Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3872
Peer-review started: August 17, 2021
First decision: December 17, 2021
Revised: January 6, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 246 Days and 23.4 Hours
In patients who suffer from both atrial fibrillation (AF) and atrial septal defect (ASD), cryoballoon pulmonary vein isolation (PVI), sequential left atrial appendage (LAA) occlusion and ASD closure could be a strategy for effective prevention of stroke and right heart failure.
A 65-year-old man was admitted to our institution due to recurrent episodes of palpitations and shortness of breath for 2 years, which had been worsening over the last 48 h. He had a history of AF, ASD, coronary heart disease with stent implantation and diabetes. Physical and laboratory examinations showed no abnormalities. The score of CHA2DS2VASc was 3, and HAS-BLED was 1. Echocardiography revealed a 25-mm secundum ASD. Pulmonary vein (PV) and LAA anatomy were assessed by cardiac computed tomography. PV mapping with 10-pole Lasso catheter was performed following ablation of all four PVs with complete PVI. Following the cryoballoon PVI, the patient underwent LAA occlusion under transesophageal echocardiographic monitoring. Lastly, a 34-mm JIYI ASD occlude device was implanted. A follow-up transesophageal echocardiography at 3 mo showed proper position of both devices and neither thrombi nor leakage was found.
Sequential cryoballoon PVI and LAA occlusion prior to ASD closure can be performed safely in AF patients with ASD.
Core Tip: Patients who suffer from atrial septal defect (ASD) with atrial fibrillation are prone to right heart dysfunction and embolism. We report the first case treated with a 3-in-1 procedure (cryoballoon pulmonary vein isolation and left atrial appendage occlusion prior to ASD closure), which may not be performed routinely. However, for ASD patients complicated with poorly controlled atrial fibrillation and unable to tolerate long-term oral anticoagulants, this 3-in-1 procedure can be considered.
- Citation: Wu YC, Wang MX, Chen GC, Ruan ZB, Zhang QQ. Cryoballoon pulmonary vein isolation and left atrial appendage occlusion prior to atrial septal defect closure: A case report. World J Clin Cases 2022; 10(12): 3872-3878
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3872.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3872
Pulmonary vein isolation (PVI) has been established as a treatment for patients with atrial fibrillation (AF)[1]. Cryoballoon PVI has become a relatively simple alternative for radiofrequency ablation[2]. Left atrial appendage (LAA) occlusion is performed as an alternative treatment to oral anticoagulation in patients with non-valvular AF[3]. Atrial septal defect (ASD), as the most common congenital heart disease, may lead to right heart dysfunction and paradoxical embolism[4]. In patients who suffer from both AF and ASD, cryoballoon PVI combined with ASD closure and the LAA occlusion combined with ASD closure have been reported[5-7]. However, there has been no report on the 3-in-1 procedure (cryoballoon PVI, LAA occlusion and ASD closure), which may be effective for preventing stroke and right heart failure. Here, we report a patient who underwent sequential cryoballoon PVI, LAA occlusion and ASD closure during the same operation.
A 65-year-old man was admitted to our hospital due to recurrent episodes of palpitations and shortness of breath for 2 years.
His symptoms started 2 years ago with recurrent episodes of palpitations and shortness of breath, which had worsened over the last 48 h.
His past illness included AF, ASD, coronary heart disease with stent implantation and diabetes.
None.
The patient’s temperature was 36.6 °C, heart rate was 74 bpm, respiratory rate was 16 breaths per minute, blood pressure was 120/70 mmHg and oxygen saturation in room air was 98%. There was no filling of jugular vein; cardiac auscultation showed arrhythmia and no cardiac murmur in each valve area; and no edema was found in both lower limbs.
On admission, his blood tests including routine blood test, renal function, liver function, thyroid function and coagulation function showed no abnormalities.
Pulmonary vein (PV) anatomy was assessed in detail by cardiac computed tomography (CT) (Figure 1A). Reconstruction and measurement of LAA and selection of suitable implantation angle and position were also completed by cardiac CT (Figure 1B-C). Electrocardiography showed AF with a ventricular rate of 76 bpm. Echocardiography showed normal left ventricular ejection fraction, moderate dilatation of the left atrium (50 mm), severe enlarged right atrium and right ventricle and moderate tricuspid regurgitation (estimated pulmonary arterial systolic pressure was 47 mmHg). Abnormal flow from the left to right atrium through the interatrial septum was found by color Doppler image. Echocardiography revealed a 25-mm secundum ASD with adequate margins for ASD closure. There were no obvious abnormalities on chest CT and abdominal color Doppler ultrasound.
CHA2DS2VASc score was 3 (diabetes mellitus, vascular disease, age 65 years to 74 years) and HAS-BLED was 1 (age ≥ 65 years). He refused a long-term anti-coagulation treatment.
AF, ASD, coronary heart disease and diabetes.
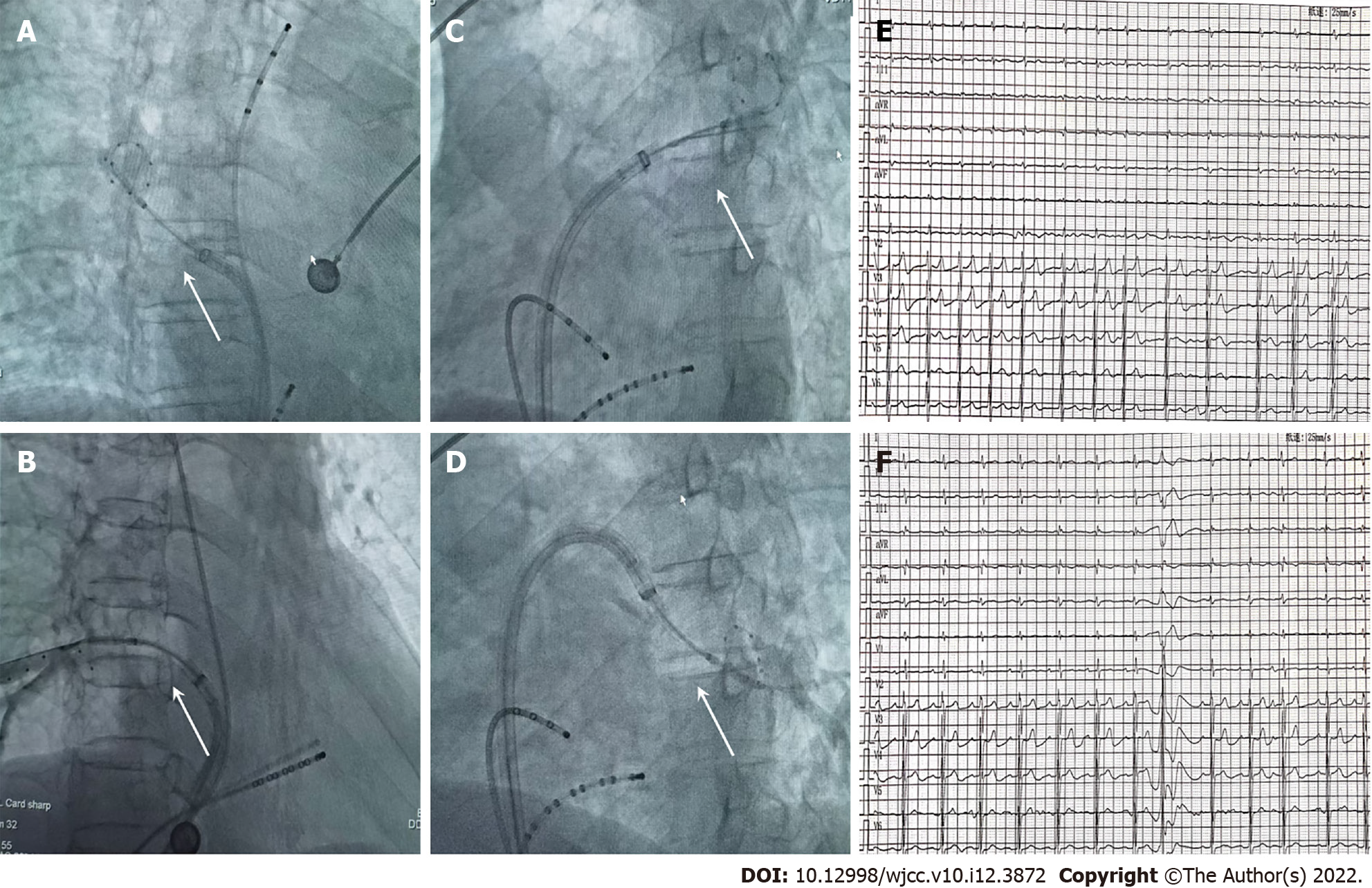
The patient had recurrent AF, which was poorly controlled with antiarrhythmic drugs, so PVI was attempted. Under general anesthesia, a 12F FlexCath steerable sheath (Medtronic Inc., Minneapolis, MN, United States) was advanced into the left atrium without transseptal puncture. A cryoballoon catheter (Medtronic Inc.) was introduced inside the 12F sheath. Following good balloon occlusion, we applied two ablation freezes for 120-180 s (Figure 2A-D). PV mapping was performed following ablation of all four PVs with a 10-pole Lasso catheter (Biosense-Webster Inc., Diamond Bar, CA, United States). We used bidirectional conduction block between the left atrium and PVs[8] to conform the complete elimination of PV electrical activity. Preoperative and postoperative electrocardiograms are shown in Figure 2E-F.
Anticoagulant therapy was recommended, but the patient refused to take long-term oral anticoagulants, so LAA occlusion was selected. Following the cryoballoon PVI, the patient underwent LAA occlusion under transesophageal echocardiographic (TEE) monitoring[9]. A special sheathing canal was placed to perform LAA angiography, and a pigtail angiographic catheter was directed to the LAA with the following positions: Right anterior oblique 30° + cranial 20° and right anterior oblique 30° + caudal 20°. Suitable LAA occluder (Watchman, 3.0 cm) was selected following measurement of LAA orifice width and depth. The LAA occluder was introduced into the LAA along the sheathing canal. The position of the occluder was monitored by TEE. A pull test was conducted to determine the stability of the occluder. After suitable position of the occluder and good plugging effect were confirmed, the occluder was released (Figure 1D).
The indications and benefits of atrial septal occlusion are clear. Figure 3A shows a secundum ASD by echocardiography. After cryoballoon PVI and LAA occlusion, the diameter of the interatrial defect was measured on TEE images in various planes, and a 34-mm JIYI ASD occluder device (Shanghai Shape Memory Co., Ltd, Shanghai, China) was implanted[10]. Secured and stable positioning of the occluder was confirmed through a push-pull test (Figure 3B). After unscrewing the occluder from the cable, good positioning of the device was demonstrated by a final TEE examination.
The patient was subsequently treated with propafenone 150 mg three times a day for 3 mo. Antiplatelet and anticoagulation therapy (clopidogrel and rivaroxaban) was administered following the doctor's advice. Before discharge, correct device positions were confirmed by echocardiography (Figure 3C). A follow-up TEE was performed to confirm proper seating of the devices and to identify thrombi or residual leak at 3 mo (Figure 3D). Both devices were located in proper position, and neither thrombi nor leakage was present. Subsequently, the patient discontinued rivaroxaban and changed to aspirin and clopidogrel.
We report an ASD patient with AF who underwent the cryoballoon PVI and LAA occlusion prior to ASD closure, which indicates that this 3-in-1 operation is feasible, but it is not recommended as a routine procedure. For patients with ASD complicated with poorly controlled AF and unable to tolerate long-term oral anticoagulants, this 3-in-1 procedure can be considered.
AF is the most common cardiac arrhythmia, which occurs in 1%-2% of the general population[11]. Since PVs were demonstrated as major sources of ectopic beats, PVI has been considered as the cornerstone for AF procedures[12]. Cryoballoon AF ablation has been established as a useful and safe method in treating paroxysmal and persistent AF, providing an alternative approach to radiofrequency ablation[13]. The incidence of AF is strikingly high in patients with ASD, even after surgical closure[14]. Furthermore, compared with the general population, patients with ASD suffer earlier from atrial arrhythmia[15]. Closure of the ASD could decrease the volume overload and reverse remodeling of the atrium[16]. In the present case, we performed cryoballoon ablation followed by closure of LAA and ASD, which we thought could maintain sinus rhythm, reverse atrium remodeling and prevent embolism.
Koermendy et al[17] reported that LAA occlusion through ASD or patent foramen ovale was a feasible access. Cardiac tamponade and perforation of adjacent organs could be obviated by avoiding a transseptal puncture[18]. Another advantage is not to create an iatrogenic septal defect. It is not easy to perform LAA occlusion after ASD occlusion, as the ASD occluder makes it difficult to transseptal puncture[19]. Thus, before ASD occlusion, it is necessary to evaluate the indication for LAA occlusion carefully. According to the reported guidelines, a CHA2DS2VASc score of ≥ 2 point is considered as an indication for LAA occlusion[20]. Our case strictly followed this standard, and as this patient refused to take long-term anti-coagulants, LAA occlusion was conducted before ASD closure.
Invasive and surgical procedures are becoming less frequent because of the improvement in percutaneous techniques, especially in cardiac interventions[21]. The present case report indicates that cryoballoon PVI and LAA occlusion prior to percutaneous ASD closure can be performed safely and can prevent several difficulties and complications. In addition, this 3-in-1 procedure was beneficial simultaneously to maintain sinus rhythm, reverse atrium remodeling and prevent embolism.
Cryoballoon PVI and LAA occlusion prior to ASD closure can be performed sequentially in ASD patients with AF, which may not be performed routinely. However, for ASD patients complicated with poorly controlled AF and unable to tolerate long-term oral anticoagulants, this 3-in-1 procedure can be considered.
The authors thank Zhang B and Wu DH for their assistance in cardiac CT analysis.
| 1. | Reissmann B, Metzner A, Kuck KH. Cryoballoon ablation versus radiofrequency ablation for atrial fibrillation. Trends Cardiovasc Med. 2017;27:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Kuck KH, Fürnkranz A. Cryoballoon ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Piayda K, Afzal S, Nielsen-Kudsk JE, Schmidt B, Mazzone P, Berti S, Fischer S, Lund J, Montorfano M, Hildick-Smith D, Gage R, Zhao H, Zeus T. Length of stay following percutaneous left atrial appendage occlusion: Data from the prospective, multicenter Amplatzer Amulet Occluder Observational Study. PLoS One. 2021;16:e0255721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Hatipoglu S, Almogheer B, Mahon C, Houshmand G, Uygur B, Giblin GT, Krupickova S, Baksi AJ, Alpendurada F, Prasad SK, Babu-Narayan SV, Gatzoulis MA, Mohiaddin RH, Pennell DJ, Izgi C. Clinical significance of partial anomalous pulmonary venous connections (isolated and atrial septal defect associated) determined by cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2021;14:e012371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Aytemir K, Sunman H, Canpolat U, Oto A. Cryoballoon pulmonary vein isolation prior to percutaneous atrial septal defect closure: a case report. Turk Kardiyol Dern Ars. 2013;41:728-731. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Song S, Lee OH, Kim JS, Cho IJ, Shim CY, Hong GR, Pak HN, Jang Y. Simultaneous closure of a left atrial appendage through an atrial septal defect and the atrial septal defect. Yonsei Med J. 2017;58:1237-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Paulo M, García E, Hernández-Antolín RA, Almería C. [Simultaneous percutaneous closure of patent foramen ovale and left atrial appendage]. Rev Esp Cardiol. 2011;64:1215-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Rottner L, Fink T, Kuck KH. Cryoballoon ablation beyond paroxysmal atrial fibrillation. Curr Opin Cardiol. 2019;34:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Cimmino G, Loffredo FS, Gallinoro E, Prozzo D, Fabiani D, Cante L, Salerno G, Cappelli Bigazzi M, Golino P. Percutaneous Left Atrial Appendage Occlusion: An Emerging Option in Patients with Atrial Fibrillation at High Risk of Bleeding. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Cruz-González I, Rama-Merchan JC, Rodríguez-Collado J, Martín-Moreiras J, Diego-Nieto A, Barreiro-Pérez M, Martín-García A, Sanchez PL. Simultaneous Percutaneous Closure of Left Atrial Appendage and Atrial Septal Defect After Mitral Valve Replacement. JACC Cardiovasc Interv. 2016;9:e129-e130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | De Marchis GM, Sposato LA, Kühne M, Dittrich TD, Bonati LH, Fischer U, Chaturvedi S. New avenues for optimal treatment of atrial fibrillation and stroke prevention. Stroke. 2021;52:1490-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Chun KR, Fürnkranz A, Schmidt B, Metzner A, Tilz R, Zerm T, Köster I, Koektuerk B, Konstantinidou M, Ouyang F, Kuck KH. Right ventricular rapid pacing in catheter ablation of atrial fibrillation: a novel application for cryoballoon pulmonary vein isolation. Clin Res Cardiol. 2009;98:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Hoffmann E, Straube F, Wegscheider K, Kuniss M, Andresen D, Wu LQ, Tebbenjohanns J, Noelker G, Tilz RR, Chun JKR, Franke A, Stellbrink C, Garcia-Alberola A, Dorwarth U, Metzner A, Ouarrak T, Brachmann J, Kuck KH, Senges J; FREEZE Cohort Study Investigators. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace. 2019;21:1313-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Deveci OS, Aytemir K, Okutucu S, Tulumen E, Aksoy H, Kaya EB, Evranos B, Kabakci G, Tokgozoglu L, Oto A, Ozkutlu H. Evaluation of the relationship between atrial septal aneurysm and cardiac arrhythmias via P-wave dispersion and signal-averaged P-wave duration. Ann Noninvasive Electrocardiol. 2010;15:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Guray U, Guray Y, Yýlmaz MB, Mecit B, Sasmaz H, Korknaz S, Kutuk E. Evaluation of P wave duration and P wave dispersion in adult patients with secundum atrial septal defect during normal sinus rhythm. Int J Cardiol. 2003;91:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Dolgner SJ, Steinberg ZL, Jones TK, Reisman M, Buber J. Stroke in patients with secundum atrial septal defect and sequelae after transcatheter closure. Heart. 2021;107:1850-1851. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Koermendy D, Nietlispach F, Shakir S, Gloekler S, Wenaweser P, Windecker S, Khattab AA, Meier B. Amplatzer left atrial appendage occlusion through a patent foramen ovale. Catheter Cardiovasc Interv. 2014;84:1190-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Tzeis S, Andrikopoulos G, Deisenhofer I, Ho SY, Theodorakis G. Transseptal catheterization: considerations and caveats. Pacing Clin Electrophysiol. 2010;33:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | He L, Du YJ, Cheng GS, Zhang YS. Device-related thrombosis on atrial septal defect occluder after simultaneous closure of left atrial appendage and atrial septal defect: a case report. J Geriatr Cardiol. 2019;16:490-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYH, Fauchier L, Betts TR, Lewalter T, Saw J, Tzikas A, Sternik L, Nietlispach F, Berti S, Sievert H, Bertog S, Meier B; ESC Scientific Document Group. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion - an update. Europace. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Kądziela J, Kochman J, Grygier M, Michałowska I, Tomaniak M, Wojakowski W, Araszkiewicz A, Dąbrowski M, Hawranek M, Huczek Z, Kralisz P, Kusa J, Roleder T, Januszewicz A, Witkowski A, Adlam D, Bartuś S. The diagnosis and management of spontaneous coronary artery dissection - expert opinion of the Association of Cardiovascular Interventions (ACVI) of Polish Cardiac Society. Kardiol Pol. 2021;79:930-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lal A, United States; Tumminello G, Italy S-Editor: Li X L-Editor: Filipodia P-Editor: Li X