Published online Sep 25, 2025. doi: 10.5501/wjv.v14.i3.107905
Revised: May 9, 2025
Accepted: August 4, 2025
Published online: September 25, 2025
Processing time: 178 Days and 18.1 Hours
A knowledge of the epidemiology and clinical aspects of non-hepatotropic viruses is becoming increasingly important in lieu of the rising incidence of acute liver injury caused by them in various circumstances. Broadly, they include the Herpesviridae group, the hemorrhagic fever viruses and certain respiratory viruses that infect the liver. They can affect both the immunocompetent and the immunocompromised individual, more commonly the latter as part of disseminated systemic infection with symptoms ranging from self-limited transaminitis to acute liver failure Various reasons for their rising importance are increased exposure to these viruses by way of: (1) Overcrowding, climatic and environmental changes, in
Core Tip: Liver infection by non-hepatotropic viruses is increasingly being encountered in the setting of emerging and re-emerging viral diseases the world over and the increasing use of immune altering medications in situations like organ transplant, immune mediated diseases and cancers, especially in the aging world population living with many comorbidities. Symptomatology can range from self-limited transaminitis in the immunocompetent to disseminated disease with acute liver failure in the immunocompromised. They are seldom detected as most infections have non-specific presentation and are self-limited. Early diagnosis can prevent complications. This review summarizes their epidemiology, clinical manifestations and management.
- Citation: Ray G. Non hepatotropic virus induced hepatitis - rising importance in a changing world. World J Virol 2025; 14(3): 107905
- URL: https://www.wjgnet.com/2220-3249/full/v14/i3/107905.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i3.107905
Acute liver injury [ALI, including acute hepatitis and acute liver failure (ALF)] can be caused by multiple factors, hepatotropic viral infections (A-E) and drug/toxin being the two commonest. Other etiologies include autoimmune, biliary, metabolic, vascular (acute Budd-Chiari syndrome, veno-occlusive disease), other infectious causes, pregnancy [acute fatty liver of pregnancy and the hemolysis, elevated liver enzyme, low platelet (HELLP) syndrome], heatstroke, liver hypoxia-ischemia and malignant infiltration. However, in spite of detailed investigations, the etiology remains unidentified in significant number of cases. The incidence of ALI due to unknown cause vary considerably in different geographical regions mostly determined by local prevalence of viral etiology, drug/toxin exposure, environmental conditions and extent of investigations done. In older series it varied from 17%-44% in different parts of the world[1]. In more recent series from the western world (which include extensively investigated cases), it still varies from 5.5%-20% in adults[2-4], and 30%-50% in children[5]. The latest Asia Pacific Association for the study of Liver data projects the incidence of ALI and acute-on-chronic liver failure (an important way of initial presentation of ALI in the Asia Pacific) of unknown etiology as 5%-15% in the Asia Pacific[6]. In one Indian study it was found to be 10.1% [caused by cytomegalovirus (CMV) and Epstein Barr virus (EBV)] in adult patients, being much more common in ALI (9.9%) than in acute-on-chronic liver failure (0.01%) group[7]. Among cases of ALI needing liver transplant, the etiology is indeterminate in 4.5% in the European region[1]. Though the etiology in some is likely to be the uncommon ones mentioned above, a significant number may be caused by non-hepatotropic viruses which remain under-studied and under-reported due to their non-specific presentation, relative rarity, and self-limited clinical course and hence remain uninvestigated for. A list of the most common non hepatotropic viruses causing ALI is shown in Table 1.
| Characteristics | Description |
| Herpes virus group | |
| Type 1, 2 | Herpes simplex virus |
| Type 3 | Varicella zoster virus |
| Type 4 | Epstein Barr virus |
| Type 5 | Cytomegalovirus |
| Type 6 | Human herpes virus |
| Emerging and re-emerging viruses | |
| Hemorrhagic fever viruses | |
| Flavivirus | Dengue virus subtypes 1-4, Yellow fever virus |
| Buniyaviridae | Crimean Congo haemorrhagic fever virus, Rift Valley fever virus, Lassa fever virus, Hantavirus |
| Filovirus | Ebola virus |
| Coronavirus | SARS-COV-2, SARS-COV-1, MERS COV |
| Orthomyxovirus | Influenza virus type A and B |
| Viruses predominantly affecting pediatric population but also immunocompromised adult host | Adenovirus type 41, Adeno-associated virus type 2, Parvovirus B19, Paramyxovirus (measles), Togavirus (rubella), Enterovirus (Coxsackie type A4, A9, B5 and echovirus), Norovirus |
Many of the above mentioned viruses (e.g. exotic ones causing hemorrhagic fevers) are emerging or re-emerging in different parts of the world at different times causing high mortality. Possible reasons are: (1) Climatic changes like global warming; (2) Overpopulation, crowding and poor vector control; (3) Rising global tourism especially involving long-distance travel to hitherto unexplored, inaccessible areas with exposure to virgin indigenous flora and fauna helping the spread of local pathogens and vectors to other regions, as also the colonisation of endemic areas by unvaccinated migrants; (4) Increasing contact with wild animals resulting from deforestation, keeping exotic pets, consumption of exotic meat, spreading zoonosis; and (5) Increasing global trade in animal food products. In addition, there is the risk of these agents being used for bioterrorism in the modern times.
In the last two decades, a number of major viral epidemics or pandemics have affected human populations, caused by viruses which have the potential to cause hepatitis like the filovirus, coronavirus, flavivirus, alphavirus, norovirus and myxovirus family. In addition, sporadic outbreaks have been caused by zoonotic RNA viruses like bunyaviruses and arenaviruses. In parallel, zoonotic viruses frequently spill into livestock and other animals, which can serve as reservoir hosts for further spillover into humans. Mosquito-borne flaviviruses (that cause dengue, West Nile fever, yellow fever and Rift Valley fever) are novel or re-emerging pathogens defined as category A/B pathogens by the National Institutes of Health. For the dengue virus (DENV), novel strains have caused major epidemics every few years since the early 2000 (2002, 2010, 2014, and 2020). In 2016, the yellow fever virus re-emerged in some west African countries, spreading to China through travel, before being controlled by mass vaccination. Influenza viruses continue to re-emerge after the 1918 H1N1 flu pandemic which killed nearly 50 million people globally e.g. 1957 (H2N2 Asian flu), 1968 (H3N2 Hong Kong flu), 2009 (H1N1 swine flu pandemic) which caused about 250000 deaths globally. In addition, there had been repeated sporadic outbreaks of avian H5N1, H7N9 and other influenza strains, with high mortality rates in humans. Two epidemics of the Ebola virus (defined as category A pathogen by the National Institute of Health) in Africa from 2013-2016 and from 2018-2020 had mortality rates of 40% and 65% respectively. The order Bunyavirales contains multiple viruses (causing hemorrhagic fever) that have caused outbreaks or have outbreak potential, and are listed as category A pathogens by the National Institute of Health owing to their high mortality rates (10%-40%). Among the coronaviruses, severe acute respiratory syndrome coronavirus (SARS-CoV) 1 had fatality rate of about 10%, the Middle East respiratory syndrome coronavirus (MERS-CoV) had fatality rates of 35% (in spite of fewer total cases) and finally, SARS-CoV-2 caused the current coronavirus disease 2019 (COVID-19) pandemic. All of these coronaviruses were traced back to bat origin, some using intermediate hosts such as camels and civet cats. Noro virus is the leading cause of gastrointestinal (GI) infections, triggering multiple epidemics in the last two decades leading to severe disease in children, the elderly and immunocompromised individuals and a projected 70000-200000 deaths per year globally.
With the increasing rate of liver transplantation globally, especially for the indication of acute hepatitis, it has become even more relevant to identify non hepatotropic viral infection as the primary hepatic insult. In general the increasing prevalence of chronic diseases worldwide (due to the rising geriatric population) that drive the need for organ transplants along with the technological advancements that enable transplants lead to their increasing demand. The immunosuppression needed post-transplant is an established cause of reactivation of as well as new infection by a number of viruses that could then indirectly cause hepatic injury (e.g. herpesviruses and others affecting the immunocompromised infants and adults)[9]. In addition, many of these viruses can be transmitted through transplanted organs. The anticipated magnitude of such problem can be gauged from the following. The latest 2022 data from the Global Observatory on Donation and Transplantation indicate that more than 150000 solid organ transplants (≤ 10% of global needs) and about 84000 hematopoietic stem cell transplants are performed annually[10,11]. The global organ transplant immunosuppressant drugs market size valued at 5.51 billion dollars in 2024 is projected to grow at a compound annual growth rate (CAGR) of 4.7% from 2025 to 2030[12]. In addition, the rising incidence of autoimmune disorders, non-autoimmune inflammatory diseases and different cancers furthers the use of immunomodulatory therapies (corticosteroids, mo
Non hepatotropic viral hepatitis can occur as an isolated illness but more commonly occurs as part of a systemic illness and its clinical manifestations may range from fever, chills, upper respiratory symptoms, abdominal pain, diarrhoea, myalgia, mild jaundice and transaminitis which are self-limiting. Uncommonly a disseminated disease can lead to ALF. Co-infection with some hepatotropic viruses may further complicate or delay diagnosis[16].
General mechanisms of liver injury in non-hepatotropic virus infections are: (1) Direct hepatocellular damage: Some non-hepatotropic viruses, such as CMV and herpes simplex virus (HSV), can infect hepatocytes directly, replicating within it or inducing a cytolysis. This can cause liver inflammation, necrosis, and even ALF in extreme cases; (2) Immune-mediated liver injury: Many non-hepatotropic viruses trigger immune responses that either damage liver tissue (e.g., an exaggerated T-cell response during EBV infection) or cause systemic inflammation (as in CMV or HSV infection) which adds to the liver dysfunction; (3) Cytokine storm: Viral infections can trigger the sudden release of large number of pro-inflammatory cytokines leading to systemic inflammation, including hepatic injury e.g. the influenza and COVID 19 virus; and (4) Co-infections and secondary effects: Co-infections with hepatotropic and non-hepatotropic viruses (e.g., EBV and hepatitis B) or viral reactivation in immunocompromised individuals (e.g., CMV) may potentiate liver damage.
CMV spreads mainly through body fluids (saliva, urine, blood, sexual fluids) and organ transplants. It can establish latency in tissues like epithelium, smooth muscle, and fibroblasts, with reactivation occurring in immunocompromised state. The infection is usually asymptomatic in immunocompetent individuals but may be severe in immunocompromised state with severe cholestatic hepatitis, granulomatous hepatitis, and acute fulminant hepatic failure[19]. In primary infection, the virus spreads to the liver sinusoids hematogenously, then disseminating to the hepatocytes and biliary duct cells causing direct as also cytokine mediated injury[20]. Solid organ transplantation, especially liver transplant, is another independent risk factor where it can mimic acute cellular rejection[9]. It commonly occurs in the first three months post transplantation with an incidence of 2.1%-29%, the risk being highest when the donor is CMV positive and the recipient is negative[21]. It is also observed in patients following COVID-19 infection and the DRESS syndrome[22,23]. It may present as a viral infection (fever, cytopenias) or as tissue invasive disease. The elevation of transaminase level differs among immunocompetent and immunosuppressed hosts. It is up to 10-fold in immunocompetent individuals due to the robust immune response with modest rise in bilirubin (up to 5-9 mg/dL), whereas it is up to two fold in the immunosuppressed but with high rise of alkaline phosphatase (1-10 fold) and gamma-glutamyl trans
Diagnosis is done based on a combination of serologic, molecular, culture-based methods, and histology tailored to the clinical context and patient population. CMV specific IgM is elevated within 3-4 weeks of infection and can last for six weeks or longer, hence it can be positive both in new infection or reactivation[16]. The differentiation (important for treatment and risk of complications) is done by the IgG avidity test where low IgG avidity indicates a recent primary infection (needing more aggressive therapy and closer monitoring), while high avidity suggested a past infection or reactivation[25]. Quantitative assay of CMV DNA by polymerase chain reaction (PCR) from the whole blood is more sensitive and predicts the risk and severity of infection but it may be absent in tissue invasive disease. The affected cells show typical nuclear and cytoplasmic inclusions (owl’s eye) in the absence of which immunohistochemical staining (for pp65 antigen) can be used. Mild cases are self-limiting, and needs only supportive treatment. Tissue invasive severe cases need intravenous ganciclovir 5 mg/kg twice a day or oral valganciclovir 900 mg twice a day for three weeks. Since the drug acts on DNA, myelosuppression, hepatotoxicity, and teratogenesis can occur so its use should initially be for 2-3 weeks. Treatment response should be monitored by measuring CMV DNA PCR weekly and discontinued when the virus is shown to be eradicated by repeat testing on one or two occasions[26]. Foscarnet and leflunomide may be considered in case of drug resistance (failure to lower CMV level even after 6 weeks)[27].
EBV infects the B lymphocytes (and is implicated in their immortalization and hyper-replication as in Burkitt’s lymphoma, MALToma of stomach, etc.). It spreads through saliva, genital secretions, organ transplants, and blood transfusions[28,29]. It infects over 90% of the world’s population, especially children and adolescents, presenting as infectious mononucleosis with fever, sweats, myalgias, with or without lymphadenopathy and organomegaly. Hepatic involvement occurs in 90%, majority being self-limited or subclinical. The transaminase elevation is 2-3 folds in majority but jaundice is seen in < 5% cases[30], with a cholestatic biochemical picture in about 65%[31] particularly in individuals > 40 years old[32]. Rarely liver involvement can manifest as isolated chronic hepatitis (mimicking autoimmune hepatitis, granulomatous hepatitis or vanishing bile duct syndrome). In the rare patients who progress to ALF, high transaminases > 1000 IU/L have been reported and in the United States its incidence was 0.21% in liver transplantation recipients with a high mortality[33]. Due to the B cell proliferation, it causes 60%-85% of post-transplant lymphoproliferative disorders with some involving the liver allograft[34]. In severe cases, it involves lymph nodes, GI tract, central nervous system, liver, lung, tonsils, and salivary gland needing multimodality treatment with surgery, chemotherapy and radiotherapy, whereas milder variety may improve with reduction of immunosuppressive drug dosage. It should be investigated for in cases of unexplained hepatitis in immunosuppressed individuals[35].
Liver injury is mostly immune mediated and diagnosed by serology and confirmatory PCR testing. The primary serological markers used are viral capsid antigen (VCA) IgM and IgG, Epstein-Barr Nuclear Antigen IgG, and heterophile antibodies. VCA IgM antibodies typically indicates an acute or recent primary infection, but it lacks specificity due to cross reactivity with other viruses and non-related antigens. VCA and Epstein-Barr Nuclear Antigen IgG antibodies indicate past infection[36]. Heterophile antibodies (detected by the monospot test) usually become detectable between
Both HSV-1 and HSV-2 can cause florid hepatitis in all age groups, but more than 60% of the cases have been attributed to HSV-2. It is a rare and aggressive form of acute hepatitis that can rapidly progress to ALF without significant grade of encephalopathy. It accounts for about 0.7% and 1.4% of all ALF cases and 8.7% and 4.7% of the viral causes of ALF in the American and French series, respectively[18,41,42]. HSV hepatitis may occur as a primary infection or secondary to reactivation brought on by “stressors” such as immunocompromised state, malignancy, pregnancy, or inhalation ane
Primary Varicella Zoster virus (VZV) infection typically causes vesicular rash (chickenpox) mostly in children while reactivation (from the dormant state in the dorsal root ganglia) causes zoster (shingles) in adults, especially when immune compromised. Liver involvement ranges from mild hepatitis to ALF (rarely), especially in immunocompromised state [with or without additional involvement of lungs (interstitial pneumonitis), heart (myocarditis), pancreas (pancreatitis), and brain (meningoencephalitis)]. Elevation in liver enzymes is reported in up to 3.4% of the children with chickenpox and but in adult transplant recipients VZV infection can occur without cutaneous lesions. A modest 2-5 folds increase in transaminase is usual but in fulminant hepatic failure, the enzymes may be > 10 folds above normal[47-49].
Diagnosis is confirmed through histological examination (inclusion bodies), immunohistochemistry, and molecular techniques such as VZV PCR which is very sensitive for establishing the diagnosis from skin lesions. IgM tests have poor specificity (as specific IgM antibodies are transiently produced) and cannot discriminate between a primary infection and reinfection or reactivation. Prompt treatment with intravenous acyclovir is recommended for immunocompromised patients as also varicella zoster immunoglobulin[50,51]. For immunocompetent patients oral valacyclovir 1000 mg three times a day for seven days or acyclovir 800 mg five times a day for 7-10 days is recommended[48,52]. Varicella zoster immunoglobulin prophylaxis is recommended for susceptible seronegative patients following virus exposure. An inactivated vaccine (Shingrix) is administered especially in pre-liver transplant setting with questionable efficacy in such setting.
Human herpes virus-6 (human B cell-lymphotropic virus) variant B is mostly responsible for symptomatic infections and most primary infection occurs within the first 3 years of life (rubeola). It may get reactivated in post transplant setting due to immunosuppression causing fever, rash, cytopenia, interstitial pneumonitis, and hepatitis. PCR is the best diagnostic method. Antiviral agents include those active against CMV, including ganciclovir, foscarnet, and cidofovir[53].
Majority of infections caused by these viruses are either subclinical or produce mild flu like symptoms (fever, malaise, body aches, nausea and bowel disturbance) which are self-limiting. Liver is affected as part of disseminated systemic disease with multiorgan involvement (kidney, brain, lungs) in a minority of patients. Transaminases are elevated 5-10 folds (or even more in dengue), which correlates with severity, but with relatively less elevation of bilirubin (except yellow fever). Platelet counts are low and the prothrombin time is deranged with/without disseminated intravascular coagulation. There is direct cytopathic effect of the virus and also the effect of the inflammatory cytokines produced by the infection on hepatocyte and vascular endothelial cells which result in liver failure and hemorrhage. Hepatic involvement is most extensive in yellow fever, dengue hemorrhagic fever, Crimean Congo hemorrhagic fever and Rift Valley fever. The epidemiological features of these viruses are summarized in Table 2 and other clinical features of the most widely prevalent of these i.e. the DENV are discussed below.
| Virus name | Geographic areas, incubation period | Vector, host and transmission | Diagnosis, treatment, case fatality rate (in severe cases) | Prevention |
| Crimean Congo hemorrhagic fever | Africa, Asia and southern Europe (Balkan region) | Ixodid tick of Hyalomma spp | RT-PCR | Avoid tick bite; no vaccine available |
| Cattle and other mammals | Ribavirin, methyl prednisolone | |||
| 1-5 days following a tick bite or 5-7 days following contact with infected blood | Humans acquire disease as accidental host by direct tick bite or contact with infected blood | 5%-40% | ||
| Ebola fever | Mostly central and West Africa | Fruit bats reservoir | RT-PCR or ELISA for Ab | 2 vaccines: (1) Ervebo (rVSV-ZEBOVGP), a live-attenuated vaccine, has very high efficacy after a single shot (up to 97.5%); and (2) One shot of Zabdeno (Ad26. ZEBOV-GP) followed by a shot of Mvabea (MVA-BN-Filo) 8 weeks later requires more time to induce protection (so not suitable for immediate effect in an outbreak) but protects over a longer period |
| 2-21 (6-10) days | Transmitted by direct contact or via bodily fluids of vector host (primates including humans) | Inmazeb (REGN-EB3), a mixture of 3 monoclonal Ab (atoltivimab/ maftivimab/odesivimab) given: 50 mg/kg in a single IV infusion, and Ebanga (Ansuvimab-zykl), a human monoclonal Ab 50 mg/kg administered within 1 hour | ||
| 50% (30% with treatment) | ||||
| Lassa fever | West Africa | Rodents (multimammate rat of Mastomys spp) | RT-PCR. ELISA for IgM and IgG Ab | Avoid exposure to rhodents |
| 1-3 weeks | Inhalation of virus containing aerosolised rat excreta (urine, feces) or consuming contaminated foods or by direct contact with abraded skin. Human-to-human transmission may occur through direct contact with blood or bodily secretions from infected persons | Supportive and start ribavirin as early as possible: 15 mg/kg/day × 10 days | No vaccine available | |
| 15%-20% (among 20% severe cases) | ||||
| Hanta fever | Liver involvement only in HFRS (caused by Seoul type virus) found in Europe and Asia | Rodents | ELISA for Ab or immunofluorescence assay | Avoid contact with rhodents |
| 2-4 weeks | Virus containing aerosols in rodent excreta | Early ribavirin | No vaccine available | |
| Rift Valley fever | Africa and the Arabian Peninsula | Arthropod borne, predominantly mosquitoes (Aedes and Culex species) from natural host i.e. livestock | RT-PCR, ELISA | Vaccinating livestock. Early detection in them and avoid contact |
| 2-6 days | Human transmission is by contact with animals | Ribavirin, favipiravir show in vitro effect only | No human vaccine available | |
| 50% (among 5% severe cases) | ||||
| Dengue fever | (Sub) tropical areas of America, Africa, Middle East, Asia and Pacific islands | Aedes aegypti, Aedes albopictus mosquitoes | RT-PCR, ELISA for IgM and IgG Ab, NS1 antigen | Mosquito repellent. Two WHO approved vaccines: (1) Dengvaxia (3 doses six months apart) for people aged 6-60 years but to be given only in laboratory-confirmed previous dengue virus infection; and (2) Qdenga (2 doses three months apart) in children aged 6-16 (upto 60 years for those with comorbidities) in high dengue transmission settings |
| 4-7 days | Mammals (mostly humans) | Supportive | ||
| 5% severe, acute liver failure in 0.5% | ||||
| Yellow fever | (Sub) tropical Africa and South America | Sylvatic (forest) cycle: Africa (Aedes africanus), South America (Haemagogus and Sabethes species and urban cycle: Aedes aegypti (both Africa and South America). Primates | IgM Ab by ELISA | Mosquito repellent |
| 3-6 days | Supportive, ribavirin, sofosbuvir has been tried | Live attenuated vaccine given to 9 months or older individuals | ||
| 20%-60% (among 15% severe cases) | Other live vaccines are in phase III-IV trial |
50% of the world population is at risk of DENV infection and each year 100-400 million cases occur of which about 70% is in Asia[55,56]. All 4 serotypes are associated with hepatitis and ALF. The symptom range varies from asymptomatic infection to mild fever with body ache (80%) to life-threatening dengue hemorrhagic fever and dengue shock syndrome (5%). Severe infections are associated with secondary infections by heterologous serotypes. Reinfections are possible as there is no cross immunity.
The liver is commonly involved in DENV infection and manifest with no or minimal elevation of transaminases to moderate elevation > 10 folds (4-15% cases)[57]. Severe injury caused massive rise in transaminases > 1000 (rarely > 20000) IU/L and rarely progress to ALF with relatively mild hyperbilirubinemia (0.3%-0.7% cases)[58,59]. Such level of transaminase elevation along with hepatomegaly and ascites are listed as warning signs of severe dengue infection by the World Health Organization (WHO)[60]. Usually, AST is more than ALT levels.
ALF presents with catastrophic suddenness in the afebrile or defervescence (recovery) phase of the disease. In a large Indian series spanning from 2014 to 2017, 36 of 10108 patients with DENV infection (0.35%) exhibited features of ALF (72% having features of hyperacute liver failure)[57]. Another study from Thailand reported 6/1926 (0.3%) ALF among dengue cases[61].
However, the degree of transaminase elevation, though correlates with disease severity, does not distinguish between survivors and non survivors[58], and so also the degree of bilirubinemia and the presence of coagulopathy despite their levels being higher in non-survivors. The predictors of mortality were other features of systemic involvement like admi
Pathogenesis of liver injury involves various combinations of direct cytopathic effects, host immune response affecting the hepatocytes, apoptosis, micro vesicular or macrovesicular steatosis (via disruption of mitochondrial machinery), circulatory compromise (causing hypoxic or ischemic hepatitis), intrahepatic sinusoidal microcirculatory dysfunction, localized vascular leakage and rhabdomyolysis (causing more AST elevation). A second bout of infection which elicit a specific range of antibody may cause severe illness and liver injury by a mechanism known as antibody-dependent enhancement of disease[65].
DENV infection in children appears to cause more severe disease than adults resulting from hemophagocytic lymphohistiocytosis which presents with prolonged fever, worsening cytopenia, hepatosplenomegaly and confirmed by a bone marrow aspiration/biopsy.
The diagnosis is established by the NS1 antigen test and detection of IgM antibody against DENV. Treatment is largely supportive. Patients with severe disease need treatment in advanced facilities through maintaining hydration, blood pressure, and supporting organ failure when it happens which is the key to reducing morbidity and mortality. Although used, the role of N-acetyl cysteine in patients with ALF is controversial and not evidence based.
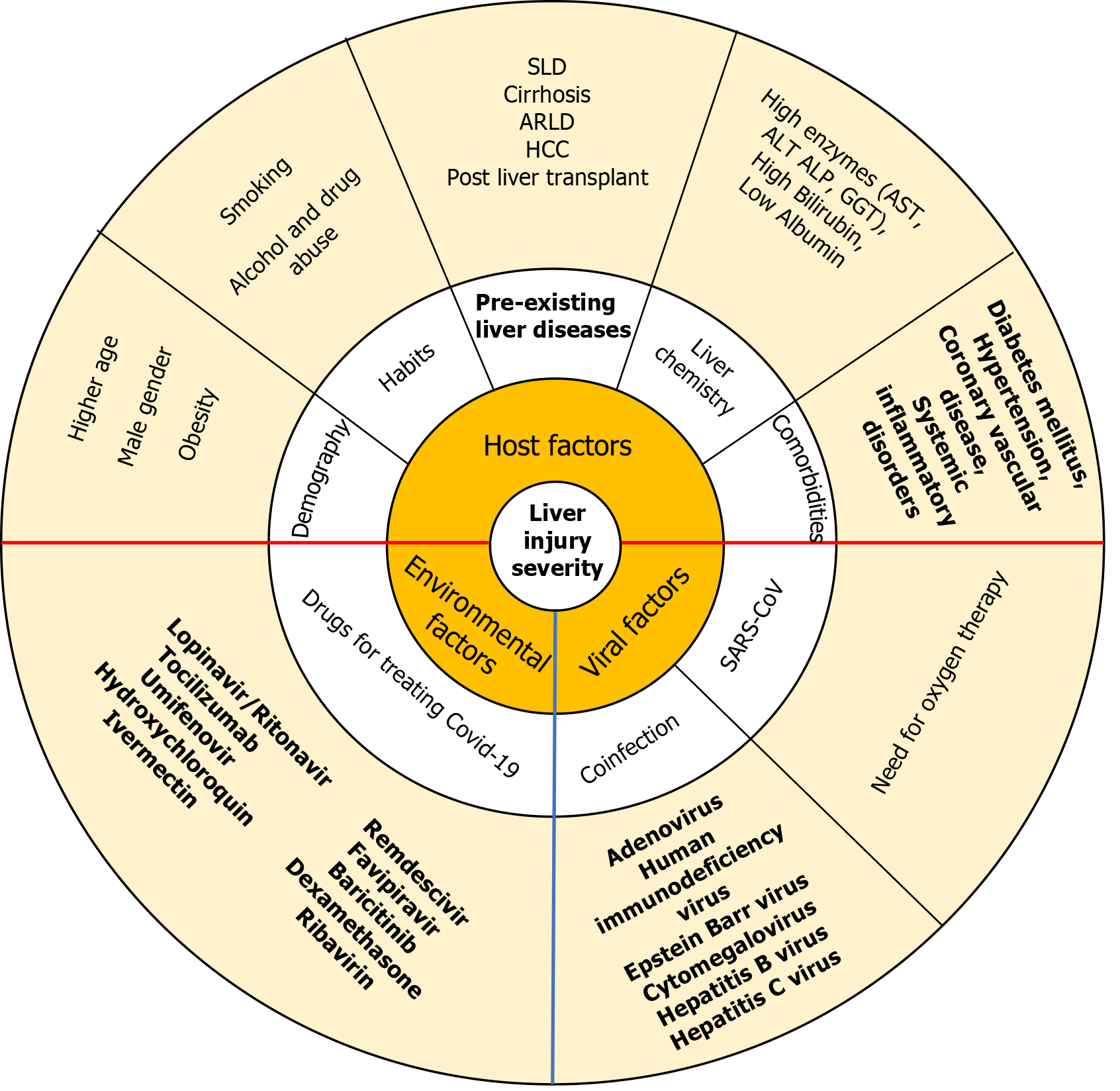
Liver involvement in SARS-CoV-1 infection was 60%, MERS CoV was 60%, and SARS-CoV-2 infection (which caused COVID-19) is 14.8%-53%, more in severe disease and in those with digestive symptoms, as diagnosed based on liver enzyme elevation and decrease in albumin level[66,67]. In COVID-19, hepatic injury is defined by elevation in ALT or AST > 3 folds, alkaline phosphatase or gamma-glutamyl transferase > 2 folds, or total bilirubin[66,68]. The incidence of liver injury in severe COVID-19 patients (74.4%) was higher than in mild disease (43%) and that in COVID-19-associated deaths was 58%. Pathological findings were non-specific including steatosis, prominent mitosis, acidophilic bodies, and mild to moderate portal tract and lobular lymphocytic inflammation. COVID-19-associated liver symptoms are generally mild and self-limiting and treatment is supportive. Factors involved in hepatic injury include direct viral cytopathic effects, exaggerated immune responses/systemic inflammatory response syndrome, hypoxia-induced changes, vascular changes and endothelitis due to coagulopathy and microcirculatory dysfunction, hepatic congestion from right heart failure, gut dysbiosis with disruption of gut–mucosal barrier and drug-induced liver injury[67,69]. Other contributory factors are shown in Figure 1. The management is mostly supportive in majority of patients without pre-existing liver disease (with possible avoidance or use of hepatotoxic drugs in lower dose). However, special emphasis is needed in patients with steatotic liver disease, alcohol related liver disease, hepatitis B and C infections, cirrhosis, hepatocellular carcinoma, and in liver transplant recipients. Treatment is continued for hepatitis B and C, autoimmune hepatitis, post liver transplant, and hepatocellular carcinoma. Steatotic liver disease (with associated obesity, diabetes mellitus, alcohol use) have higher risk of severe COVID-19 and higher liver involvement. Cirrhosis patients have higher mortality than non cirrhotics, and so also those with higher Child-Turcotte-Pugh grade[66].
Degree of liver dysfunction (higher ALT/AST and lower albumin) are directly related to severity and mortality in patients with COVID-19. Those with severe liver injury (ALT > 5 folds elevated) had more severe clinical outcomes, including higher intensive care unit (ICU) admission rates (69% vs 42% vs 16%), intubation (65% vs 38% vs 13%), renal replacement therapy (33% vs 15% vs 7.5%), and in hospital mortality (42% vs 23% vs 21%) as compared to those with lesser degree or no elevation of transaminases respectively. Among patients with severe liver injury, 70% required vasopressors, 39% were paralyzed, 12% received inotropes, 10% needed proning, and 2.8% required extracorporeal membrane oxygenation[70].
An international registry found higher mortality in cirrhotic patients (32%) compared to non cirrhotics (8%) with a strong correlation between the stage of liver disease and the rate of ICU admissions, renal replacement therapy and death, though respiratory symptoms were similar among the groups at admission. Majority (71%) of deaths were respiratory cause related but 19% had liver related cause. GI side effects were comparatively higher in cirrhotics. Furthermore, baseline liver disease stage and alcohol-related liver disease were risk factors for death[71].
While influenza primarily affects the respiratory system, it has been linked to liver dysfunction and hepatitis in some instances, especially during severe infection or in immunocompromised individuals. In the 2004 H5N1 influenza (bird flu) outbreak, about 60% of patients with pneumonia had deranged liver function tests with GI symptoms such as vomiting, abdominal pain and diarrhoea on initial presentation[72]. A global pooled analysis showed higher odds of hospitalisation, ICU admission and mortality in influenza affected chronic liver disease patients in the 2009 HINI pandemic[73]. Similarly a multicenter study from 2013 to 2014 reported a two-fold increased risk of hospitalization, specifically due to influenza virus infection in liver disease patients[74]. Influenza causes hepatic decompensation and high mortality in cirrhosis patients[75-77].
In the waning period of the COVID-19 pandemic in 2022, a sudden surge of acute hepatitis of unknown etiology in children surfaced in the United Kingdom[78] following which the WHO issued the Multicountry Disease Outbreak News on Acute hepatitis of unknown aetiology on 23 April 2022. Since then, such affliction among young children have been reported from all over the world. As of 22 June 2022, 920 probable cases fitting the WHO case definition [any child up to 16 years of age presenting with non A-E hepatitis with serum transaminases > 500 IU/L (AST or ALT)] have been reported from 33 countries in five WHO Regions. The majority (n = 460; 50%) are from the WHO European Region (22 countries), among whom the majority (70%) are from the Great Britain and Northern Ireland alone. Others are from the Region of the Americas (n = 383, 39.1%, 50% from United States), Western Pacific Region (n = 61), the South-East Asia Region (n = 14) and Eastern Mediterranean Region (n = 2). Of the etiologies identified, 327 (35.5%) were caused by adenovirus, 44 (4.8%) by adenovirus type 41 and 64 (7%) by SARS-COV-2 + adenovirus[79]. Some had co-infections with CMV, EBV and enterovirus also[80]. While most patients recovered uneventfully with conservative management, 45 children required liver transplantation[81], but case reports of using corticosteroids and intravenous cidofovir as treatment are also available[82].
In a Romanian study of adenoviruses in children mostly from the prepandemic period, overall viral co-infection was present in 22.9% (mostly rotavirus and norovirus but also EBV, CMV, SARS-COV-2, enterovirus, influenza, measles and respiratory syncytial virus). 21.5% had altered liver function test of which 75% had increased AST only (6 had > 500 IU/L, all with 3 coinfecting viruses). Coinfection rate was similar among those with (34.1%) and without (30.8%) transaminitis. This not only shows that adenovirus itself can cause hepatitis but coinfection with other non-hepatotropic viruses can often occur in such patients[83].
It is postulated that adenovirus acts as a trigger in presence of a cofactor like adeno associated virus (AAV) type 2 (AAV2, which needs the adenovirus to complete its lytic replication cycle) or human herpesvirus type 6[17,84] leading to liver injury by immunological mechanisms, This is supported by the presence of higher concentrations of AAV2 in liver and body fluids of patients than in controls, the absence of adenovirus in explant livers and increased incidence of AAV2 in patients post COVID-19[85,86]. In a recent report from Egypt, adenovirus etiology has also been identified in adults with acute hepatitis due to unknown cause, some with coinfection with hepatotropic viruses[87]. In addition to severe hepatitis, adenoviruses can cause a variety of other clinical syndromes especially in immunocompromised individuals, as it can establish latency in human tissue over prolonged periods. Its transmission with the donated organ is a risk factor for hepatitis in pediatric liver transplantation[88].
The following viruses predominantly affects children, most being mildly symptomatic and self contained. Rarely they can lead to hepatitis and ALF in the setting of multisystem involvement[89]. They can potentially affect adults also, especially if immunocompromised.
Coxsackievirus A (type 4, 9) and B (type 5) and echovirus can cause viral hepatitis in severe cases, often seen in children and is a relevant cause of ALF in neonates as part of multisystem involvement. Patients generally have a good prognosis with mild symptoms with rare instances of severe jaundice or elevated ALT, AST level[90].
Hepatitis typically occurs with systemic infection in pediatric patients. Histologic findings include extensive hepatocyte necrosis, marked extramedullary hematopoiesis and characteristic “ground-glass” intranuclear inclusions within the hematopoietic cells[91]. It is also believed to cause chronic hepatitis, though evidence remains limited.
Measles can lead to a variety of systemic complications, including hepatitis in some cases. Out of a total of 80 adult patients with measles, liver enzymes were elevated in 65 (81%) upon admission, over 5 folds in 18 (22.5%) and over 10 folds in 5 (6.25%) patients[92]. Rubella Virus can sometimes cause hepatitis as part of the infection process[93,94].
In a detailed literature search from 1967 to 2019, only 17 adult cases were identified[95].
NHV are an emerging cause of acute hepatitis in both healthy and immunocompromised individuals. These viruses include the Herpesviridae group, hemorrhagic fever viruses and some respiratory ones that can infect the liver (adenovirus, coronavirus, influenza virus). Most infections are self-limiting, presenting with non-specific signs and symptoms with or without mild transaminitis and jaundice. Liver involvement can be prominent in HSV, VZV, adenovirus, yellow fever, dengue hemorrhagic fever, Crimean Congo hemorrhagic fever and Rift Valley fever infections. Knowledge of the epidemiology and clinical manifestations of these viruses can help clinicians, in both endemic and nonendemic areas, to suspect them early and to apply adequate diagnostic methods in appropriate clinical setting like recent travel, exposure to vectors and viruses, and immunosuppressed state. Elevation of transaminase can be of varying levels but correlates with disease severity and often with AST > ALT. Viral detection by serum PCR is the best method for diagnosis but IgM antibodies can also help in some cases. Some of the herpesviruses responds well to acyclovir, ganciclovir and their prodrugs if initiated early. Other drugs such as vidarabine, cidofovir, foscarnet, omacyclovir, maribavir, leflunomide have limited efficacy and are under trial. For other viruses, the treatment is virtually always supportive and, except for yellow fever, vaccines are still under development. Therefore, it is best to initiate necessary control measures in case of outbreaks, as also to detect these viruses timely to prevent morbidity and mortality.
| 1. | European Association for the Study of the Liver. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 669] [Article Influence: 74.3] [Reference Citation Analysis (1)] |
| 2. | Ganger DR, Rule J, Rakela J, Bass N, Reuben A, Stravitz RT, Sussman N, Larson AM, James L, Chiu C, Lee WM; Acute Liver Failure Study Group. Acute Liver Failure of Indeterminate Etiology: A Comprehensive Systematic Approach by An Expert Committee to Establish Causality. Am J Gastroenterol. 2018;113:1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Otzurk B. Defining and Managing Acute Liver Failure. AASLD Family of Websites. American Association for the Study of Liver Diseases. Jan 7, 2025. [cited 30 March 2025]. Available from: https://www.aasld.org/liver-fellow-network/core-series/back-basics/defining-and-managing-acute-liver-failure. |
| 4. | Brennan PN, Donnelly MC, Simpson KJ. Systematic review: non A-E, seronegative or indeterminate hepatitis; what is this deadly disease? Aliment Pharmacol Ther. 2018;47:1079-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Centers for Disease Control and Prevention. Overview: Children with Hepatitis of Unknown Cause. June 7, 2022. [cited 30 March 2025]. Available from: Available from: https://archive.cdc.gov/#/details?q=https://www.cdc.gov/ncird/investigation/hepatitis-unknown-cause/overview-what-to-know.html&start=0&rows=10&url=https://www.cdc.gov/ncird/investigation/hepatitis-unknown-cause/overview-what-to-know.html. |
| 6. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Rastogi A, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Alam S, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 640] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 7. | Gupta E, Ballani N, Kumar M, Sarin SK. Role of non-hepatotropic viruses in acute sporadic viral hepatitis and acute-on-chronic liver failure in adults. Indian J Gastroenterol. 2015;34:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Meganck RM, Baric RS. Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nat Med. 2021;27:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 9. | Gupta M, Manek G, Dombrowski K, Maiwall R. Newer developments in viral hepatitis: Looking beyond hepatotropic viruses. World J Meta Anal. 2021;9:522-542. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | World Health Organization. Seventy-seventh World Health Assembly. [cited 30 March 2025]. Available from: https://www.who.int/home/search-results?indexCatalogue=genericsearchindex1&searchQuery=Seventy-seventh%20World%20Health%20Assembly&wordsMode=AnyWord. |
| 11. | Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, Elhaddad A, Frutos C, Galeano S, Hamad N, Hamidieh AA, Hashmi S, Ho A, Horowitz MM, Iida M, Jaimovich G, Karduss A, Kodera Y, Kröger N, Péffault de Latour R, Lee JW, Martínez-Rolón J, Pasquini MC, Passweg J, Paulson K, Seber A, Snowden JA, Srivastava A, Szer J, Weisdorf D, Worel N, Koh MBC, Aljurf M, Greinix H, Atsuta Y, Saber W. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022;107:1045-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 12. | Grand View Research. Organ Transplant Immunosuppressant Drugs Market Size, Share & Trends Analysis Report By Drug Class (Calcineurin Inhibitors, Antiproliferative Agents), By Transplant Type, By Region, And Segment Forecasts, 2025 – 2030. [cited 30 March 2025]. Available from: https://www.grandviewresearch.com/industry-analysis/organ-transplant-immunosuppressant-drugs-market. |
| 13. | The Business Research Company. Immunosuppressants Global Market Report 2025. [cited 30 March 2025]. Available from: https://www.marketresearch.com/Business-Research-Company-v4006/Immunosuppressants-Global-40678484/. |
| 14. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12461] [Article Influence: 6230.5] [Reference Citation Analysis (6)] |
| 15. | IQVIA Institute for Human Data Science. The Global Use of Medicines 2024: Outlook to 2028. Jan 16, 2024. [cited 30 March 2025]. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/the-global-use-of-medicines-2024-outlook-to-2028. |
| 16. | Khan MM, Ali MJ, Hanif H, Maqsood MH, Ahmad I, Alvarez JEG, Catana MA, Lau DTY. The dilemma of cytomegalovirus and hepatitis B virus interaction. Gastroenterol Rep (Oxf). 2022;10:goac018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Bathobakae L, Bashir R, Wilkinson T, Phuu P, Koodirile A, Yuridullah R, Balikani L, Amer K, Cavanagh Y, Baddoura W, Suh JS. Non-hepatotropic viral hepatitis: a narrative review. Scand J Gastroenterol. 2024;59:1322-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Devarbhavi HC, Patil M, Kurien SS, Shafiq S. Non-hepatotropic viruses causing viral hepatitis: current perspectives. Trop Gastroenterol. 2024. |
| 19. | Schottstedt V, Blümel J, Burger R, Drosten C, Gröner A, Gürtler L, Heiden M, Hildebrandt M, Jansen B, Montag-Lessing T, Offergeld R, Pauli G, Seitz R, Schlenkrich U, Strobel J, Willkommen H, von König CH. Human Cytomegalovirus (HCMV) - Revised. Transfus Med Hemother. 2010;37:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Da Cunha T, Wu GY. Cytomegalovirus Hepatitis in Immunocompetent and Immunocompromised Hosts. J Clin Transl Hepatol. 2021;9:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Paya CV, Hermans PE, Wiesner RH, Ludwig J, Smith TF, Rakela J, Krom RA. Cytomegalovirus hepatitis in liver transplantation: prospective analysis of 93 consecutive orthotopic liver transplantations. J Infect Dis. 1989;160:752-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Gentile I, Talamo M, Borgia G. Is the drug-induced hypersensitivity syndrome (DIHS) due to human herpesvirus 6 infection or to allergy-mediated viral reactivation? Report of a case and literature review. BMC Infect Dis. 2010;10:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Gatto I, Biagioni E, Coloretti I, Farinelli C, Avoni C, Caciagli V, Busani S, Sarti M, Pecorari M, Gennari W, Guaraldi G, Franceschini E, Meschiari M, Mussini C, Tonelli R, Clini E, Cossarizza A, Girardis M; Modena COVID-19 Working Group. Cytomegalovirus blood reactivation in COVID-19 critically ill patients: risk factors and impact on mortality. Intensive Care Med. 2022;48:706-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Seehofer D, Rayes N, Tullius SG, Schmidt CA, Neumann UP, Radke C, Settmacher U, Müller AR, Steinmüller T, Neuhaus P. CMV hepatitis after liver transplantation: incidence, clinical course, and long-term follow-up. Liver Transpl. 2002;8:1138-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Vauloup-Fellous C, Lazzarotto T, Revello MG, Grangeot-Keros L. Clinical evaluation of the Roche Elecsys CMV IgG Avidity assay. Eur J Clin Microbiol Infect Dis. 2014;33:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A; Transplantation Society International CMV Consensus Group. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 577] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 27. | Mozaffar M, Shahidi S, Mansourian M, Badri S. Optimal Use of Ganciclovir and Valganciclovir in Transplanted Patients: How Does It Relate to the Outcome? J Transplant. 2018;2018:8414385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Khoo A. Acute cholestatic hepatitis induced by Epstein-Barr virus infection in an adult: a case report. J Med Case Rep. 2016;10:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Hinedi TB, Koff RS. Cholestatic hepatitis induced by Epstein-Barr virus infection in an adult. Dig Dis Sci. 2003;48:539-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J. 2006;99:544-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Kofteridis DP, Koulentaki M, Valachis A, Christofaki M, Mazokopakis E, Papazoglou G, Samonis G. Epstein Barr virus hepatitis. Eur J Intern Med. 2011;22:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Vine LJ, Shepherd K, Hunter JG, Madden R, Thornton C, Ellis V, Bendall RP, Dalton HR. Characteristics of Epstein-Barr virus hepatitis among patients with jaundice or acute hepatitis. Aliment Pharmacol Ther. 2012;36:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Mellinger JL, Rossaro L, Naugler WE, Nadig SN, Appelman H, Lee WM, Fontana RJ. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014;59:1630-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Kremers WK, Devarbhavi HC, Wiesner RH, Krom RA, Macon WR, Habermann TM. Post-transplant lymphoproliferative disorders following liver transplantation: incidence, risk factors and survival. Am J Transplant. 2006;6:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Bunchorntavakul C, Reddy KR. Epstein-Barr Virus and Cytomegalovirus Infections of the Liver. Gastroenterol Clin North Am. 2020;49:331-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 37. | De Paschale M, Clerici P. Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World J Virol. 2012;1:31-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (4)] |
| 38. | Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S 3rd, Theel ES, Thomson RB Jr, Weinstein MP, Yao JD. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67:e1-e94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 393] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 39. | Suh N, Liapis H, Misdraji J, Brunt EM, Wang HL. Epstein-Barr virus hepatitis: diagnostic value of in situ hybridization, polymerase chain reaction, and immunohistochemistry on liver biopsy from immunocompetent patients. Am J Surg Pathol. 2007;31:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Pagano JS, Whitehurst CB, Andrei G. Antiviral Drugs for EBV. Cancers (Basel). 2018;10:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Schiødt FV, Davern TJ, Shakil AO, McGuire B, Samuel G, Lee WM. Viral hepatitis-related acute liver failure. Am J Gastroenterol. 2003;98:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 43. | Kaufman B, Gandhi SA, Louie E, Rizzi R, Illei P. Herpes simplex virus hepatitis: case report and review. Clin Infect Dis. 1997;24:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Natu A, Iuppa G, Packer CD. Herpes Simplex Virus Hepatitis: A Presentation of Multi-Institutional Cases to Promote Early Diagnosis and Management of the Disease. Case Reports Hepatol. 2017;2017:3180984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Sarkar M, Brady CW, Fleckenstein J, Forde KA, Khungar V, Molleston JP, Afshar Y, Terrault NA. Reproductive Health and Liver Disease: Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:318-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 46. | Then EO, Gayam V, Are VS, Sunkara T, Gaduputi V. Herpes Simplex Virus Hepatitis: A Brief Review of an Oft-overlooked Pathology. Cureus. 2019;11:e4313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Feldman S, Crout JD, Andrew ME. Incidence and natural history of chemically defined varicella-zoster virus hepatitis in children and adolescents. Scand J Infect Dis. 1997;29:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Mantadakis E, Anagnostatou N, Danilatou V, Markaki EA, Spanaki AM, Briassoulis G, Kalmanti M. Fulminant hepatitis due to varicella zoster virus in a girl with acute lymphoblastic leukemia in remission: report of a case and review. J Pediatr Hematol Oncol. 2005;27:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Anderson DR, Schwartz J, Hunter NJ, Cottrill C, Bisaccia E, Klainer AS. Varicella hepatitis: a fatal case in a previously healthy, immunocompetent adult. Report of a case, autopsy, and review of the literature. Arch Intern Med. 1994;154:2101-2106. [PubMed] |
| 50. | Brewer EC, Hunter L. Acute Liver Failure due to Disseminated Varicella Zoster Infection. Case Reports Hepatol. 2018;2018:1269340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Hsing LC, Kim JY, Kwon JS, Shin EC, Kim SH. Successful Treatment of Fulminant Hepatitis due to Varicella Zoster Virus using Immunoglobulin in a Kidney Transplant Patient. Infect Chemother. 2019;51:310-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML, McKendrick MW, Nurmikko TJ, Oaklander AL, Oxman MN, Pavan-Langston D, Petersen KL, Rowbotham MC, Schmader KE, Stacey BR, Tyring SK, van Wijck AJ, Wallace MS, Wassilew SW, Whitley RJ. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44 Suppl 1:S1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 53. | Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev. 2015;28:313-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 54. | van Leeuwen LPM, de Jong W, Doornekamp L, van Gorp ECM, Wismans PJ, Goeijenbier M. Exotic viral hepatitis: A review on epidemiology, pathogenesis, and treatment. J Hepatol. 2022;77:1431-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6667] [Cited by in RCA: 6539] [Article Influence: 503.0] [Reference Citation Analysis (1)] |
| 56. | World Health Organization. Dengue and Severe Dengue. [cited 30 March 2025]. Available from: https://www.who.int/home/search-results?indexCatalogue=genericsearchindex1&searchQuery=Dengue%20and%20Severe%20Dengue%2C%20Fact%20Sheets&wordsMode=AnyWord. |
| 57. | Samanta J, Sharma V. Dengue and its effects on liver. World J Clin Cases. 2015;3:125-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 130] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (7)] |
| 58. | Devarbhavi H, Ganga D, Menon M, Kothari K, Singh R. Dengue hepatitis with acute liver failure: Clinical, biochemical, histopathological characteristics and predictors of outcome. J Gastroenterol Hepatol. 2020;35:1223-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Seneviratne SL, Malavige GN, de Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg. 2006;100:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 60. | Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nat Rev Dis Primers. 2016;2:16055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 494] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 61. | Kye Mon K, Nontprasert A, Kittitrakul C, Tangkijvanich P, Leowattana W, Poovorawan K. Incidence and Clinical Outcome of Acute Liver Failure Caused by Dengue in a Hospital for Tropical Diseases, Thailand. Am J Trop Med Hyg. 2016;95:1338-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Thanachartwet V, Oer-Areemitr N, Chamnanchanunt S, Sahassananda D, Jittmittraphap A, Suwannakudt P, Desakorn V, Wattanathum A. Identification of clinical factors associated with severe dengue among Thai adults: a prospective study. BMC Infect Dis. 2015;15:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Lien CE, Chou YJ, Shen YJ, Tsai T, Huang N. A Population-Based Cohort Study on Chronic Comorbidity Risk Factors for Adverse Dengue Outcomes. Am J Trop Med Hyg. 2021;105:1544-1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Badawi A, Velummailum R, Ryoo SG, Senthinathan A, Yaghoubi S, Vasileva D, Ostermeier E, Plishka M, Soosaipillai M, Arora P. Prevalence of chronic comorbidities in dengue fever and West Nile virus: A systematic review and meta-analysis. PLoS One. 2018;13:e0200200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 65. | Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 806] [Cited by in RCA: 877] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 66. | Kariyawasam JC, Jayarajah U, Abeysuriya V, Riza R, Seneviratne SL. Involvement of the Liver in COVID-19: A Systematic Review. Am J Trop Med Hyg. 2022;106:1026-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 68. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 69. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (3)] |
| 70. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (3)] |
| 71. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 72. | Yuen KY, Wong SS. Human infection by avian influenza A H5N1. Hong Kong Med J. 2005;11:189-199. [PubMed] |
| 73. | Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, Carlino LO, Owen R, Paterson B, Pelletier L, Vachon J, Gonzalez C, Hongjie Y, Zijian F, Chuang SK, Au A, Buda S, Krause G, Haas W, Bonmarin I, Taniguichi K, Nakajima K, Shobayashi T, Takayama Y, Sunagawa T, Heraud JM, Orelle A, Palacios E, van der Sande MA, Wielders CC, Hunt D, Cutter J, Lee VJ, Thomas J, Santa-Olalla P, Sierra-Moros MJ, Hanshaoworakul W, Ungchusak K, Pebody R, Jain S, Mounts AW; WHO Working Group for Risk Factors for Severe H1N1pdm Infection. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 74. | Puig-Barberà J, Natividad-Sancho A, Trushakova S, Sominina A, Pisareva M, Ciblak MA, Badur S, Yu H, Cowling BJ, El Guerche-Séblain C, Mira-Iglesias A, Kisteneva L, Stolyarov K, Yurtcu K, Feng L, López-Labrador X, Burtseva E; Global Influenza Hospital Surveillance Study Group (GIHSN). Epidemiology of Hospital Admissions with Influenza during the 2013/2014 Northern Hemisphere Influenza Season: Results from the Global Influenza Hospital Surveillance Network. PLoS One. 2016;11:e0154970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Schütte A, Ciesek S, Wedemeyer H, Lange CM. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70:797-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 76. | Duchini A, Viernes ME, Nyberg LM, Hendry RM, Pockros PJ. Hepatic decompensation in patients with cirrhosis during infection with influenza A. Arch Intern Med. 2000;160:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Premkumar M, Devurgowda D, Dudha S, Maiwall R, Bihari C, Grover S, Gupta E, Kumar S, Sarin SK. A/H1N1/09 Influenza is Associated With High Mortality in Liver Cirrhosis. J Clin Exp Hepatol. 2019;9:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 78. | Kelgeri C, Couper M, Gupte GL, Brant A, Patel M, Johansen L, Valamparampil J, Ong E, Hartog H, Perera MTPR, Mirza D, van Mourik I, Sharif K, Hartley J. Clinical Spectrum of Children with Acute Hepatitis of Unknown Cause. N Engl J Med. 2022;387:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 79. | World Health Organization. Severe acute hepatitis of unknown aetiology in children ‐ Multi‐country. [cited 30 March 2025]. Available from: https://www.who.int/home/search-results?indexCatalogue=genericsearchindex1&searchQuery=Severe%20acute%20hepatitis%20of%20unknown%20aetiology%20in%20children%20%E2%80%90%20Multi%E2%80%90country&wordsMode=AnyWord. |
| 80. | Kelly DA, Stamataki Z. Sudden onset hepatitis in children. Nat Rev Gastroenterol Hepatol. 2022;19:553-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Elsheikh R, Tien HT, Makram AM, Van NT, Le TTB, Vasanthakumaran T, Huy NT. Acute hepatitis of unknown origin in children: Behind the statistics. Hepatology. 2023;77:2118-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Gutierrez Sanchez LH, Shiau H, Baker JM, Saaybi S, Buchfellner M, Britt W, Sanchez V, Potter JL, Ingram LA, Kelly D, Lu X, Ayers-Millsap S, Willeford WG, Rassaei N, Bhatnagar J, Bullock H, Reagan-Steiner S, Martin A, Rogers ME, Banc-Husu AM, Harpavat S, Leung DH, Moulton EA, Lamson DM, St George K, Hall AJ, Parashar U, MacNeil A, Tate JE, Kirking HL. A Case Series of Children with Acute Hepatitis and Human Adenovirus Infection. N Engl J Med. 2022;387:620-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 83. | Săndulescu O, Streinu-Cercel A, Miron VD, Covăcescu SM, Streinu-Cercel A, Craiu M. Liver Transaminases in Pediatric Adenovirus Infection-A Five-Year Study in Two Major Reference Centers from Romania. Microorganisms. 2023;11:302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 84. | Rodriguez-Frias F, Rando-Segura A, Quer J. Solved the enigma of pediatric severe acute hepatitis of unknown origin? Front Cell Infect Microbiol. 2023;13:1175996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Servellita V, Sotomayor Gonzalez A, Lamson DM, Foresythe A, Huh HJ, Bazinet AL, Bergman NH, Bull RL, Garcia KY, Goodrich JS, Lovett SP, Parker K, Radune D, Hatada A, Pan CY, Rizzo K, Bertumen JB, Morales C, Oluniyi PE, Nguyen J, Tan J, Stryke D, Jaber R, Leslie MT, Lyons Z, Hedman HD, Parashar U, Sullivan M, Wroblewski K, Oberste MS, Tate JE, Baker JM, Sugerman D, Potts C, Lu X, Chhabra P; Pediatric Hepatitis of Unknown Etiology Working Group, Ingram LA, Shiau H, Britt W, Gutierrez Sanchez LH, Ciric C, Rostad CA, Vinjé J, Kirking HL, Wadford DA, Raborn RT, St George K, Chiu CY. Adeno-associated virus type 2 in US children with acute severe hepatitis. Nature. 2023;617:574-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 86. | Gates S, Andreani J, Dewar R, Smith DB, Templeton K, Child HT, Breuer J, Golubchik T, Bassano I, Wade MJ, Jeffries AR, Simmonds P, Harvala H. Postpandemic rebound of adeno-associated virus type 2 (AAV2) infections temporally associated with an outbreak of unexplained severe acute hepatitis in children in the United Kingdom. J Med Virol. 2023;95:e28921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 87. | Ramadan HK, Sayed IM, Elkhawaga AA, Meghezel EM, Askar AA, Moussa AM, Osman AOBS, Elfadl AA, Khalifa WA, Ashmawy AM, El-Mokhtar MA. Characteristics and outcomes of acute hepatitis of unknown etiology in Egypt: first report of adult adenovirus-associated hepatitis. Infection. 2023;51:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 88. | Spengler U. Liver Disease Associated with Non-Hepatitis Viruses. Encycl Gastroenterol. 2020. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Wang R, Xie Z. Non-hepatotropic viral hepatitis and its causative pathogens: The ongoing need for monitoring in children with severe acute hepatitis of unknown etiology. Pediatr Investig. 2022;6:151-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 90. | Yen FB, Chang LY, Kao CL, Lee PI, Chen CM, Lee CY, Shao PL, Wang SC, Lu CY, Huang LM. Coxsackieviruses infection in northern Taiwan--epidemiology and clinical characteristics. J Microbiol Immunol Infect. 2009;42:38-46. [PubMed] |
| 91. | Leon LAA, Alves ADR, Garcia RCNC, Melgaço JG, de Paula VS, Pinto MA. Parvovirus B19 Infection in a Fatal Case of Acute Liver Failure. Pediatr Infect Dis J. 2017;36:e355-e358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Dinh A, Fleuret V, Hanslik T. Liver involvement in adults with measles. Int J Infect Dis. 2013;17:e1243-e1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 93. | Tameda Y, Kosaka Y, Shiraki K, Ohashi Y, Hamada M, Miyazaki M, Ito N, Takase K, Nakano T. Hepatitis in an adult with rubella. Intern Med. 1993;32:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Arai M, Wada N, Maruyama K, Nomiyama T, Tanaka S, Okazaki I. Acute hepatitis in an adult with acquired rubella infection. J Gastroenterol. 1995;30:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Ho CLT, Oligbu O, Asaid F, Oligbu G. Does norovirus induce acute hepatitis? AIMS Public Health. 2020;7:148-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/