Published online Sep 25, 2025. doi: 10.5501/wjv.v14.i3.107903
Revised: May 17, 2025
Accepted: July 24, 2025
Published online: September 25, 2025
Processing time: 178 Days and 19.1 Hours
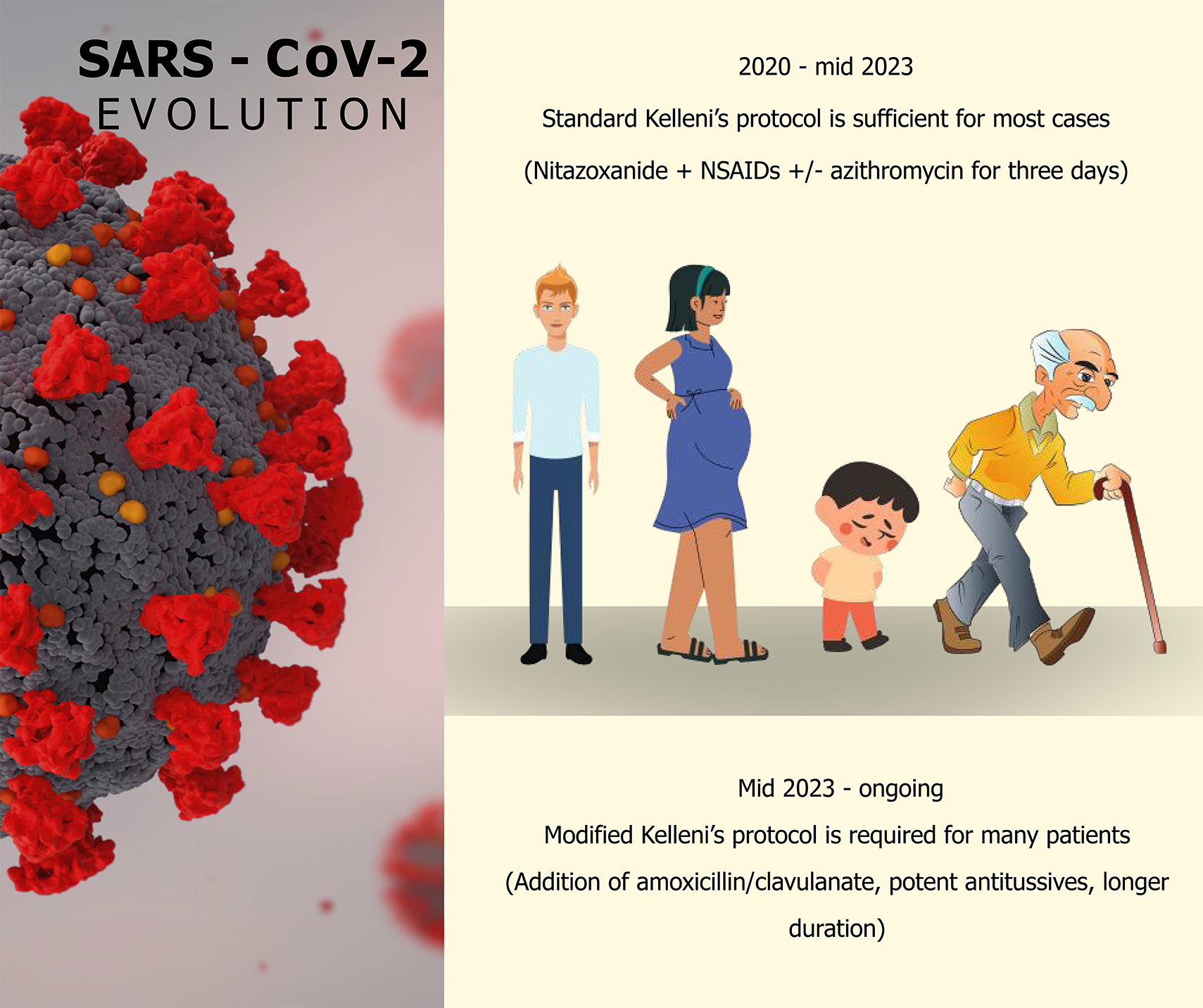
This article discusses the evolving real-world practice using nitazoxanide, non-steroidal anti-inflammatory drugs (NSAIDs) and/or azithromycin (Kelleni’s protocol) to manage the evolving manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron EG.5.1, its descendant HV.1 as well as BA.2.86 and its descendant JN.1 subvariants in Egypt in 2024. These subvariants are well-known for their highly evolved immune-evasive properties and the manifestations include some peculiar manifestations as persistent cough besides high fever in young children as well as high fever, persistent severe cough, change of voice, loss of taste and smell, epigastric pain, nausea, vomiting, diarrhea, generalized malaise and marked bone aches in adults including the high-risk groups. It’s suggested that the ongoing SARS-CoV-2 evolution is continuing to mostly affect the high-risk groups of patients, to some of whom we’ve also successfully prescribed nitazoxanide and/or NSAIDs for post-expo
Core Tip: Coronavirus disease 2019 (COVID-19) has claimed the lives of over seven million people worldwide. Most deaths have occurred among high-risk patients, making it crucial to find a protocol that is effective for post-exposure prophylaxis in this pandemic and future ones. This article provides insight into how Kelleni’s protocol was successfully and safely used to save lives among high-risk groups of COVID-19 patients amidst the ongoing evolution of severe acute respiratory syndrome coronavirus 2.
- Citation: Kelleni MT. Real-life practice of Kelleni’s protocol in treatment and post exposure prophylaxis of SARS-CoV-2 HV.1 and JN.1 subvariants. World J Virol 2025; 14(3): 107903
- URL: https://www.wjgnet.com/2220-3249/full/v14/i3/107903.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i3.107903
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) evolution is ongoing while threatening to return the world to square one[1,2]. However, Africa has adopted early treatment approach and managed to avoid most of the burden of coronavirus disease 2019 (COVID-19)[3,4]. We would like to report the real-life practice of the Egyptian Kelleni’s protocol in both treatment and post-exposure prophylaxis of SARS-CoV-2 infection in 2024.
For almost four years we’ve been safely and effectively practicing the immune-modulatory antiviral nitazxoanide, non-steroidal anti-inflammatory drugs (NSAIDs) and/or azithromycin (the standard Kelleni’s protocol) to early manage several respiratory viral infections including all SARS-CoV-2 variants that have evolved and affected patients of all ages including pregnant ones and those suffering from various medical co-morbidities[5]. Notably, Egypt has officially acknowledged EG.5 subvariant only in August 2023 and in December 2023, Egypt has similarly acknowledged BA.2.86 and its descendant JN.1, which are currently the dominating global ones. Importantly, SARS-CoV-2 variants of interests and concern are being closely monitored, due to their highly evolved immune-evasive properties which can lead to a potential reclassification of their global COVID-19 morbidity and mortality impact.
We’d like to report that since October 2023 until April 2024, we’ve been experiencing a surge of unusually encountered more virulent respiratory infections which significantly affect the high-risk groups of patients; especially young children and immune-compromised adults. Interestingly, the abrupt onset of persistent high fever reaching occasionally exceeding 40 °C was a hallmark of those high risk patients, during the first two to three days of manifestations. Notably, for several times during COVID-19 pandemic, new SARS-CoV-2 variants of concern were highly suspected clinically before being officially named. The most recently encountered manifestations in otherwise healthy adults, since March 2024, are high fever persistent marked epigastric pain, nausea, vomiting and/or diarrhea which were preceded or followed by the respiratory manifestations of rhinorrhea, persistent cough, loss of taste and smell, generalized malaise and severe bone ache. Notably, these predominant gastrointestinal manifestations were previously suggested to imply an evolutionary change of SARS-CoV-2 tropism[5].
The high fever was efficiently relieved by regular use of NSAIDs two to three times a day, along with cold packs and it’s noteworthy that we’ve not encountered such intensity of fever in that frequency before and we’ve added amoxicillin/clavulanic acid to the standard Kelleni’s protocol if the expected clinical improvement was not found at the end of the third day. Notably, this modification has adequately managed the debilitated high risk patients who might have experienced resistance to macrolides or a more severe secondary bacterial infection which is a known serious complication of COVID-19[6].
Importantly, though Mycoplasma pneumoniae infections, characterized by persistent dry cough and prolonged manifestations, were reported to surge especially in children in different countries after the lifting of COVID-19 restrictions, yet Egypt has had almost zero COVID-19 restrictions for over two years and these symptoms were reported in both high and low risk groups of patients of all ages. Moreover, though amoxicillin/calvulanic acid efficacy is lacking as regards to Mycoplasma pneumoniae which has no cell wall, yet enhanced antimicrobial activity of azithromycin against mycoplasma pneumoniae in the presence of amoxicillin/clavulanic acid could be suggested[7] as observed clinically in some treated pediatric patients during this surge.
As per our clinical experience, it’s become increasingly not uncommon for those high-risk patients to require an extended course of Kelleni’s antiviral protocol including a 5-day-course of double antibiotics; azithromycin and amoxicillin/clavulanic acid in addition to the standard nitazoxanide and NSAIDs[5].
Moreover, persistent troublesome cough was quite evident and prevalent in this current surge of SARS-CoV-2 infections. It was managed by both natural antitussives and loratadine[3], but a personalized administration of cloperastine suspension or pholcodine containing syrup was required in selected severe cases. Furthermore, change of voice was occasionally the main presentation in young healthy adults who have been re-infected during this current surge, along with mild to moderate diarrhea, headache, fatigue, malaise and some patients experienced marked retro-orbital pain or persistent severe cough. These symptoms, other than cough, were effectively managed by NSAIDs with or without nitazoxanide.
Importantly, persistent cough and high fever were less frequently encountered when we included azithromycin, together with nitazoxanide and ibuprofen, in the prompt management of early SARS-CoV-2 infection in young children.
Interestingly, a young female child presented with several bouts of hematochezia without respiratory symptoms or abdominal colic. She was effectively managed using nitazoxanide and azithromycin, without NSAIDs to avoid exacerbating bleeding attacks. We advised the parents to use acetaminophen if the child developed subsequent fever, although it was not ultimately required. Noteworthy, hematochezia associated with COVID-19 has been previously described as more common in geriatric COVID-19 male patients suffering from other co-morbidities[8], yet pediatric and female patients have also been reported[9,10].
Notably, immune-compromised patients, and an adult receiving immunotherapy with nivolumab to manage his slowly regressive hepatocellular carcinoma, experienced more frequent persistent troublesome cough, marked bone aches and fatigue. However, when these patients were early managed, the standard Kelleni’s protocol was sufficient. Moreover, from our clinical practice, when immune-competent young adults were prescribed nitazoxanide and NSAIDs at the first symptoms of sore throat, sneezing and early rhinorrhea the infection was effectively managed in three days in the vast majority of cases (Figure 1).
Notably, nitazoxanide is currently heavily and effectively prescribed in Egypt especially to manage the prevalent gastrointestinal manifestations of SARS-CoV-2 and other viruses[5]. Notably, in a multicentre, randomized, double-blind, placebo-controlled clinical trial, nitazoxanide was shown to improve the clinical outcome, time to hospital discharge, and to reduce the oxygen requirements and the C-reactive protein, D-dimer, and ferritin levels compared to the placebo group and no serious adverse events were observed[11]. Furthermore, in a Mexican study that included 312 patients suffering from severe COVID-19 and were treated using nitazoxanide as part of the administered regimen, only 6 patients were died[12].
Furthermore, we’ve repeatedly used NSAIDs and/or nitazoxanide for post-exposure prophylaxis of high-risk house-hold contacts and this approach has clinically proven to be safe and effective. It has sometimes prevented but mostly significantly alleviated the inevitable infection. Unfortunately, a pioneering clinical trial (NCT04435314) to assess the efficacy of nitazoxanide in SARS-CoV-2 post exposure prophylaxis was aborted due to “sponsor’s strategic decision”. Moreover, we suggest that nitazoxanie is not pharmacologically fit to be tested as a drug used chronically for “pre
Interestingly, during the described surge of infections, we’ve also prescribed nitazoxanide, without NSAIDs, to a pregnant patient in her late second trimester and most of the symptoms were alleviated within two days except for prolonged severe cough that was only relieved after adding loratadine to the herbal antitussives. Moreover, nitazoxanide was used as an immediate post-exposure prophylaxis to this pregnant patient when re-infected in her third trimester, the clinical outcome was better, and the severe cough was not even encountered. However, the recently acquired previous immunity though couldn’t prevent the re-infection; it could probably have played at least a partial role in this favorable response.
Notably, in addition to recommending starting the personalized Kelleni’s protocol as early as possible for the mana
Fortunately, all cases encountered thus far have fully recovered within one week without any post coronavirus disease complaints. However, it’s quite evident from a clinical standpoint that SARS-CoV-2 evolution is ongoing and this could reignite panic particularly in countries that have not yet adopted early immune-modulation in the pharmacological management of COVID-19[4].
Given the ongoing circulation and evolution of SARS-CoV-2, as well as the emergence of new variants of potential clinical concern due to their expedited transmission and/or greater immunological evasion properties, especially among vulnerable populations, it’s crucial to prioritize standardized post-exposure prophylaxis protocols as part of global preparedness. Kelleni’s post-exposure prophylaxis protocol presents a cost-effective, readily implementable, and potentially impactful approach to minimize severe COVID-19 and hospitalization, particularly in resource-limited countries. Kelleni’s protocol could significantly enhance equitable and preventive public health strategies in both current and future pandemics.
| 1. | Hattab D, Amer MFA, Al-Alami ZM, Bakhtiar A. SARS-CoV-2 journey: from alpha variant to omicron and its sub-variants. Infection. 2024;52:767-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Hu B, Guo H, Si H, Shi Z. Emergence of SARS and COVID-19 and preparedness for the next emerging disease X. Front Med. 2024;18:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Kelleni MT. The African Kelleni's roadmap using nitazoxanide and broad-spectrum antimicrobials to abort returning to COVID-19 square one. Inflammopharmacology. 2023;31:3335-3338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Kelleni MT. COVID-19 mortality paradox (United States vs Africa): Mass vaccination vs early treatment. World J Exp Med. 2024;14:88674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kelleni MT. Real-world practice of the Egyptian Kelleni's protocol amid changing tropism of SARS-CoV-2 omicron BA.5.2.1.7, XBB 1.5 and CH.1.1 subvariants: a multi-purpose protocol. Inflammopharmacology. 2023;31:1559-1560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Shafran N, Shafran I, Ben-Zvi H, Sofer S, Sheena L, Krause I, Shlomai A, Goldberg E, Sklan EH. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci Rep. 2021;11:12703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 7. | Onodera S, Kiyota H, Endo K, Suzuki H, Hosobe T, Takahashi T, Egawa S, Kobayashi I. Enhancement of antimicrobial activities of cefteram or clavulanic acid/amoxicillin against cefixime-resistant Neisseria gonorrhoeae in the presence of clarithromycin or azithromycin. J Infect Chemother. 2006;12:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Ion D, Gherghinescu M, Andronic O, Andreescu CV, Păduraru DN, Bolocan A, Russu C, Aprodu S, Mationi G, Nicolescu C, Popa D. Prognosis Evaluation for Patients with Abdominal Trauma Using Usual Biological Parameters. Chirurgia (Bucur). 2021;116:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Frič VO, Borzan V, Borzan A, Kiš I, Dmitrović B, Roksandić-Križan I. Colitis as the Main Presentation of COVID-19: A Case Report. Medicina (Kaunas). 2023;59:576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | El Hasbani G, Taher AT, Jawad ASM, Uthman I. Henoch-Schönlein purpura: Another COVID-19 complication. Pediatr Dermatol. 2021;38:1359-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Rocco PRM, Silva PL, Cruz FF, Tierno PFGMM, Rabello E, Junior JC, Haag F, de Ávila RE, da Silva JDG, Mamede MMS, Buchele KS, Barbosa LCV, Cabral AC, Junqueira AAF, Araújo-Filho JA, da Costa LATJ, Alvarenga PPM, Moura AS, Carajeleascow R, de Oliveira MC, Silva RGF, Soares CRP, Fernandes APSM, Fonseca FG, Camargos VN, Reis JS, Franchini KG, Luiz RR, Morais S, Sverdloff C, Martins CM, Felix NS, Mattos-Silva P, Nogueira CMB, Caldeira DAF, Pelosi P, Lapa-E-Silva JR. Nitazoxanide in Patients Hospitalized With COVID-19 Pneumonia: A Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial. Front Med (Lausanne). 2022;9:844728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Romero-Cabello R, Romero-Feregrino R, Romero-Feregrino R, Muñoz-Cordero B, Sevilla-Fuentes S. Outpatient treatment of COVID-19: an experience with 552 cases in Mexico. J Infect Dev Ctries. 2023;17:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Sokhela S, Bosch B, Hill A, Simmons B, Woods J, Johnstone H, Akpomiemie G, Ellis L, Owen A, Casas CP, Venter WDF. Randomized clinical trial of nitazoxanide or sofosbuvir/daclatasvir for the prevention of SARS-CoV-2 infection. J Antimicrob Chemother. 2022;77:2706-2712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/