Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.8863
Peer-review started: April 19, 2022
First decision: May 11, 2022
Revised: May 22, 2022
Accepted: July 29, 2022
Article in press: July 29, 2022
Published online: September 6, 2022
Processing time: 128 Days and 23.5 Hours
The incidence of prostate cancer (PCa) is on the rise in China. The risk level of patients with PCa is associated with disease-free survival rate at 10 years after radical prostatectomy. Predicting prognosis in advance according to the degree of risk can provide a reference for patients, especially treatment options and postoperative adjuvant treatment measures for high-risk/extremely high-risk patients.
To explore the predictive value of the prognostic nutritional index (PNI) for biological recurrence in Chinese patients with high/extremely high-risk PCa after radical prostatectomy.
The biochemical test results and clinical data of 193 patients who underwent radical prostatectomy for the first time from January 2015 to December 2020 were retrospectively collected. The PNI value of peripheral blood within 1 wk before surgery was calculated, and during the follow-up period, prostate-specific antigen ≥ 0.2 ng/mL was considered to have biological recurrence. The receiver operating characteristic (ROC) curve was used to calculate the optimal critical value and area under the curve (AUC) of the patients. According to the critical value, the progression-free survival of the high PNI group and low PNI group was compared. The independent influencing factors of the patients' prognosis were obtained by the Cox proportional hazards regression model.
The non-biological recurrence rates at 1, 3, and 5 years were 92.02%, 84.05%, and 74.85%, respectively. The optimal critical value for PNI to predict biological recurrence was 46.23, and the AUC was 0.789 (95% confidence interval: 0.651-0.860; P < 0.001). The sensitivity and specificity were 82.93% and 62.30%, respectively. In accordance with the optimal critical value of the ROC curve (46.23), 193 patients were further divided into a high PNI group (PNI ≤ 46.23, n = 108) and low PNI group (PNI > 46.23, n = 85). The incidence of postoperative complications in the high PNI group was lower than that in the low PNI group (21.18% vs 38.96%). Kaplan-Meier survival analysis showed that the overall survival rate at 5 years in the low PNI group was 87.96% (13/108), which was lower than that in the high PNI group (61.18%, 33/85; P < 0.05). Low PNI [hazard ratio (HR) = 1.74; P = 0.003] and positive incisal margin status (HR = 2.14; P = 0.001) were independent predictors of biological recurrence in patients with high/extremely high-risk PCa.
The PNI has predictive value for the prognosis of patients with high/extremely high-risk PCa, and is an independent prognostic factor. Patients with low PNI value have a shorter time of non-biological recurrence after prostatectomy. It is expected that the combined prediction of other clinicopathological data will further improve the accuracy and guide postoperative adjuvant therapy to improve the quality of prognosis.
Core Tip: Radical prostatectomy is the main treatment for prostate cancer (PCa). However, the incidence of postoperative complications and tumor progression remains high, and patients are highly prone to recurrence or metastasis. Therefore, it is crucial to identify effective prognostic biomarkers. The pro
- Citation: Yang F, Pan M, Nie J, Xiao F, Zhang Y. Evaluation of the prognostic nutritional index for the prognosis of Chinese patients with high/extremely high-risk prostate cancer after radical prostatectomy. World J Clin Cases 2022; 10(25): 8863-8871
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/8863.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.8863
The results of the Globocan project showed that prostate cancer (PCa) is one of the most common cancers in men. In the United States, PCa ranks first in incidence and third in mortality among male cancer patients. The incidence of PCa is related to race, diet structure, and other factors. In China, the incidence of PCa is relatively low, but with the aging of the population, the westernization of lifestyle, the progress of detection methods, and the development of tumor screening, the incidence of PCa is also rising. Another study reported that the incidence of PCa in China is 5.3/100000, and the mortality rate is 2.5/100000[1,2]. Currently, radical prostatectomy is the main treatment option, and there is still a great possibility of recurrence or metastasis after surgery. According to PAS, T classification, and Gleason's cancer score, patients with PCa are classified as low risk, medium risk, and high risk. The risk level is related to the 10-year disease-free survival rate after radical prostatectomy; the 10-year disease-free survival rates at low, medium, and high risk are 83%, 46%, and 29%, respectively[3,4]. Predicting the prognosis information in advance according to the degree of risk can provide a reference for patients, especially for the treatment options and postoperative adjuvant treatment measures of high/extremely high-risk patients.
Prognostic nutritional index (PNI) was initially proposed to predict the immunogenicity and risk of gastrointestinal surgery by albumin and lymphocytes, but it was later found that PNI is correlated with the prognosis of many tumors. At present, PNI is considered to have predictive significance in the prognosis of lung cancer[5], melanoma[6], esophageal cancer[7], and it is considered that low PNI is related to a poor prognosis. Currently, there are few studies on the relationship between PNI and the prognosis of patients with high-risk/extremely high-risk PCa.
This study investigated the predictive value of the PNI for the prognosis of patients with high-risk/extremely high-risk PCa after radical prostatectomy.
The clinical data of 193 patients with PCa, who were first treated in our hospital from January 2013 to December 2016, were retrospectively collected. All patients and their families provided written informed consent. Inclusion criteria were as follows: PCa was first diagnosed by pathology, and surgical resection was performed; the blood test report and other data were complete and informed consent was obtained; and as per the National Comprehensive Cancer Network (NCCN)[8] criterion, the patient was diagnosed with high/extremely high-risk PCa (high risk: T3a or prostate-specific antigen (PSA) > 20 ng/mL or Gleason score ≥ 8, and extremely high-risk: T3b-4). Exclusion criteria were as follows: the patient had other serious diseases, tumors, or postoperative infection. This study was approved by the Medical Ethics Committees of Tongji Hospital, Tongji Medical College, and Huazhong University of Science and Technology.
The clinical data of all patients were collected by consulting medical records and calling for follow-up including age, history of chronic diseases, incisal margin status, capsule invasion, nerve invasion, and postoperative adjuvant therapy.
PNI value: Peripheral venous blood was collected from all patients within 1 wk before operation for blood routine, blood biochemistry, and PSA value detection. The PNI value was calculated from the total number of peripheral serum albumin and blood lymphocytes according to the following formula: PNI = albumin value (g/L) + 5 × total lymphocyte count (109/L).
Follow-up and outcome indicators: Patients were followed up by telephone, outpatient visits, and reviewing medical records. Paper questionnaires were filled in to record patients’ postoperative data and disease progression. Follow-up included patients’ survival status (recurrence and death) and survival time. The biological recurrence indicator was PSA. When PSA was ≥ 0.2 ng/mL, biological recurrence occurred. The starting point of follow-up was postoperative pathological diagnosis, and the end point was outcome events (recurrence and death). The overall survival (OS) was the time from the starting point to the end point or last follow-up. Death due to reasons other than PCa or no recurrence before the end of the follow-up period was regarded as withdrawal or termination.
The data were entered into Epidata and corrected logically. SPSS21.0 was used to analyze the data. Rate or constituent ratio was used to describe the count data. The rate or composition ratio between groups was compared by the chi-square test. The area under the curve (AUC) was obtained by receiver operating characteristic (ROC) curve. The sensitivity and specificity of the PNI were evaluated by the AUC. Calculating the Yordon index was used to determine the optimal critical value of PNI. Progression-free survival (PFS) was compared between the two groups of patients with PNI by Kaplan-Meier survival analysis. The influencing factors of biological recurrence were analyzed by Cox proportional hazards regression model. P < 0.05 was considered statistically significant.
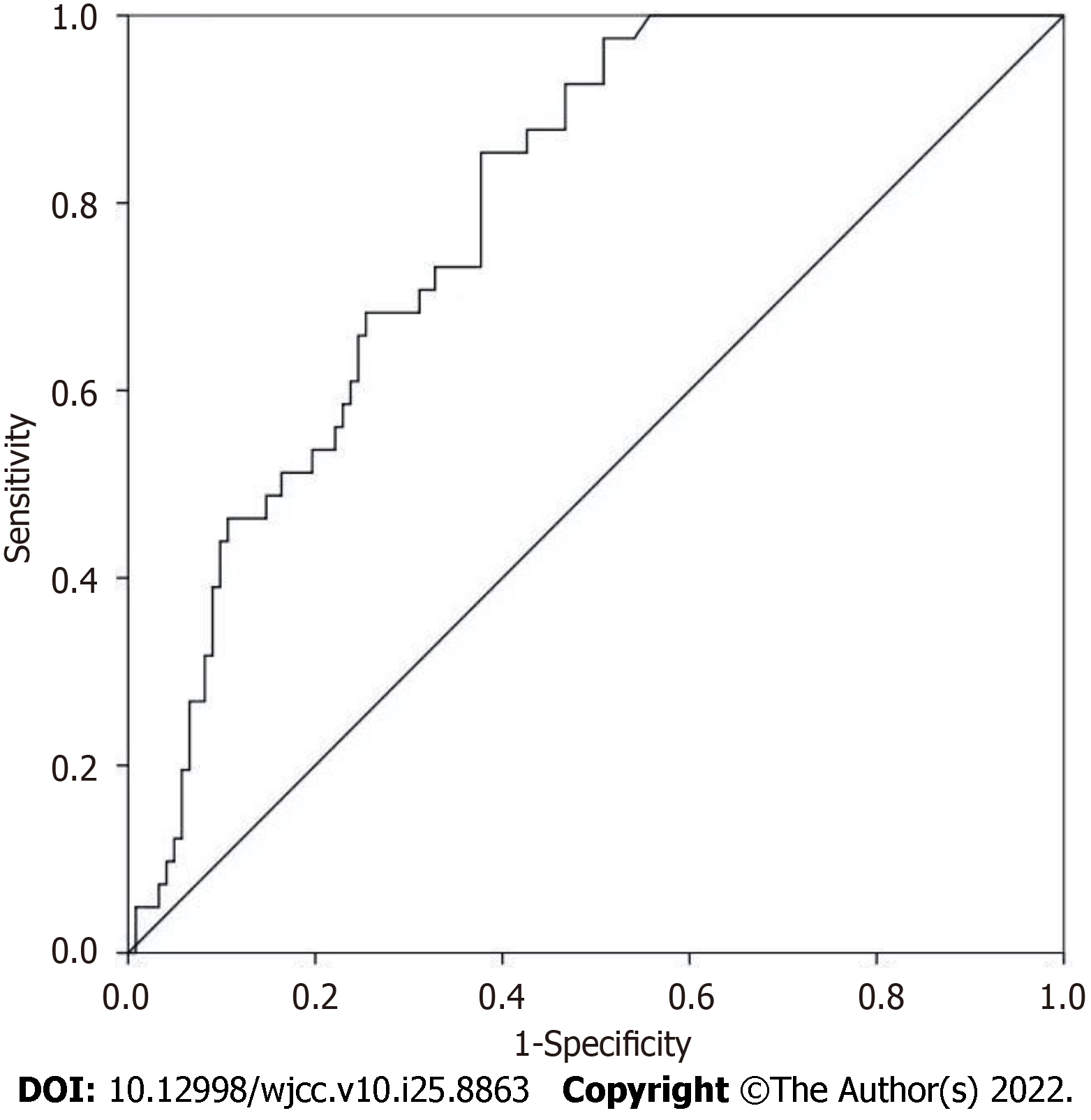
The survival outcome was regarded as the dependent variable to draw the ROC curve of the PNI value. The results showed that the AUC was 0.789 (95% confidence interval: 0.651-0.860; P < 0.001). When the critical value of PNI for the diagnosis of biological recurrence was 46.23, the Joden index was the maximum, so the optimal critical value of PNI was 46.23. Under this threshold, the sensitivity and specificity of PNI diagnosis were 82.93% and 62.30%, respectively, as shown in Figure 1.
In line with the optimal critical value of ROC curve (46.23), 193 patients were further divided into a high PNI group (PNI ≤ 46.23, n = 108) and low PNI group (PNI > 46.23, n = 85). There were no significant differences in the rates of high PNI group and low PNI group in patients with regard to age, chronic disease history, incisal margin status, capsule invasion, nerve invasion, and postoperative adjuvant therapy. There was comparability between the two groups of patients with different PNI, as shown in Table 1.
| n | PNI (n = 193) | χ2 | P value | |||
| ≤ 46.23 (n = 108), n (%) | > 46.23 (n = 85), n (%) | |||||
| Age | ≤ 65 | 69 | 43 (62.3) | 26 (37.7) | 0.078 | 0.779 |
| > 65 | 124 | 65 (52.4) | 59 (47.6) | |||
| Chronic disease | Find | 122 | 68 (55.7) | 54 (44.3) | 0.007 | 0.935 |
| Nil | 71 | 40 (56.3) | 31 (43.7) | |||
| Margin status | Positivity | 64 | 35 (54.7) | 29 (45.3) | 0.063 | 0.802 |
| Feminine | 129 | 73 (56.6) | 56 (43.4) | |||
| Envelope violations | Yes | 59 | 29 (49.2) | 30 (50.8) | 1.597 | 0.206 |
| No | 134 | 79 (59.0) | 55 (41.0) | |||
| Postoperative adjuvant therapy | Find | 173 | 101 (58.4) | 72 (41.6) | 1.310 | 0.252 |
| Nil | 20 | 7 (35.0) | 13 (65.0) | |||
| Nerve invasion | Yes | 105 | 62 (59.0) | 43 (41.0) | 0.892 | 0.345 |
| No | 88 | 46 (52.3) | 42 (47.7) | |||
After radical prostatectomy in 193 patients with high/extremely high-risk PCa, there were 8 cases of incision infection, 8 cases of postoperative bleeding, 7 cases of abdominal infection, 5 cases of rectal injury, 7 cases of postoperative penile erection dysfunction, 6 cases of bladder-urethral anastomotic stenosis, 8 cases of urethral stenosis, 3 cases of lymphatic cyst, 9 cases of urinary fistula, and 6 cases of pulmonary embolism. There were 59 cases of postoperative complications. The incidence of postoperative complications in high PNI group was lower than that in low PNI group (21.18% vs 38.96%), as shown in Table 2.
| n | PNI | χ2 | P value | ||
| ≤ 46.23, n (%) | > 46.23, n (%) | ||||
| Postoperative complication | 59 | 41 (38.96) | 18 (21.18) | 6.315 | 0.012 |
| Incision infection | 8 | 6 | 2 | ||
| Postoperative bleeding | 8 | 4 | 4 | ||
| Abdominal infection | 7 | 6 | 1 | ||
| Rectal injury | 5 | 3 | 2 | ||
| Postoperative penile erectile dysfunction | 7 | 4 | 3 | ||
| Permanent/temporary urinary incontinence | 0 | 0 | 0 | ||
| Bladder-urethral anastomotic stenosis | 6 | 4 | 2 | ||
| Urethral stricture | 8 | 7 | 1 | ||
| Deep venous thrombosis | 0 | 0 | 0 | ||
| Lymphatic cyst | 3 | 2 | 1 | ||
| Urinary fistula | 9 | 4 | 5 | ||
| Pulmonary embolism | 6 | 2 | 4 | ||
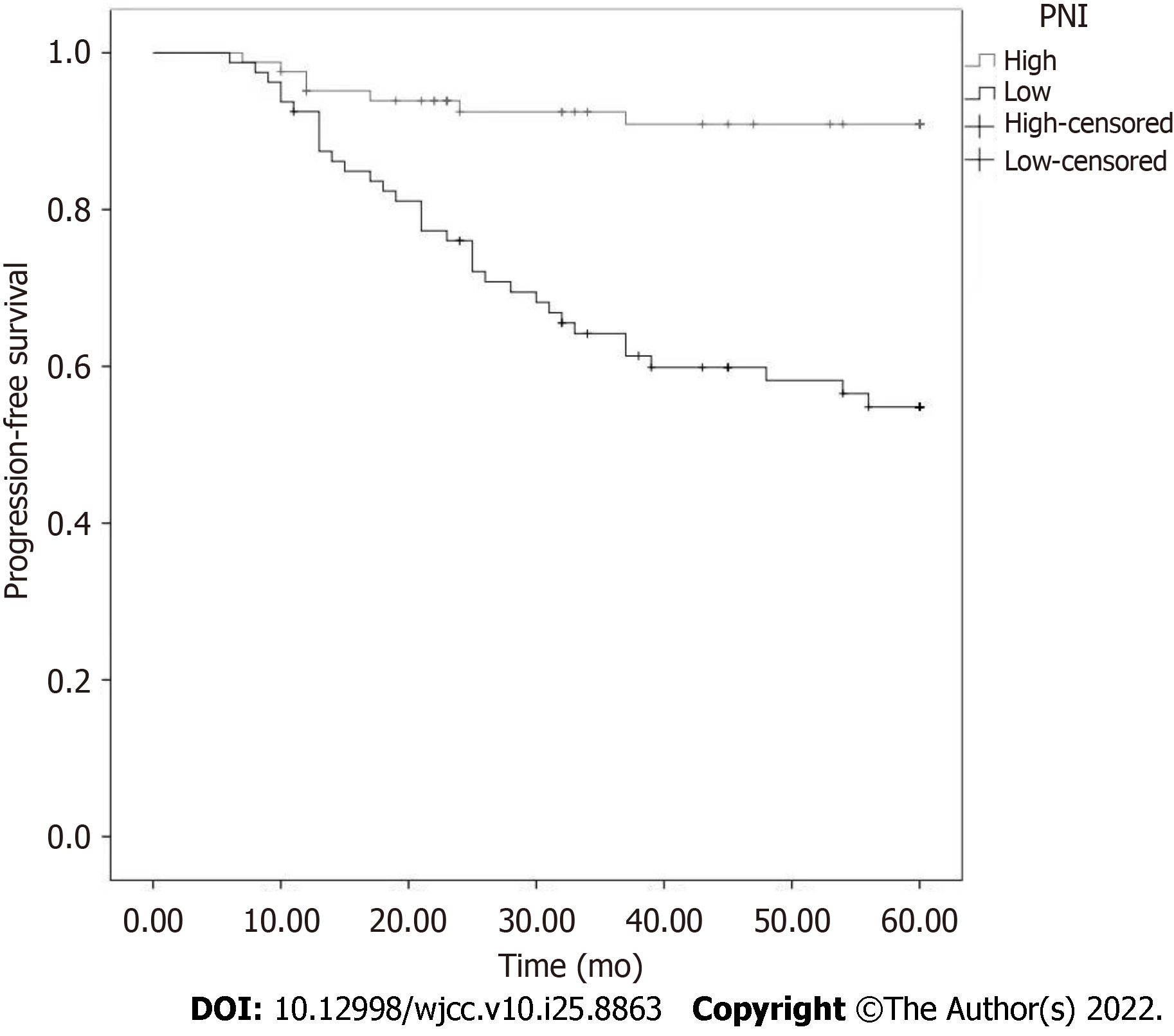
A total of 46 patients died of PCa 5 years after operation, and the survival rate at 5 years was 76.17%. Postoperative PFS curves were plotted using Kaplan-Meier survival analysis in patients with high/extremely high risk PCa. The results showed that the OS rate at 5 years in the low PNI group was 87.96% (13/108), which was lower than that in the high PNI group (61.18%, 33/85), and the difference was statistically significant (P < 0.05). The 5-year low PNI group had a shorter PFS (P < 0.001), poor prognosis, and higher postoperative biological recurrence rate (Figure 2).
At the end of follow-up, biological recurrence was considered the dependent variable (biological recurrence Y = 1, no biological recurrence Y = 0); and PNI group, age, chronic disease history, incisal margin status, capsule invasion, nerve invasion, and postoperative adjuvant therapy were considered independent variables in the Cox proportional hazards regression model. Single factor results showed that the factors affecting the biological recurrence of PCa patients included PNI grouping, age, history of chronic diseases, incisal margin status, capsule invasion, and nerve invasion (P < 0.05). The above factors with statistically significant effects on biological recurrence of patients were included in the multivariate Cox proportional hazards regression model. The results showed that low PNI [hazard ratio (HR) = 1.74; P = 0.003] and positive incisal margin status (HR = 2.14; P = 0.001) were independent predictors of biological recurrence in patients with high/extremely high-risk PCa. Specifically, the prognostic biological recurrence in patients with low PNI was 1.74 times that in patients with high PNI, and the prognostic biological recurrence was 2.14 times higher in patients with positive incisal margin status than that in patients with negative incisal margin status (Table 3).
| Parameter estimation | Standard error | HR (95%CI) | P value | |
| PNI, 0: > 46.23; 1: ≤ 46.23 | 0.61 | 0.27 | 1.74 (1.03-2.85) | 0.003 |
| Age, 0: ≤ 65; 1: > 65 | 0.27 | 0.16 | 1.31 (0.95-1.80) | 0.102 |
| Chronic disease, 0: Nil; 1: Find | 0.35 | 0.22 | 1.41 (0.92-2.18) | 0.120 |
| Margin status, 0: Feminine; 1: Positivity | 0.71 | 0.28 | 2.14 (1.22-3.62) | 0.001 |
| Envelope violations, 0: No; 1: Yes | 0.17 | 0.10 | 1.08 (0.88-1.32) | 0.473 |
| Nerve invasion, 0: No; 1: Yes | 0.44 | 0.31 | 1.59 (0.86-2.96) | 0.147 |
| Postoperative adjuvant therapy, 0: Nil; 1: Find | -0.11 | 0.18 | 0.90 (0.63-1.28) | 0.548 |
Lymphocytes and albumin were used to reflect the immune nutritional status of patients for PNI. PNI in a variety of cancers is expected to be considered a predictor of therapeutic effect, and a large number of related studies have suggested that low PNI is correlated with a poor cancer prognosis[9]. Surgical stress may cause a series of metabolic changes in patients, and allow consumption of nutrients such as protein and fat in the early metabolic period[10]. Although serum albumin cannot be directly used as a nutrient by the body, its half-life of up to 20 d can reflect the reserve level of nutrients such as amino acids in patients. Low albumin level reduces plasma osmotic pressure, resulting in reduced tolerance and absorption capacity of intestinal nutrients. Intestinal hyperosmotic nutrition agents also absorb liquid in the intestinal wall such as the intestinal cavity[11], and serum albumin is closely related to a variety of tumor regulators. Some studies have shown that serum albumin contributes to cell stability and DNA replication[12]. Lymphocytes are an important part of the immune system, which can secrete tumor necrosis factor alpha, interferon gamma, and other cytokines to inhibit tumor progression. At the same time, lymphocytes have important inhibitory effects on postoperative inflammation. The low level of lymphocytes mostly indicate immune dysfunction[13]. Immunity and nutrition play important roles in the disease progression and prognosis of patients. Peripheral blood albumin and lymphocytes make a reliable predictive analysis for the prognosis of cancer patients.
PCa is a heterogeneous disease, ranging from inertia to high invasiveness. Approximately 15% of patients with PCa are diagnosed as high risk. However, the high mortality rate in men with high-risk/very high-risk PCa remains a challenge, estimated at 14.2% after radical prostatectomy[14,15]. To identify the risk of PCa earlier and accurately, in order to develop effective diagnosis, treatment, and rehabilitation models, the risk of PCa is managed by classification. At present, there are many PCa classification methods, but it is difficult to determine the core percentage involved in the tumor reliably and repeatedly, and most of them are based on T stage, PSA, and Gleason score to classify patients. This study refers to the classification criteria of the NCCN[16].
The ROC curve results of this study showed that the optimal critical value of PNI for predicting PCa biological recurrence was 46.23 and the AUC was 0.789, indicating that the results of PNI for predicting biological recurrence had good authenticity and was a relatively accurate prediction index. The optimal diagnostic critical value of PNI for postoperative recurrence of non-small cell lung cancer was 45[17], and the optimal critical value for the survival outcome of urothelial carcinoma was 46.91[18]. However, in another PCa study, the optimal prognostic critical value was 50.2[19]. Combined with the results of this study, the optimal predictive critical value of PNI for the prognosis of cancer patients may be mostly between 45 and 50. Elevated PSA is an important early detection marker of PCa in most clinically significant PCa, but prostatic hyperplasia and prostatitis can also lead to PSA elevation. The results of this study (sensitivity 82.93%, specificity 62.30%) are consistent with the characteristics of low specificity. The PFS of patients in the low PNI group was shorter than that of patients in the high PNI group, which was similar to the previous findings in a variety of cancers. However, in this survey, there might be misdiagnosis in judging biological recurrence only by PSA value.
AUC = 0.789 indicated that the diagnostic value of prognosis was still limited. The fitting results of Cox proportional hazards regression model also showed that PNI < 46.23 was an independent factor influencing the poor prognosis of patients with PCa after operation, and the positive incisal margin status could also promote biological recurrence. At the same time, some results showed that PNI was correlated with some pathological data[19], suggesting that PNI combined with other clinical or pathological data can improve accuracy in predicting the prognosis of PCa patients.
This was a retrospective study of patients in a hospital. There may have been bias in the admission rate. The clinical and pathological data of the included patients were not comprehensive enough, and other nutritional or immune indicators of the patients were not considered. There may have been information bias. There was no further grouping analysis for the cases of PNI < 46.23. It is expected that future multicenter prospective studies with a large sample will provide more comprehensive information and further explore the prognostic impact of PNI in PCa patients.
In summary, PNI value has predictive significance for prognosis in patients with high/extremely high-risk PCa after radical prostatectomy, which can help doctors evaluate the prognosis of patients at an early stage. The prognosis of patients with high PNI is better than that of patients with low PNI. Preventive postoperative adjuvant therapy can help patients to prolong PFS.
Patients with prostate cancer (PCa) were divided into low-risk, medium-risk, and high-risk groups. The risk level was associated with 10-year disease-free survival after radical prostatectomy. The 10-year disease-free survival rates of the low-risk, medium-risk, and high-risk groups were 83%, 46%, and 29%, respectively.
Predicting the prognosis in advance according to the degree of risk can provide a basis for individualized treatment options and postoperative adjuvant treatment measures for PCa patients, especially for high/extremely high-risk patients.
Prognostic nutritional index (PNI) is considered to have predictive significance in the prognosis of lung cancer, melanoma, and esophageal cancer. PNI is also associated with the prognosis of PCa.
Patients with PCa were divided into a high PNI group and low PNI group based on the PNI threshold. The progression-free survival was compared between the two groups. Meanwhile, the influencing factors of biological recurrence were analyzed.
The progression-free survival of patients in the low PNI group within 5 years was shorter, and the low PNI and positive incisal margin status were independent predictors of biological recurrence in patients with high/extremely high-risk PCa.
PNI value has predictive significance on the prognosis of patients with high/extremely high-risk PCa.
Calculating the Youden index was used to determine the optimal critical value of PNI. The progression-free survival of low PNI group and high PNI group were compared by Kaplan-Meier survival analysis. The influencing factors of biological recurrence was analyzed by Cox proportional hazards regression model.
| 1. | Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol. 2018;25:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 272] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 2. | GLOBOCAN. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available from: http://globocan.iarc.fr/Default.aspx. |
| 3. | D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3325] [Cited by in RCA: 3486] [Article Influence: 124.5] [Reference Citation Analysis (12)] |
| 4. | D'Amico AV, Whittington R, Malkowicz SB, Weinstein M, Tomaszewski JE, Schultz D, Rhude M, Rocha S, Wein A, Richie JP. Predicting prostate specific antigen outcome preoperatively in the prostate specific antigen era. J Urol. 2001;166:2185-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Wang Z, Wang Y, Zhang X, Zhang T. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: Review and meta-analysis. Clin Chim Acta. 2018;486:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Mirili C, Yılmaz A, Demirkan S, Bilici M, Basol Tekin S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. 2019;24:1301-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 7. | Liao G, Zhao Z, Yang H, Chen M, Li X. Can Prognostic Nutritional Index be a Prediction Factor in Esophageal Cancer? Nutr Cancer. 2020;72:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Huang J, Vicini FA, Williams SG, Ye H, McGrath S, Ghilezan M, Krauss D, Martinez AA, Kestin LL. Percentage of positive biopsy cores: a better risk stratification model for prostate cancer? Int J Radiat Oncol Biol Phys. 2012;83:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Qi F, Zhou X, Wang Y, Zhang Q, Cong R, Yang J, Song N. Pre-treatment prognostic nutritional index may serve as a potential biomarker in urinary cancers: a systematic review and meta-analysis. Cancer Cell Int. 2018;18:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Lv DP, Xu GW. Albumin and surgical nutrition. Zhongguo Shiyong Waike Zazhi. 2003;7:65-66. |
| 11. | Eisenberg P. An overview of diarrhea in the patient receiving enteral nutrition. Gastroenterol Nurs. 2002;25:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 12. | Seaton K. Albumin concentration controls cancer. J Natl Med Assoc. 2001;93:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Chen L, Yan Y, Zhu L, Cong X, Li S, Song S, Song H, Xue Y. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2017;9:849-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Goldberg H, Baniel J, Yossepowitch O. Defining high-risk prostate cancer. Curr Opin Urol. 2013;23:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003;21:2163-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 345] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Chang AJ, Autio KA, Roach M 3rd, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014;11:308-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 379] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 17. | Hayasaka K, Shiono S, Suzuki K, Endoh M, Okada Y. Postoperative prognostic nutritional index as a prognostic factor after non-small cell lung cancer surgery. Gen Thorac Cardiovasc Surg. 2020;68:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Xue W, Tan P, Xu H, Yang L, Wei Q. Impact of the preoperative prognostic nutritional index on survival outcomes in upper tract urothelial carcinomas. Cancer Med. 2019;8:2971-2978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Li B, Lu Z, Wang S, Hou J, Xia G, Li H, Yin B, Lu W. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer. 2020;20:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Heidenreich A, Germany; Tanabe K, Japan S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP