©The Author(s) 2025.
World J Clin Cases. Nov 16, 2025; 13(32): 104208
Published online Nov 16, 2025. doi: 10.12998/wjcc.v13.i32.104208
Published online Nov 16, 2025. doi: 10.12998/wjcc.v13.i32.104208
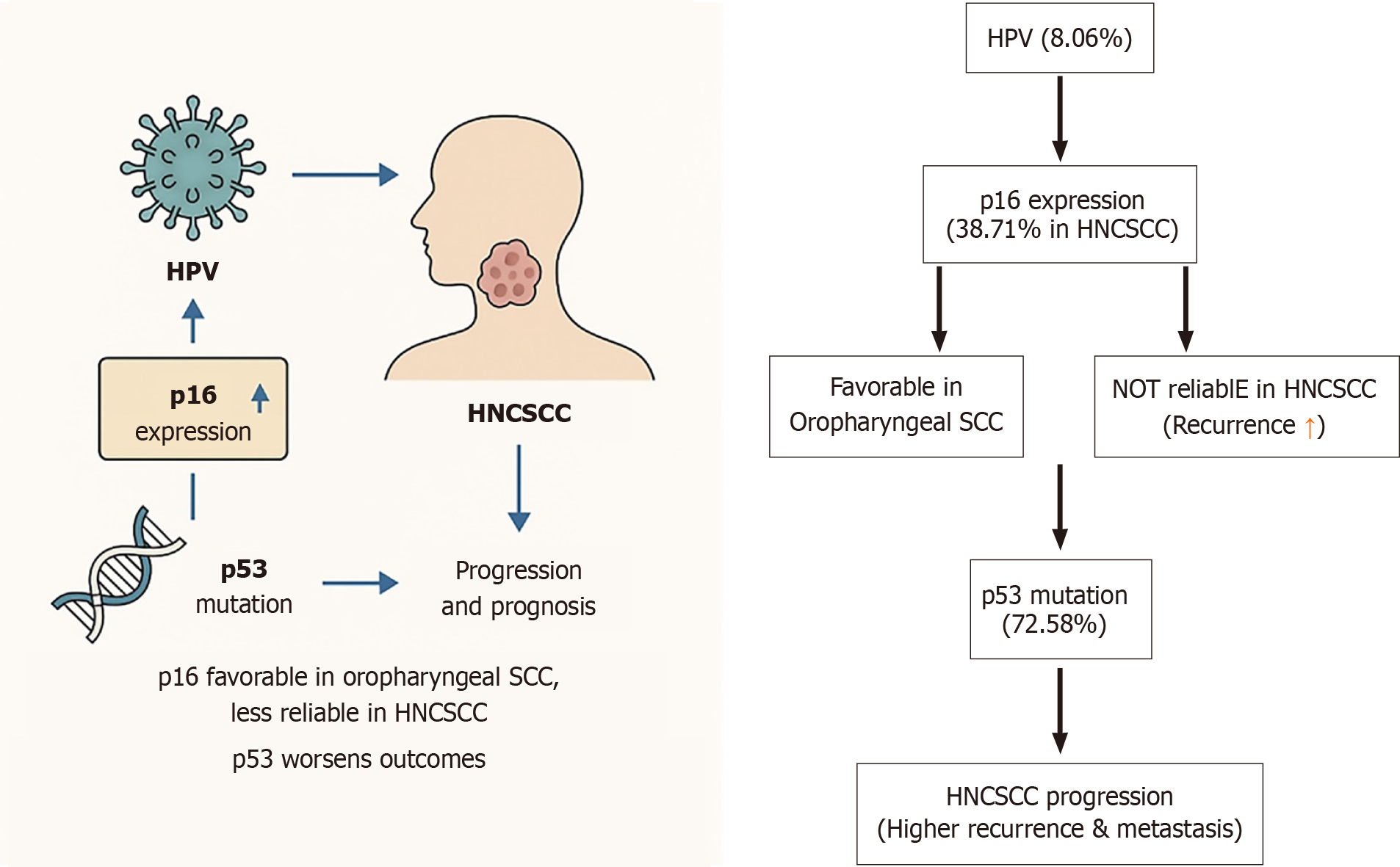
Figure 1 This diagram illustrates the relationship between human papillomavirus, p16 expression, p53 mutation, and their roles in head and neck squamous cell carcinoma progression and prognosis.
Human papillomavirus infection, p16 expression, and p53 mutations are interconnected factors influencing head and neck squamous cell carcinoma (HNCSCC) development, progression, and prognosis. While p16 is favorable in oropharyngeal squamous cell carcinoma, it is less reliable in HNCSCC, especially when combined with p53 mutations, which worsen outcomes. These biomarkers are essential for understanding and managing HNCSCC. HPV: Human papillomavirus; HNCSCC: Head and neck squamous cell carcinoma; SCC: Squamous cell carcinoma.
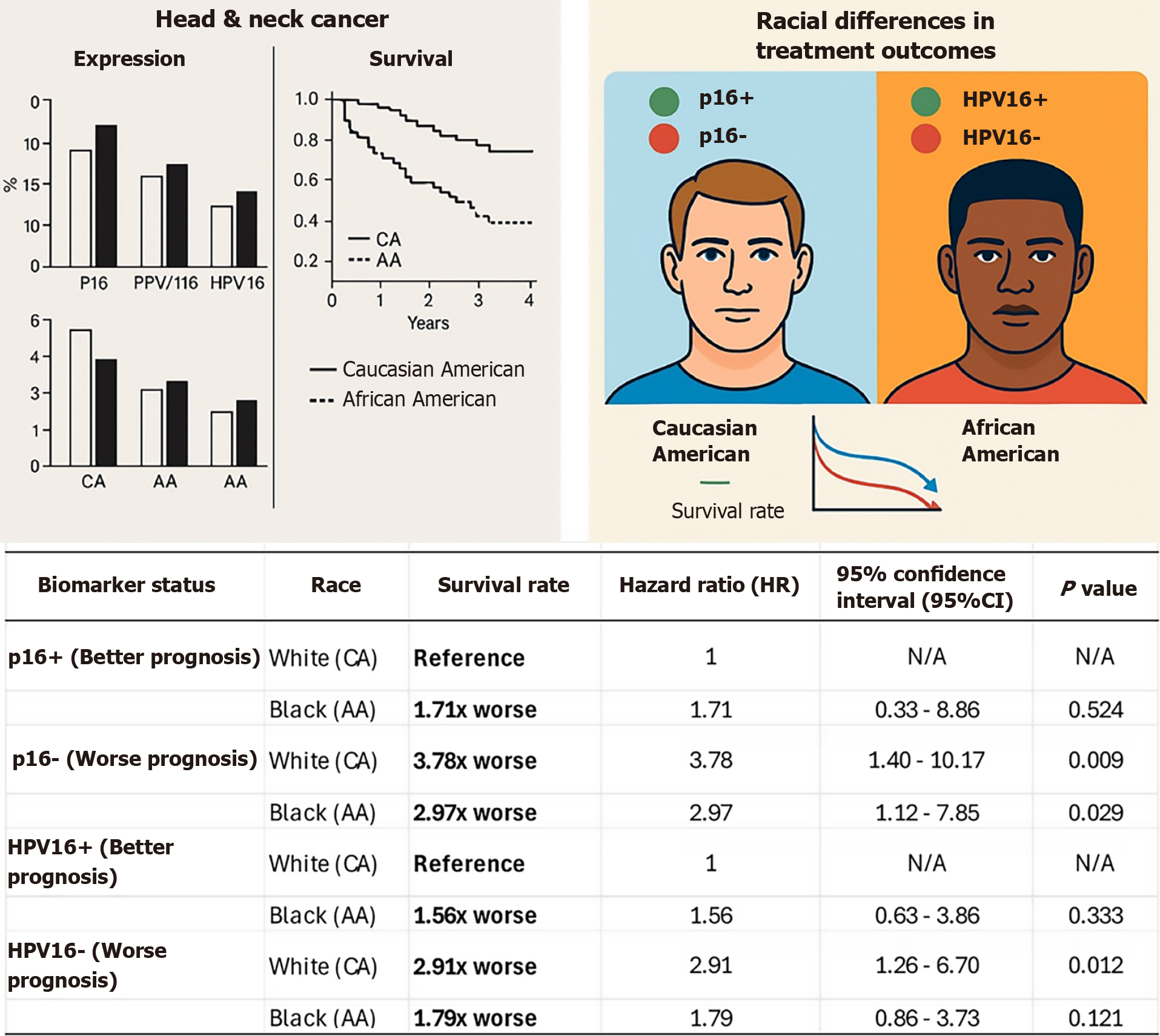
Figure 2 An illustration depicting Caucasian and African American individuals with a graphical representation of head and neck cancer biomarkers, survival markers, and associated prognosis.
The table compares survival rates, hazard ratios, and P values for head and neck cancer patients based on biomarker status and race (Caucasian American vs African American). Specifically, p16+ (better prognosis): P16+ is generally associated with better prognosis, but survival disparities exist between races. p16- (worse prognosis): P16 is linked to significantly worse survival rates, especially among Caucasian patients. Human papillomavirus (HPV) 16+ (better prognosis): HPV16+ is associated with improved survival, though the effect varies between racial groups. HPV16- (worse prognosis): HPV16 results in significantly worse survival outcomes, particularly for Caucasian patients. HPV: Human papillomavirus; CA: Caucasian American; AA: African American; HR: Hazard ratio; CI: Confidence interval; N/A: Not applicable.
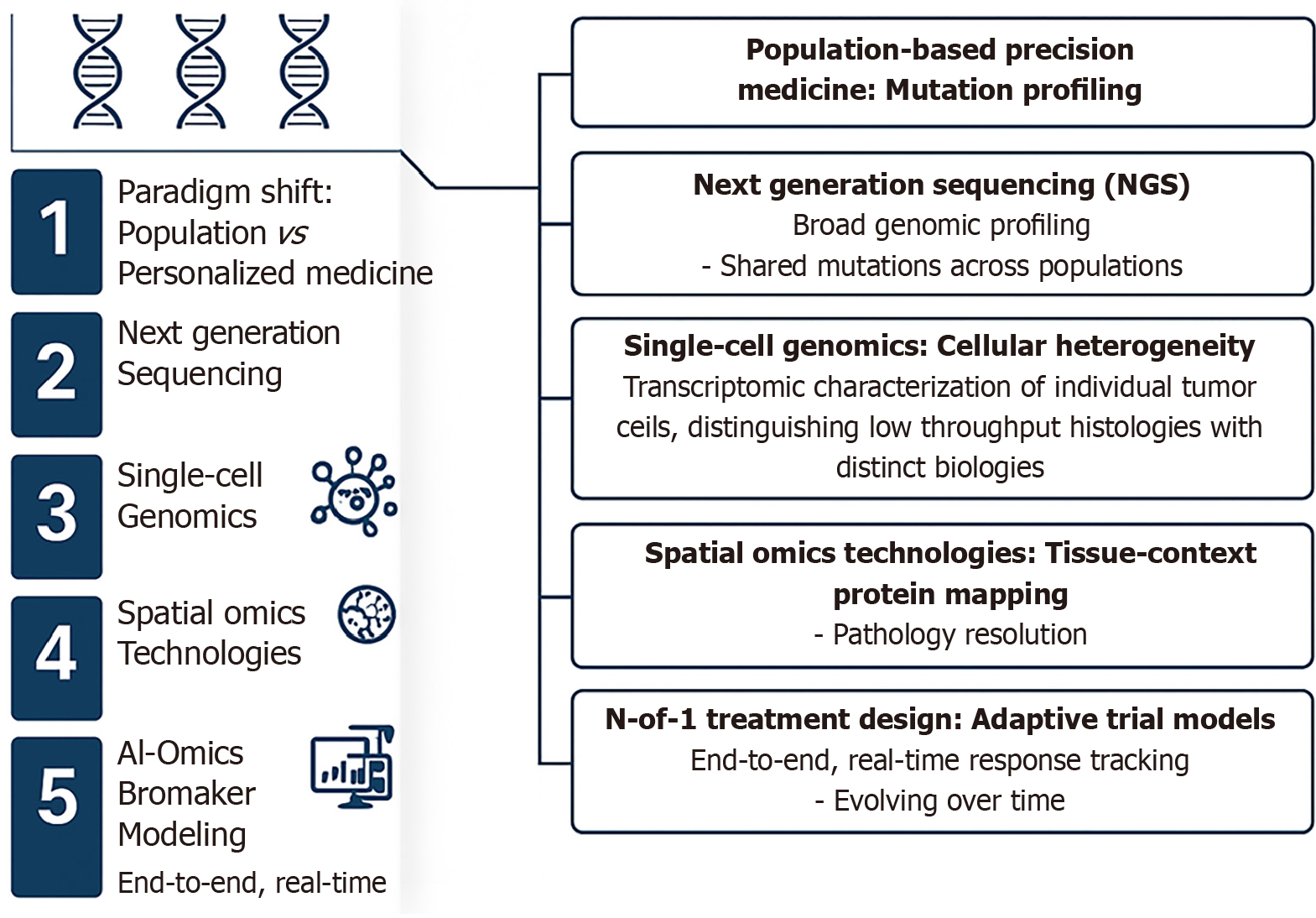
Figure 3 Comprehensive and visually structured overview of the evolution of precision medicine, tracing its development from population-based mutation profiling toward fully personalized, spatiotemporal cancer care.
It begins with the paradigm shift from generalized population-level strategies to personalized medicine, where the integration of individual genomic and lifestyle data takes center stage. The next major advancement depicted is next-generation sequencing, enabling broad genomic profiling and the identification of shared mutations across populations, a foundational step in large-scale precision oncology. This leads into the application of single-cell genomics, which addresses cellular heterogeneity by mapping the transcriptional landscapes of individual tumor cells, uncovering subpopulations with distinct biological characteristics. Further refinement is achieved through spatial omics and proteomics technologies, which provide tissue-contextual protein mapping at high resolution, crucial for pathology insights and tumor microenvironment analysis. The figure then emphasizes the role of artificial intelligence-assisted imaging and biomarker modeling, which supports real-time, end-to-end response tracking and dynamic patient monitoring. As precision deepens, the N-of-1 treatment design is introduced, highlighting adaptive trial models tailored to individual patients through the integration of genomic data and lifestyle factors. The final component in the figure encapsulates spatiotemporal assessment of prognosis and monitoring, where therapy responses, resistance patterns, recurrence risks, and lifestyle influences are evaluated across time to enable personalized lifetime cancer care. This integrated, stepwise visualization underscores the convergence of omics technologies, artificial intelligence, and individualized data in revolutionizing modern cancer diagnostics and therapeutics. AI: Artificial intelligence; NGS: Next-generation sequencing.
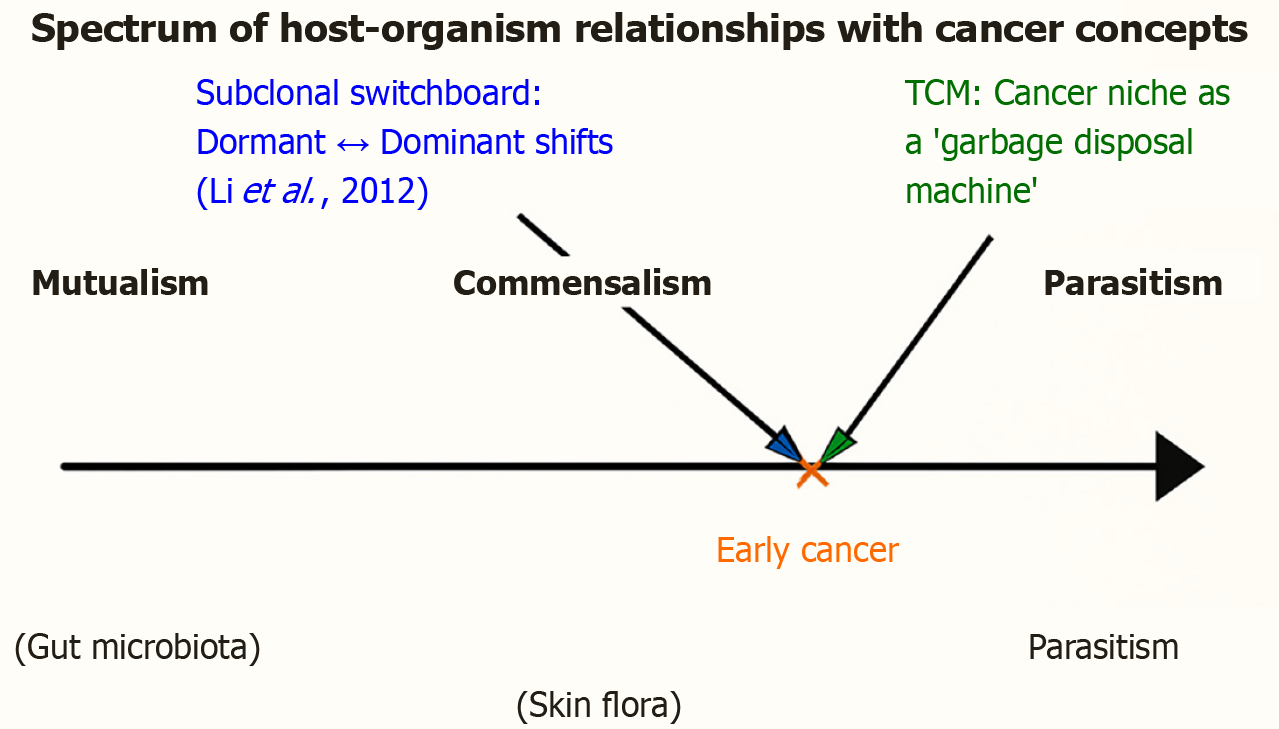
Figure 4 Spectrum of host-organism relationships, applied to cancer.
The continuum of biological interactions ranges from mutualism (benefiting both partners; e.g., gut microbiota), through commensalism (benefiting one partner without harming the other; e.g., skin flora), to parasitism (benefiting one at the expense of the other; e.g., malaria parasite). Early cancer is positioned closer to parasitism but not at the extreme, reflecting its dual role as both a host threat and a potential trainer of immunity. Two conceptual frameworks are overlaid: (1) Cancer niche as a “garbage disposal machine”, Li et al[72], 2019, for subclones, Li et al[73], 2012; green annotation: Highlighting how tumors may buffer metabolic imbalance and function transiently as regulatory sinks within the body-disease continuum, echoing traditional Chinese medicine concepts of balance; and (2) Subclonal switchboard signaling, Li et al[73], 2012; blue annotation: Emphasizing that dominant and dormant cancer subclones dynamically exchange roles, shifting the tumor’s position along the spectrum and driving progression or recurrence. Together, these perspectives reframe cancer not merely as a destructive parasite, but as part of a coevolutionary dialogue with the host, spanning the continuum of mutualism, commensalism, and parasitism.
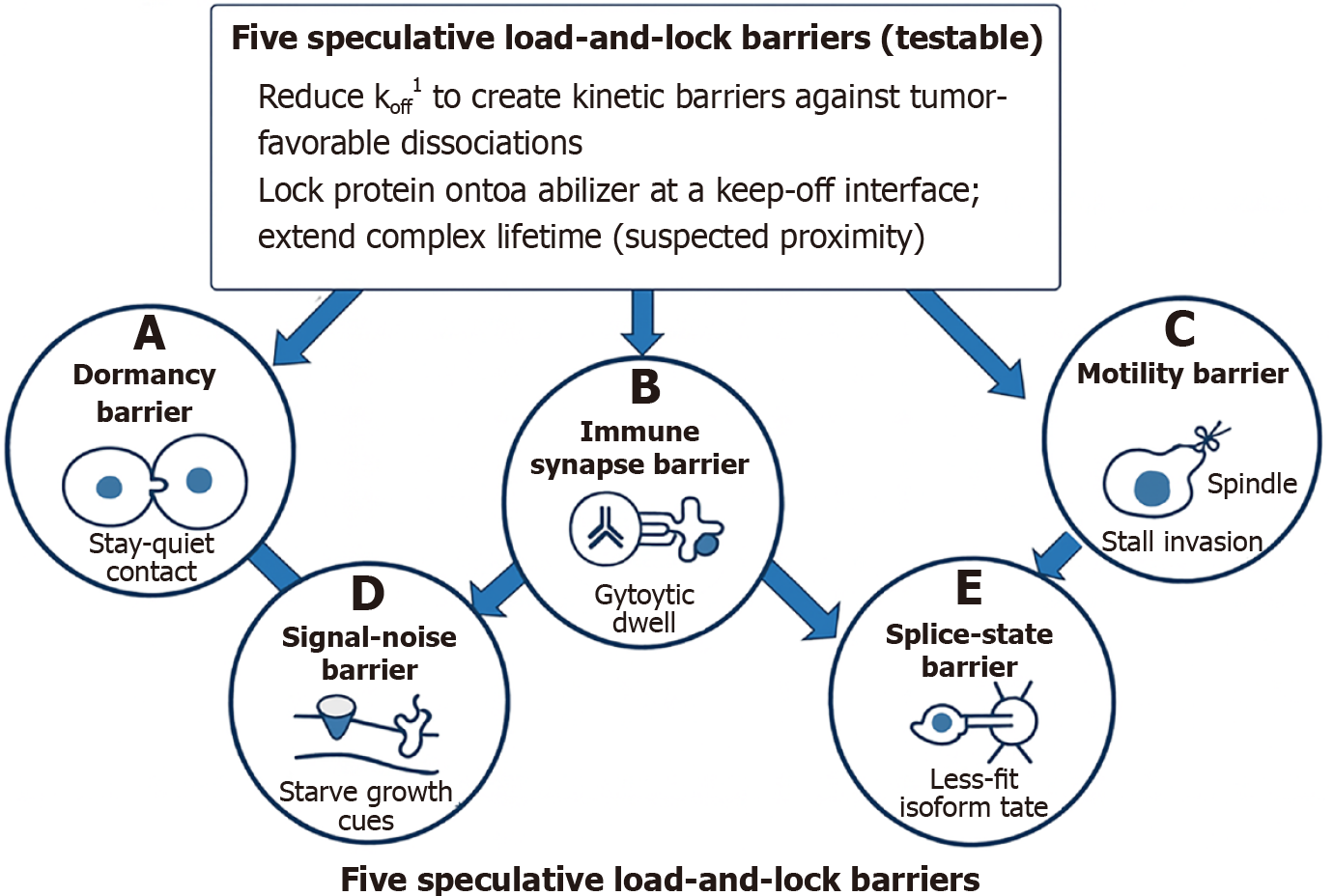
Figure 5 Conceptual diagram of five speculative load-and-lock barriers (testable).
This illustration summarizes a unifying load-and-lock strategy designed to kinetically trap tumor-promoting cellular states by reducing the dissociation rate (koff) of critical molecular interactions. At the center, the strategy emphasizes stabilizing complexes at key interfaces to extend residence time and block rapid transitions. A-E: Radiating from this core are five mechanistically distinct barriers: Dormancy barrier: Locks “stay-quiet” tumor-niche contacts to maintain subclone quiescence and prevent reactivation (A); immune synapse barrier: Locks cytotoxic T cell-tumor synapsed well while blocking immune checkpoint rebinding to enhance killing (B); motility barrier: Locks mitotic motors like KIF18A to stall cell cycle progression and metastatic invasion (C); signal-noise barrier: Locks latent growth inhibitors and decoys ligands to reduce signaling noise and plasticity (D); splice-state barrier: Locks spliceosomal assemblies to bias tumors toward less-fit isoform states (E). Each barrier is represented with a simplified icon and linked to the central load-and-lock concept, illustrating how kinetic stabilization across diverse molecular axes can synergistically constrain tumor evolution. 1koff is the “escape rate” of a molecular handshake. Reducing it means tightening the grip, increasing residence time, and functionally locking tumor biology into less dangerous states. In molecular binding kinetics, koff (the dissociation rate constant) describes how quickly a bound complex falls apart. Mathematically, it has units of per second and represents the probability per unit time that a complex (e.g., protein-ligand, receptor-drug, or synapse contact) will dissociate back into its separate components. A lower koff = slower unbinding - the interaction is more stable, with longer residence time (the duration the complex stays intact). This is distinct from kon (association rate constant), which describes how quickly binding occurs. The load-and-lock strategy is essentially about slowing koff across different molecular systems. By making complexes last longer (kinetically trapping them), you extend functional effects: (1) Dormancy niches hold tumor cells in quiescence; (2) T cell-tumor synapses persist longer, boosting killing; (3) Motor proteins like KIF18A remain stuck, blocking mitosis; (4) Growth inhibitors stay bound, muting noise; and (5) Spliceosomes stay locked in certain states, biasing isoform output.
- Citation: Lee HM, Li SC. Rethinking p16, p53, and HPV in HNCSCC through lessons from glioblastoma subclonal evolution toward patient-centric N-of-1 single-cell RNA sequencing paradigm. World J Clin Cases 2025; 13(32): 104208
- URL: https://www.wjgnet.com/2307-8960/full/v13/i32/104208.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i32.104208