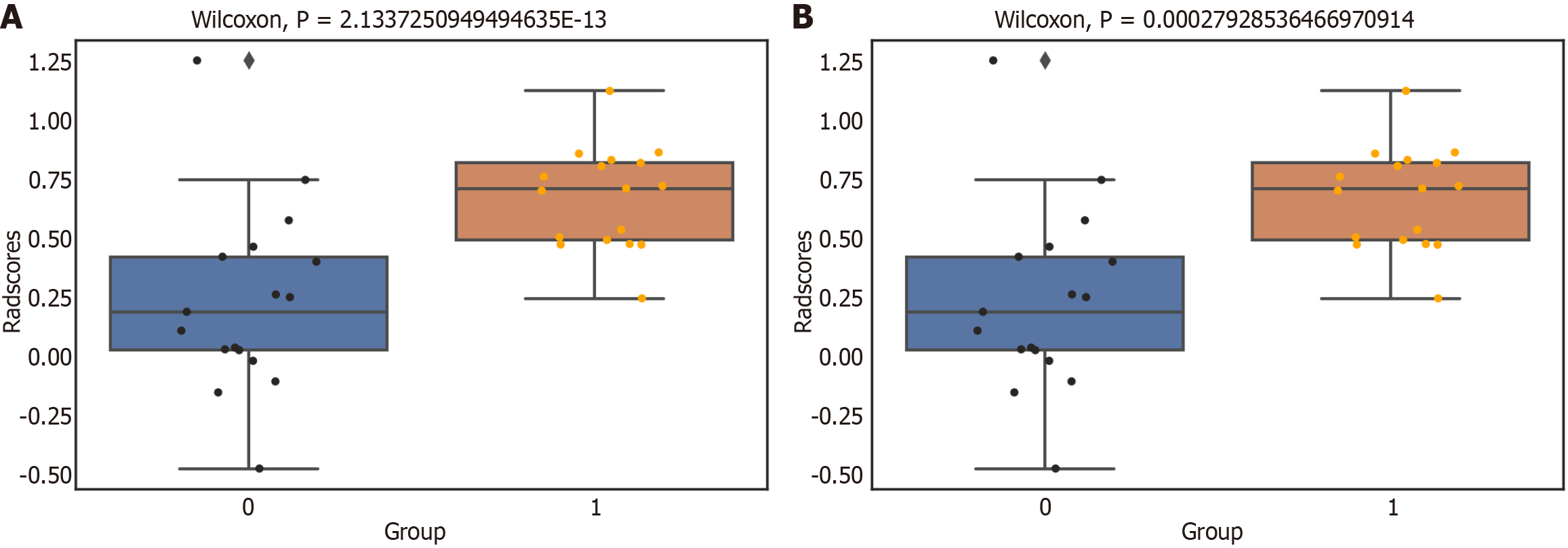
The study observed that the radscore exhibited greater values in the high-risk subgroup in comparison to the low-risk subgroup. Additionally, statistical analysis demonstrated that the radscore had a strong capability to differentiate between EC patients with low risk and those with high risk, as observed in both the training and validation cohorts. Low-risk EC patients had a radscore of 0.25 ± 0.20 in the training cohort compared to high-risk patient radscores of 0.75 ± 0.15 (P = 0.000); in the validation cohort, low-risk EC patients had a radscore of 0.24 ± 0.39 compared to high-risk patient radscores of 0.67 ± 0.21 (P = 0.000) (Figure 3).
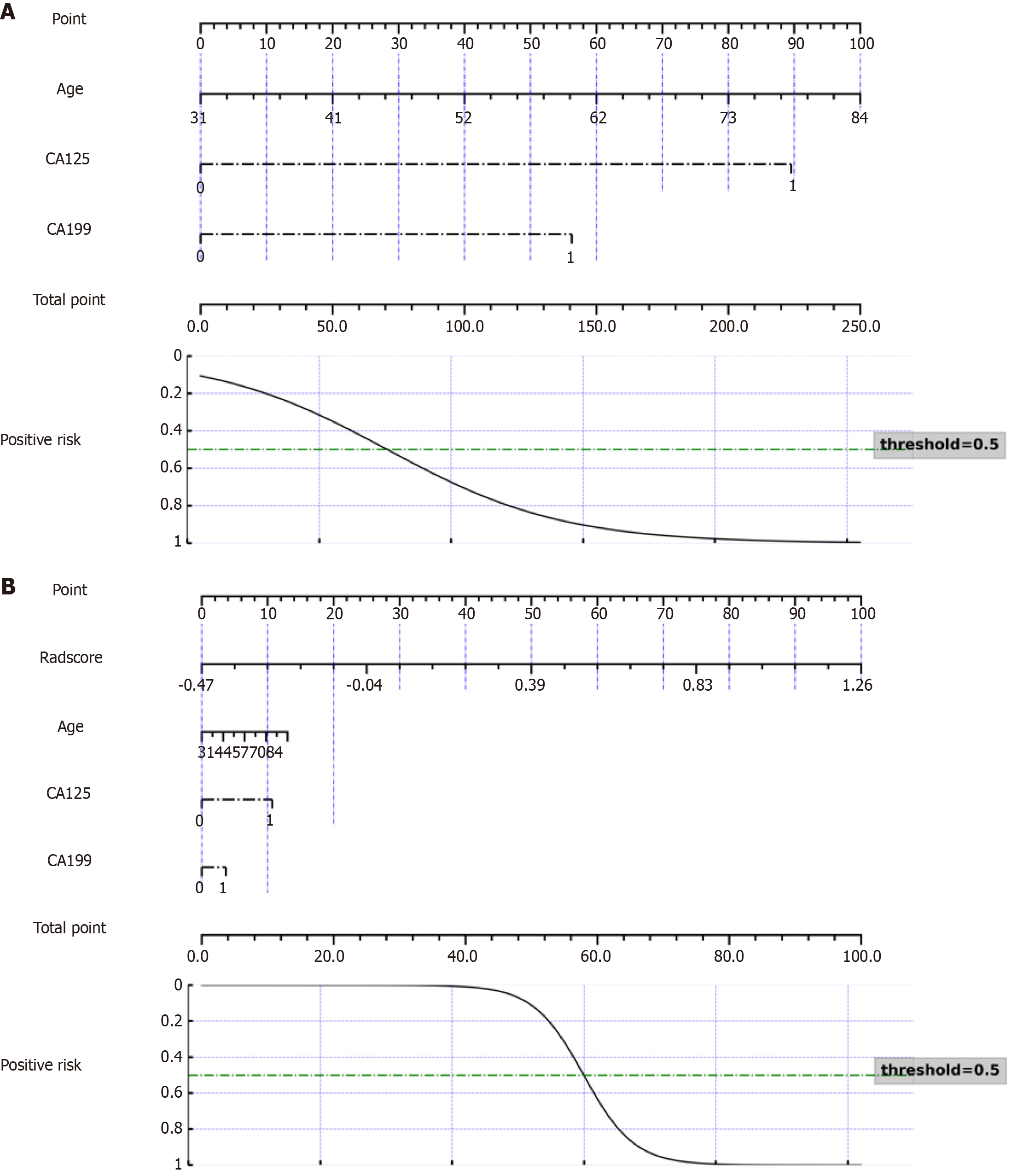
Logistic regression analysis, both univariate and multivariate, was used to filter out all of the clinically suspicious risk factors (Table 3). The findings revealed that age, CA125, and CA19-9 were clinical independent predictors. A clinical model was constructed with the predictors. The AUC was 0.751 [95% confidence interval (CI): 0.611-0.899] in validation set, and accuracy was 0.706. Figure 4A depicts the clinical prediction model nomogram.
| Characteristics | Univariate regression OR (95%CI) | P value | Multivariate regression OR (95%CI) | P value |
| Age | Reference 1.091 (1.038-1.147) | 0.000 | Reference 1.10 (1.000-1.019) | 0.048 |
| BMI | ||||
| > 24 | Reference | |||
| ≤ 24 | 2.333 (0.933-5.833) | 0.070 | ||
| Hypertension | ||||
| Positive | Reference | |||
| Negative | 0.438 (0.152-1.256) | 0.124 | ||
| Diabetes mellitus | ||||
| Positive | Reference | |||
| Negative | 0.848 (0.274-2.619) | 0.774 | ||
| Hyperlipidemia | ||||
| Positive | Reference | |||
| Negative | 0.649 (0.102-4.113) | 0.646 | ||
| Menstruation | ||||
| Regular | Reference | |||
| Irregular | 1.591 (0.615-4.114) | 0.338 | ||
| Menopausal status | ||||
| Premenopausal | Reference | |||
| Postmenopausal | 2.560 (0.898-7.296) | 0.079 | ||
| Reproductive history | ||||
| Positive | Reference | |||
| Negative | 0.777 (0.192-3.142) | 0.724 | ||
| CA125 | ||||
| < 35 U/mL | Reference | Reference | ||
| ≥ 35 U/mL | 26.435 (3.284-29.776) | 0.002 | 1.515 (1.163-1.973) | 0.003 |
| CA19-9 | Reference | |||
| < 27 U/mL | Reference | 1.355 (1.030-1.783) | 0.033 | |
| ≥ 27 U/mL | 5.550 (1.114-27.648) | 0.036 | ||
| Family history of cancer | ||||
| Negative | Reference | |||
| Positive | 1.000 (0.265-3.772) | 1.000 | ||
| Drinking history | ||||
| Negative | Reference | |||
| Positive | 4.343 (0.463-40.749) | 0.199 | ||
| Smoke history | ||||
| Negative | Reference | |||
| Positive | 0.316 (0.031-3.177) | 0.328 | ||
| Radscore | 1.027 (1.002-1.054) | 0.038 | 1.025 (0.990-1.060) | 0.016 |
Radiomics models were created using LR, KNN, DT, RF, GB, XGBoost, and GBDT. The model performance information is displayed in Table 4. The discrimination of the RF algorithm model outperformed the others.
| Algorithms | AUC (95%CI) | Accuracy | Sensitivity | Specificity |
| LR | 0.794 (0.706-0.976) | 0.794 | 0.765 | 0.824 |
| KNN | 0.893 (0.766-0.971) | 0.765 | 0.823 | 0.705 |
| DT | 0.676 (0.529-0.824) | 0.676 | 0.529 | 0.824 |
| RF | 0.915 (0.806-0.986) | 0.824 | 0.824 | 0.824 |
| GB | 0.907 (0.792-0.979) | 0.794 | 0.706 | 0.882 |
| XGBoost | 0.869 (0.739-0.965) | 0.765 | 0.706 | 0.824 |
| GBDT | 0.872 (0.742-0.958) | 0.765 | 0.647 | 0.882 |
The LR technique was employed to generate a nomogram that integrated the variables of age, CA125, CA19-9, and radscore into a combined model. The AUC in the test collection demonstrated outstanding discrimination, with a value of 0.869 (95%CI: 0.702-0.986). Figure 4B displays the nomogram representing the combined prediction models.
Figure 5 including the receiver operating characteristic curves, calibration curves, and decision curve analysis, presents a comparative analysis of the performance between a clinical model, the best radiomics model, and the combined model. According to the Delong test (P < 0.05), the radiomics model exhibited superior discriminatory performance compared to both the clinical and combined models.
This study involved the development of three models that were based on clinical independent risk factors, radiomics features, and the combination of clinical risk factors with radscore. The objective was to predict risk stratification for EC. This study demonstrated that the MRI-based radiomics models and the clinical models have good efficacy when assessing the risk grade of EC. The diagnostic efficacy of the combined model (AUC: 0.869, 95%CI: 0.702-0.986) was higher than the clinical model (AUC: 0.75, 95%CI: 0.611-0.899), and the radiomics model (AUC: 0.915, 95%CI: 0.806-0.986) developed based on the imaging features extracted from the T2WI and DWI sequence had the highest efficacy. The RF model performed best in this investigation out of various classifiers utilized to build the radiomics predictive model. This study showcased the efficacy of utilizing a comprehensive tumor radiomics-based analysis to evaluate the risk classification of EC before surgical intervention. The radiomics model may be clinically effective in the individualized surgical care of EC patients since radiomics may extract relevant data about high-risk indicators prior to surgery.
Some studies have explored preoperative prediction of EC risk stratification. Yan et al[26] found that variables for high-risk EC included metabolic syndrome, CA125, age, and tumor grade after curettage. Saarelainen et al[27] also concluded that CA125 paired with other serum indicators was superior to CA125 alone in predicting the recurrence of disease in EC patients. CA125 is a factor that relates to high-risk EC. In line with this, age, CA125, and CA19-9 were independent risk factors associated with EC risk stratification in this study.
Previous studies have noted that patients with EC, particularly those exhibiting unfavorable prognostic factors such as tumor recurrences, grade 3 tumors, DMI, lymph node metastasis, and extrauterine disease, utilize CA125 as a biomarker for tumor detection[28]. CA125 has been integrated into many pre-surgery prediction models, with varying levels of effectiveness. In clinical settings, the utilization of a threshold value of 35 U/mL for CA125 can prove to be a valuable diagnostic tool due to its high sensitivity. This is evidenced by the fact that a mere 1% of females who are in good health exhibit CA125 levels surpassing this cutoff point[29]. Therefore, this widely used cutoff was also used in this study.
Furthermore, there is research that has demonstrated notable correlations between International Federation of Gynaecology and Obstetrics staging and several other characteristics. These factors encompass inadequate histological classification, progressed clinical stage, existence of metastatic lymph nodes, and heightened blood CA19-9 levels[30]. Elderly females are more likely to have metabolic syndrome, have worse basic health status, and are more likely to develop high-risk EC. A study showed that reproductive history was a high-risk indicator for the emergence of EC[31]. In the current study, however, this conclusion was not found, suggesting that reproductive history may be more related to the incidence of EC instead of risk grade.
Previous studies have found a connection between the characteristics of tumor texture and diagnosis and grading[32,33]. Celli et al[34] found that the differentiation of low-risk EC from other risk categories, as well as the identification of the existence of LVSI, can be achieved with moderate to high accuracy by the utilization of MRI-based whole-tumor radiomics and radio-genomic investigations. Using MRI texture features, Ueno et al[35] established preliminary mathematical modelling that showed a correlation with DMI, LVSI, and high tumor grade. The AUC of the model in predicting high-risk EC was 0.83, but they did not perform the volumetric analysis. High-throughput parameters were extracted for the current investigation, and 15 statistically significant features were then found to create a radiomics model. The model performed well in identifying low-risk and high-risk EC; the sensitivity and specificity of the validation cohort were 82.4% and 82.4%, and the AUC was 0.915.
The GLCM feature characterizes the spatial arrangement of grey intensity in a two-dimensional manner by quantifying the probability of observing specific pixel pairs with particular grey values[36,37]. The chosen feature in this study comprised six GLCM features, indicating a close association between the distribution of texture and spatial heterogeneity within tumors and tumor differentiation. Moreover, alterations in the internal texture of tumors play a crucial role in determining tumor risk grading.
Among the seven machine learning algorithms we used to develop a radiomics model, the RF model performed best. It is based on DT analysis; the algorithm builds DTs by employing a random selection process to determine a subset of the samples and a subset of the features to be used for the split at each node. This greatly reduces the variance term in the diagnostic error. This is an advantage over bagging of the DTs[38]. In the area of biomedical research, the characteristics of RF make it the perfect material for creating diagnostic models.
Our study had some limitations. First, our model might have been overfitted because we only included 112 patients in this early investigation. Second, even though the MRI was performed according to a set protocol, the retrospective nature of the study could result in inhomogeneity un the imaging data. Although normalization was used in the picture analysis, image standardization and normalization techniques still require research. Third, we included only T2WI map images in our model, which may potentially enable missing significant data. To limit the potential of biases from only one sequence, future studies should use multiparametric techniques.
The MRI-based radiomics model put forth in this research showed strong predictive ability and great potential value for assessing the level of EC risk. The integration of predictive models into clinical practice would greatly enhance the preoperative selection of customized therapy.
The authors express their heartfelt thanks to the staff of the Department of Obstetrics and Gynecology of our hospital.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68641] [Article Influence: 13728.2] [Reference Citation Analysis (201)] |
| 2. | Pintican R, Bura V, Zerunian M, Smith J, Addley H, Freeman S, Caruso D, Laghi A, Sala E, Jimenez-Linan M. MRI of the endometrium - from normal appearances to rare pathology. Br J Radiol. 2021;94:20201347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | PDQ Adult Treatment Editorial Board. Endometrial Cancer Treatment (PDQ®): Health Professional Version. 2024 Feb 8. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [PubMed] |
| 4. | Gultekin M, Guler OC, Yuce Sari S, Akkus Yildirim B, Onal C, Celik H, Yuce K, Ayhan A, Arik Z, Kose F, Altundag O, Zoto Mustafayev T, Atalar B, Bolukbasi Y, Yildiz F. Multi-institutional validation of the ESMO-ESGO-ESTRO consensus conference risk grouping in Turkish endometrial cancer patients treated with comprehensive surgical staging. J Obstet Gynaecol. 2021;41:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Jang JW, Lee LJ. External Beam, Brachytherapy, or Chemotherapy? Defining Adjuvant Therapy for Early-Stage and High- and High-Intermediate-Risk Endometrial Cancer. J Clin Oncol. 2019;37:1778-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Yost KJ, Cheville AL, Al-Hilli MM, Mariani A, Barrette BA, McGree ME, Weaver AL, Dowdy SC. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol. 2014;124:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 7. | Kinkel K, Forstner R, Danza FM, Oleaga L, Cunha TM, Bergman A, Barentsz JO, Balleyguier C, Brkljacic B, Spencer JA; European Society of Urogenital Imaging. Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol. 2009;19:1565-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Sala E, Crawford R, Senior E, Shaw A, Simcock B, Vrotsou K, Palmer C, Rajan P, Joubert I, Lomas D. Added value of dynamic contrast-enhanced magnetic resonance imaging in predicting advanced stage disease in patients with endometrial carcinoma. Int J Gynecol Cancer. 2009;19:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Wu LM, Xu JR, Gu HY, Hua J, Haacke EM, Hu J. Predictive value of T2-weighted imaging and contrast-enhanced MR imaging in assessing myometrial invasion in endometrial cancer: a pooled analysis of prospective studies. Eur Radiol. 2013;23:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Haldorsen IS, Husby JA, Werner HM, Magnussen IJ, Rørvik J, Helland H, Trovik J, Salvesen ØO, Espeland A, Salvesen HB. Standard 1.5-T MRI of endometrial carcinomas: modest agreement between radiologists. Eur Radiol. 2012;22:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Bi Q, Chen Y, Wu K, Wang J, Zhao Y, Wang B, Du J. The Diagnostic Value of MRI for Preoperative Staging in Patients with Endometrial Cancer: A Meta-Analysis. Acad Radiol. 2020;27:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Wang Q, Li Q, Mi R, Ye H, Zhang H, Chen B, Li Y, Huang G, Xia J. Radiomics Nomogram Building From Multiparametric MRI to Predict Grade in Patients With Glioma: A Cohort Study. J Magn Reson Imaging. 2019;49:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Manganaro L, Nicolino GM, Dolciami M, Martorana F, Stathis A, Colombo I, Rizzo S. Radiomics in cervical and endometrial cancer. Br J Radiol. 2021;94:20201314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 3950] [Article Influence: 438.9] [Reference Citation Analysis (0)] |
| 15. | Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, Mak RH, Tamimi RM, Tempany CM, Swanton C, Hoffmann U, Schwartz LH, Gillies RJ, Huang RY, Aerts HJWL. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin. 2019;69:127-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 905] [Article Influence: 129.3] [Reference Citation Analysis (6)] |
| 16. | Moro F, Albanese M, Boldrini L, Chiappa V, Lenkowicz J, Bertolina F, Mascilini F, Moroni R, Gambacorta MA, Raspagliesi F, Scambia G, Testa AC, Fanfani F. Developing and validating ultrasound-based radiomics models for predicting high-risk endometrial cancer. Ultrasound Obstet Gynecol. 2022;60:256-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Korkmaz V, Meydanli MM, Yalçın I, Sarı ME, Sahin H, Kocaman E, Haberal A, Dursun P, Güngör T, Ayhan A. Comparison of three different risk-stratification models for predicting lymph node involvement in endometrioid endometrial cancer clinically confined to the uterus. J Gynecol Oncol. 2017;28:e78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Liu Z, Wang S, Dong D, Wei J, Fang C, Zhou X, Sun K, Li L, Li B, Wang M, Tian J. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics. 2019;9:1303-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 676] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 19. | van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017;77:e104-e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 4364] [Article Influence: 484.9] [Reference Citation Analysis (0)] |
| 20. | Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512-5528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 861] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 21. | Vasquez MM, Hu C, Roe DJ, Chen Z, Halonen M, Guerra S. Least absolute shrinkage and selection operator type methods for the identification of serum biomarkers of overweight and obesity: simulation and application. BMC Med Res Methodol. 2016;16:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 22. | Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z, Liang C, Tian J, Liang C. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology. 2016;281:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 568] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 23. | Rui W, Qiao N, Wu Y, Zhang Y, Aili A, Zhang Z, Ye H, Wang Y, Zhao Y, Yao Z. Radiomics analysis allows for precise prediction of silent corticotroph adenoma among non-functioning pituitary adenomas. Eur Radiol. 2022;32:1570-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13220] [Cited by in RCA: 13268] [Article Influence: 349.2] [Reference Citation Analysis (0)] |
| 25. | Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3195] [Cited by in RCA: 3571] [Article Influence: 223.2] [Reference Citation Analysis (1)] |
| 26. | Yan BC, Li Y, Ma FH, Feng F, Sun MH, Lin GW, Zhang GF, Qiang JW. Preoperative Assessment for High-Risk Endometrial Cancer by Developing an MRI- and Clinical-Based Radiomics Nomogram: A Multicenter Study. J Magn Reson Imaging. 2020;52:1872-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Saarelainen SK, Peltonen N, Lehtimäki T, Perheentupa A, Vuento MH, Mäenpää JU. Predictive value of serum human epididymis protein 4 and cancer antigen 125 concentrations in endometrial carcinoma. Am J Obstet Gynecol. 2013;209:142.e1-142.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Shawn LyBarger K, Miller HA, Frieboes HB. CA125 as a predictor of endometrial cancer lymphovascular space invasion and lymph node metastasis for risk stratification in the preoperative setting. Sci Rep. 2022;12:19783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 29. | Bast RC Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L, Zurawski VR Jr, Knapp RC. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1509] [Cited by in RCA: 1505] [Article Influence: 35.0] [Reference Citation Analysis (5)] |
| 30. | Bian J, Sun X, Li B, Ming L. Clinical Significance of Serum HE4, CA125, CA724, and CA19-9 in Patients With Endometrial Cancer. Technol Cancer Res Treat. 2017;16:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 31. | Sponholtz TR, Palmer JR, Rosenberg L, Hatch EE, Adams-Campbell LL, Wise LA. Reproductive factors and incidence of endometrial cancer in U.S. black women. Cancer Causes Control. 2017;28:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Xu X, Li H, Wang S, Fang M, Zhong L, Fan W, Dong D, Tian J, Zhao X. Multiplanar MRI-Based Predictive Model for Preoperative Assessment of Lymph Node Metastasis in Endometrial Cancer. Front Oncol. 2019;9:1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Chu H, Pang P, He J, Zhang D, Zhang M, Qiu Y, Li X, Lei P, Fan B, Xu R. Value of radiomics model based on enhanced computed tomography in risk grade prediction of gastrointestinal stromal tumors. Sci Rep. 2021;11:12009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Celli V, Guerreri M, Pernazza A, Cuccu I, Palaia I, Tomao F, Di Donato V, Pricolo P, Ercolani G, Ciulla S, Colombo N, Leopizzi M, Di Maio V, Faiella E, Santucci D, Soda P, Cordelli E, Perniola G, Gui B, Rizzo S, Della Rocca C, Petralia G, Catalano C, Manganaro L. MRI- and Histologic-Molecular-Based Radio-Genomics Nomogram for Preoperative Assessment of Risk Classes in Endometrial Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Ueno Y, Forghani B, Forghani R, Dohan A, Zeng XZ, Chamming's F, Arseneau J, Fu L, Gilbert L, Gallix B, Reinhold C. Endometrial Carcinoma: MR Imaging-based Texture Model for Preoperative Risk Stratification-A Preliminary Analysis. Radiology. 2017;284:748-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 36. | Park HJ, Park B, Lee SS. Radiomics and Deep Learning: Hepatic Applications. Korean J Radiol. 2020;21:387-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 37. | Chu H, Lin X, He J, Pang P, Fan B, Lei P, Guo D, Ye C. Value of MRI Radiomics Based on Enhanced T1WI Images in Prediction of Meningiomas Grade. Acad Radiol. 2021;28:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Breiman L. Bagging predictors. Mach Learn. 1996;24:123-140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10697] [Cited by in RCA: 4687] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/