Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3866
Peer-review started: August 16, 2021
First decision: October 22, 2021
Revised: November 4, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 247 Days and 21.9 Hours
The outbreak of the coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 has been the most important clinical challenge worldwide since January 2020. COVID-19 inactivated vaccines play a crucial role in reducing the rates of morbidity and mortality.
We presented a 48-year-old woman from Haidian District, Beijing, China who developed ischemic colitis after receiving the second dose of COVID-19 inactivated vaccine. Computed tomography of the abdomen showed edema and bowel wall thickening with hypodensity in the sigmoid colon and descending colon. Colonoscopy revealed hyperemia, edema and erosion of the mucosa with superficial ulceration and a yellow-white coating at the descending colon and sigmoid colon. The symptoms were relieved after 1 wk of receiving pinaverium bromide (50 mg, tid) and aspirin enteric-coated tablets (0.1 g, qd).
The possible occurrence of ischemic colitis should be considered after administration of the COVID-19 inactivated vaccines.
Core Tip: Coronavirus disease 2019 (COVID-19) inactivated vaccines play a crucial role in reducing the rates of morbidity and mortality. The adverse events after administration of inactivated vaccines are varied and need to be profoundly studied in clinical practice. This report described the relationship between the administration of the COVID-19 inactivated vaccine with ischemic colitis and its possible causes. The potential mechanism behind the development of ischemic colitis needs further exploration to better understand and manage unforeseen complications of COVID-19 inactivated vaccines.
- Citation: Cui MH, Hou XL, Liu JY. Ischemic colitis after receiving the second dose of a COVID-19 inactivated vaccine: A case report. World J Clin Cases 2022; 10(12): 3866-3871
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3866.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3866
The outbreak of the coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been the most important clinical challenge worldwide since January 2020. While the spread of COVID-19 in China has been effectively controlled, the pandemic has not ceased. To date, COVID-19 has affected more than 128 million individuals causing over 2.8 million deaths worldwide[1]. The COVID-19 pandemic is globally considered as the primary health threat[2]. Although the mortality rate of COVID-19 is low, individuals with diabetes or hypertension are at a higher risk of mortality[3,4].
Individuals encountered a variety of mental health challenges during the COVID-19 pandemic[5]. Given the lack of a specific treatment strategy for COVID-19, the development of vaccines against COVID-19 is a milestone. To date, diverse types of vaccines for COVID-19 have been proposed including messenger RNA (mRNA), non-replicating and replicating vectors, inactivated viruses, protein subunits, viral-like particles, DNA vaccines and live attenuated vaccines[6-8]. Two inactivated vaccines, the China National Biotec Group SARS-CoV-2 vaccine and the CoronaVac vaccine (Sinovac Biotech Ltd., China) have been adopted for mass vaccination within mainland China. Although the inactivated vaccine is well-tolerated in the general population[9], some rare or serious adverse events may occur. We have attempted to present a case of ischemic colitis following the administration of an inactivated vaccine.
A 48-year-old woman from Haidian District, Beijing, China was admitted into the gastroenterology department due to abdominal pain accompanied by hematochezia for 1 d.
The patient had received the second dose of the COVID-19 inactivated vaccine (4 ug/0.5 mL) in her deltoid muscle of the upper arm 1 d prior to her admission to our hospital and she did not experience any obvious discomfort during the injection. She experienced acute pain in the middle of her lower abdomen 1 h after the injection followed by 2 episodes of defecation characterized by small amounts of loose yellow stools. Subsequently, this was followed by more than 10 episodes of defecation characterized by small amounts of loose stools with hematochezia. These episodes were associated with fatigue. She did not present with nausea, vomiting, fever, dizziness or palpitations. The symptoms lasted for approximately 14 h before the appearance of abdominal pain and the hematochezia was gradually resolved spontaneously.
The patient’s diet history was normal and she did not experience adverse events after receiving the first dose of COVID-19 inactivated vaccine. A previous physical examination revealed that she had hyperlipidemia but she did not receive treatment and a normal bowel movement was noted.
There is no personal and family history.
In addition to confirming a normal menstrual cycle, physical examination revealed the following outcomes: Body mass index (BMI) of 21.1 kg/m2; body temperature of 36.2°C; heart rate of 80 beats/min; respiratory rate of 19 breaths/min; and blood pressure of 140/85 mmHg. Upon undergoing abdominal examination, her abdomen was soft with tenderness in the left lower quadrant and no rebound pain or muscle tension. Upon undergoing digital rectal examination, there were no masses and no blood was found in the finger sleeve.
The results of hematological tests showed a normal C-reactive protein level, and the results of routine blood examination were as follows: D-dimer 329 ug/L; fibrinogen degradation product 2.5 mg/L; glucose 7.35 mmol/L; and lactic acid 2.60 mmol/L. The testing of nucleic acids, IgM and IgG showed negative results.
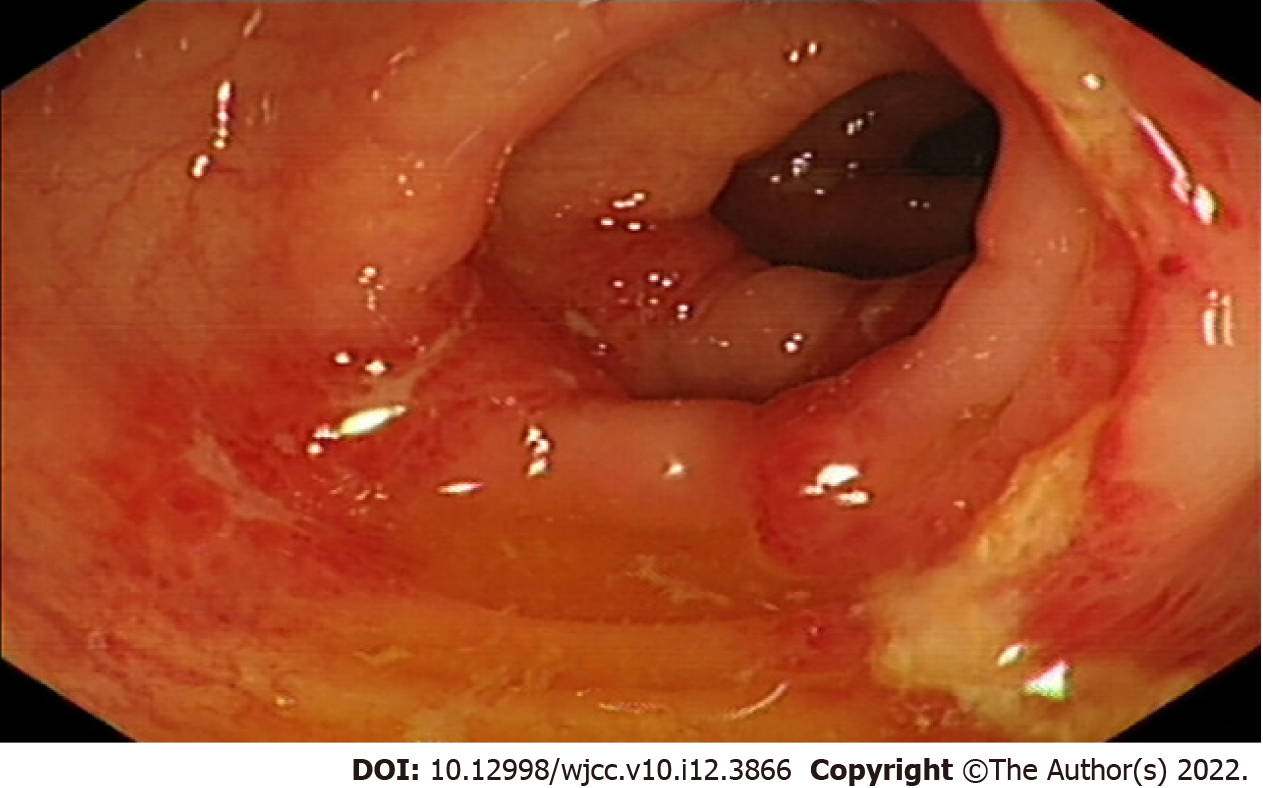
Computed tomography of the abdomen showed edema and bowel wall thickening with hypodensity in the sigmoid colon and descending colon (Figure 1). Colonoscopy revealed hyperemia, edema and erosion of the mucosa with superficial ulceration and a yellow-white coating located at the descending colon and sigmoid colon (Figure 2).
Based on the above-mentioned results, the patient was diagnosed with ischemic colitis.
The symptoms of the disease were relieved after admission to our hospital and the disease was in complete remission after administration of pinaverium bromide (50 mg, tid) and aspirin enteric-coated tablets (0.1 g, qd) for 1 wk.
The patient did not present with abdominal pain or hematochezia and the frequency of defecation was once/day with soft yellow stool.
More than 24 million doses of the COVID-19 vaccines have been administered in China as of January 31, 2021[10]. As the safety profile of vaccines could affect vaccination acceptance, resulting in the control of the COVID-19 pandemic, it is imperative to report all suspected adverse events. The incidence of anaphylaxis following the administration of the COVID-19 inactivated vaccines was recently reported at nearly 1.4 cases per one million doses[11]. The present study found that recipients of the second dose of COVID-19 inactivated vaccines experienced ischemic colitis, indicating the necessity of clinicians’ higher attention to vaccination in clinical practice.
Two doses of inactivated vaccines against COVID-19 were reported to provide a highly effective defense against antibody-dependent infection which is associated with higher efficiency and genetic stability[12]. Several studies have addressed the safety of COVID-19 inactivated vaccines. For instance, Xia et al[13,15] performed an interim analysis of two randomized clinical trials and found the most common adverse events, including injection site pain and fever which was experienced within 7 d after the administration of the vaccine, and these adverse events were mild and self-limited. Zhang et al[14] conducted a phase 1/2 clinical trial on healthy adults who were aged 18-59-years-old, and reported that the most common adverse events encountered from days 1 to 14 included fatigue and injection-site pain. However, as a newly developed inactivated vaccine, its safety still needs to be verified in the general population through long-term follow-up and rare adverse events must be considered in clinical practice. The present study, for the first time, reported a woman who experienced ischemic colitis after administration of a second dose of the COVID-19 inactivated vaccine.
Hypercoagulable states impairing arterial blood supply to the colon potentially induced by the COVID-19 vaccine could be a potential cause of ischemic colitis[16]. Other risk factors for ischemic colitis include atherosclerosis, abdominal vascular disease and autoimmune disease which all can lead to reduced intestinal blood flow. These in turn may give rise to hypercoagulable states such as suffering from malignant tumors, portal hypertension, hematological disease, hypertension, coronary heart disease, diabetes, constipation, cardiogenic embolism, chronic obstructive pulmonary disease and abdominal surgery[17,18]. The medical history of the patient was normal except for a previous diagnosis of hyperlipidemia.
The onset time of ischemic colitis was closely correlated to the administration of the COVID-19 vaccines and a potential association could not be ruled out. Although the specific pathogenesis is elusive, the inflammation and immune reaction caused by vaccine administration could induce a hypercoagulable state. However, the levels of D-dimer and fibrinogen degradation product were normal which may be due to the acute onset of disease. Other inflammatory indices were normal as well. Increased intestinal peristalsis with sustained muscle contractions after vaccine administration could lead to the increase of intestinal pressure and vasospasm, in which the reduced blood volume due to frequent episodes of diarrhea could cause abdominal wall defects, mucosal ischemia, impaired metabolic function, acidosis, necrosis, gastric mucosal injury and bleeding[19].
Our patient received an inactivated vaccine which retained the immunogenicity of the virus without its pathogenic effect. Hence, although ischemic colitis can be induced by COVID-19, ischemic colitis in this patient might not be caused by SARS-CoV-2[20-22]. Studies have demonstrated that SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) receptor to facilitate viral entry into target cells. ACE-2 is expressed in pulmonary alveolar epithelial cells and throughout the gastrointestinal tract, including the colonic mucosa, thereby playing an important role in the development of ischemic colitis[23-25]. The present episode was transient with rapidly resolving symptoms indicating a relatively benign prognosis. Therefore, the inactivated vaccine should be applied against COVID-19 owing to the extremely low incidence of ischemic colitis.
This report described the temporal relationship between the administration of the COVID-19 inactivated vaccine with ischemic colitis and discussed its possible causes. The potential mechanism behind the development of ischemic colitis in the present case remained obscure and further evaluation is required to understand and manage unforeseen complications of COVID-19 inactivated vaccines. Patients with abdominal pain accompanied by hematochezia at 1 d after administration of COVID-19 inactivated vaccines should be cautiously managed in clinical practice.
| 1. | World Health Organization. WHO coronavirus disease (COVID-19) dashboard. [cited March 2021]. Available from: https://covid19.who.int/. |
| 2. | Sun J, He WT, Wang L, Lai A, Ji X, Zhai X, Li G, Suchard MA, Tian J, Zhou J, Veit M, Su S. COVID-19: Epidemiology, Evolution, and Cross-Disciplinary Perspectives. Trends Mol Med. 2020;26:483-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 350] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 3. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19018] [Article Influence: 3169.7] [Reference Citation Analysis (9)] |
| 4. | Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1758] [Cited by in RCA: 1959] [Article Influence: 326.5] [Reference Citation Analysis (0)] |
| 5. | Meo SA, Abukhalaf AA, Alomar AA, Sattar K, Klonoff DC. COVID-19 Pandemic: Impact of Quarantine on Medical Students' Mental Wellbeing and Learning Behaviors. Pak J Med Sci. 2020;36:S43-S48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 6. | Romero JR, Bernstein HH. COVID-19 Vaccines: A Primer for Clinicians. Pediatr Ann. 2020;49:e532-e536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Sharma O, Sultan AA, Ding H, Triggle CR. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front Immunol. 2020;11:585354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 313] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 8. | Korang SK, Juul S, Nielsen EE, Feinberg J, Siddiqui F, Ong G, Klingenberg S, Veroniki AA, Bu F, Thabane L, Thomsen AR, Jakobsen JC, Gluud C. Vaccines to prevent COVID-19: a protocol for a living systematic review with network meta-analysis including individual patient data (The LIVING VACCINE Project). Syst Rev. 2020;9:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Haque A, Pant AB. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 10. | The Cumulative Vaccination of SARS-CoV-2 Vaccines in China Has Exceeded 24 Million Doses. Available from: https://news.cctv.com/2021/01/31/ARTIQJMVtbU2HQJUrS5DlsJY210131.shtml.. |
| 11. | McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, Hambidge SJ, Lee GM, Jackson LA, Irving SA, King JP, Kharbanda EO, Bednarczyk RA, DeStefano F. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 12. | Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, Xu W, Zhao Y, Li N, Zhang J, Liang H, Bao L, Xu Y, Ding L, Zhou W, Gao H, Liu J, Niu P, Zhao L, Zhen W, Fu H, Yu S, Zhang Z, Xu G, Li C, Lou Z, Xu M, Qin C, Wu G, Gao GF, Tan W, Yang X. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020;182:713-721.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 580] [Article Influence: 96.7] [Reference Citation Analysis (1)] |
| 13. | Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang W, Yang Y, Chen W, Gao X, You W, Wang X, Wang Z, Shi Z, Wang Y, Yang X, Zhang L, Huang L, Wang Q, Lu J, Guo J, Zhou W, Wan X, Wu C, Wang W, Huang S, Du J, Meng Z, Pan A, Yuan Z, Shen S, Guo W. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA. 2020;324:951-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 589] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, Chen X, Liu X, Jiang C, Li J, Yang M, Song Y, Wang X, Gao Q, Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 986] [Article Influence: 164.3] [Reference Citation Analysis (0)] |
| 15. | Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, Huang W, Xu W, Huang B, Wang W, Zhang W, Li N, Xie Z, Ding L, You W, Zhao Y, Yang X, Liu Y, Wang Q, Huang L, Xu G, Luo B, Liu P, Guo W. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 832] [Article Influence: 166.4] [Reference Citation Analysis (12)] |
| 16. | Tsimperidis AG, Kapsoritakis AN, Linardou IA, Psychos AK, Papageorgiou AA, Vamvakopoulos NC, Kyriakou DS, Potamianos SP. The role of hypercoagulability in ischemic colitis. Scand J Gastroenterol. 2015;50:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 17. | Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J. 2004;97:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Twohig PA, Desai A, Skeans J, Waghray N. Quantifying risk factors for ischemic colitis: A nationwide, retrospective cohort study. Indian J Gastroenterol. 2020;39:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Yngvadottir Y, Karlsdottir BR, Hreinsson JP, Ragnarsson G, Mitev RUM, Jonasson JG, Möller PH, Björnsson ES. The incidence and outcome of ischemic colitis in a population-based setting. Scand J Gastroenterol. 2017;52:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Chan KH, Lim SL, Damati A, Maruboyina SP, Bondili L, Abu Hanoud A, Slim J. Coronavirus disease 2019 (COVID-19) and ischemic colitis: An under-recognized complication. Am J Emerg Med. 2020;38:2758.e1-2758.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Paul T, Joy AR, Alsoub HARS, Parambil JV. Case Report: Ischemic Colitis in Severe COVID-19 Pneumonia: An Unforeseen Gastrointestinal Complication. Am J Trop Med Hyg. 2021;104:63-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | González Lázaro P, Lomas Meneses A, Del Val Zaballos F, Morandeira Rivas A. Ischemic colitis and short bowel disease due to choronavirus disease 2019 (COVID 19). Clin Nutr ESPEN. 2020;40:406-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4642] [Article Influence: 201.8] [Reference Citation Analysis (0)] |
| 24. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4193] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 25. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2016] [Article Influence: 336.0] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fan X, United States; Kumar A, India; Musoni L, Morocco S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ