Published online Sep 25, 2025. doi: 10.5501/wjv.v14.i3.107214
Revised: April 21, 2025
Accepted: June 13, 2025
Published online: September 25, 2025
Processing time: 191 Days and 18 Hours
Due to low bacteria count and high likelihood of having extrapulmonary tuberculosis (TB) among patients with advanced human immunodeficiency virus (HIV) disease, the World Health Organization (WHO) recommended the use of urine lateral flow urine lipoarabinomannan (LF-LAM) or sputum-Xpert to screen for TB.
To estimate pooled prevalence of TB screening uptake, TB diagnosis, TB treatment initiation and mortality among patients with advanced HIV disease in Africa.
PubMed, Cochrane Library and EMBASE were searched for articles published between January 2011 and December 2024. TB screening uptake was defined as percentage of patients with advanced HIV disease (CD4 ≤ 200 cells/mm3 or WHO stage III/IV) who tested for TB. Using random effects models, we computed the pooled estimate of TB screening uptake, TB prevalence, TB treatment initiation and mortality and their corresponding 95%CIs. Stratified analysis to compare uptake of TB testing and TB prevalence between children vs adults and multisite vs single site studies was performed.
A total of nineteen studies with 16065 people with advanced HIV disease were analyzed. The pooled prevalence of TB screening uptake was 64.6% (95%CI: 49.2–80.1). The pooled prevalence of TB was 29.4% (95%CI: 22.0–36.8), and TB treatment initiation was 77.9% (95%CI: 63.9–91.8), and mortality was 19.5% (95%CI: 8.9–30.0). The pooled prevalence of TB testing uptake was significantly lower among children compared to adults (28.2% vs 66.4%, P = 0.003) and lower for multi-sites compared to single site studies (58.8% vs 82.9%, P = 0.002). The pooled prevalence of TB was significantly lower among children compared to adults (24.2% vs 27.6%, P = 0.012) and higher among studies that involved multi vs single sites (30.0% vs 21.9%, P = 0.001).
Four in ten people with advanced HIV disease were not screened for TB as recommended by the WHO, indicating significant gaps in identifying patients with TB. Excluding patients with evidence of TB is critical to avoid exposing them to subtherapeutic levels of anti TB treatment.
Core Tip: Despite its benefit, the utility of lateral flow urine lipoarabinomannan assays in the diagnosis of tuberculosis (TB) among patients with advanced human immunodeficiency virus (HIV) disease in Africa is low. This systematic review analyzed 16055 participants with advanced HIV from nineteen studies involving thirteen countries in Africa. Of the participants eligible to test for TB using lateral flow urine lipoarabinomannan (LF-LAM), only 63% tested with uptake being lower among children compared to adults. Among those diagnosed with TB, 22% died. Nearly four in ten people with advanced HIV disease in Africa tested for TB using LF-LAM per the World Health Organization recommendations, indi
- Citation: Moshi L, Bakari HM, Mbishi JV, Ally ZM, Mbwana MS, Ally HM, Musoke R, Salim SM, Karia MF, Karia LF, Fussi HF, Mustafa AO, El-lmam IA, Ramadhani HO. Uptake and disparities in tuberculosis screening using urine-lipoarabinomannan among patients with advanced human immunodeficiency virus-disease in Africa: A systematic review. World J Virol 2025; 14(3): 107214
- URL: https://www.wjgnet.com/2220-3249/full/v14/i3/107214.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i3.107214
Stigma against human immunodeficiency virus (HIV) continues to impact people living with HIV and as a result some patients present late with advanced HIV disease (CD4 count ≤ 200 cells/mm3 or World Health Organization (WHO) stage III/IV) at the time of HIV diagnosis despite an expanded anti-retroviral (ART) coverage[1-4]. From 2011, studies that used CD4 count threshold of ≤ 100 cells/mm3, estimated the prevalence of advanced HIV disease for newly diagnosed individuals to range from 13.9% to 33.6%[5,6], while those which used a higher threshold of ≤ 200 cells/mm3 reported a broader range of 17.2% to 71%[7,8] reflecting differences in study population, healthcare settings, and diagnostic thresholds. Advanced HIV disease is associated with negative consequences including delayed time to viral load suppression[9,10], increased risk of onward HIV transmission, increased treatment costs[11] and life-threatening opportunistic infections such as cryptococcal meningitis and tuberculosis (TB)[12,13]. Despite increased access to ART, advanced HIV disease is the predominant concern for an ongoing acquired immunodeficiency syndrome (AIDS) related mortality[14].
Globally, it is estimated that 4.3 million adults are living with advanced HIV disease[15]. TB remains the leading cause of HIV related mortality globally, accounting for approximately 30% of HIV related mortality annually[16]. Among HIV patients co-infected with TB, provision of both ART and anti TB treatment averted 6.4 million deaths between 2010 and 2020 globally[17]. Of the 6.4 million deaths averted, three quarters were from Africa. Screening for TB and treatment of latent TB infection reduce mortality and years of potential life lost due to active TB disease[18]. These data underscore the need for accurate diagnosis and treatment.
Due to low bacteria count and high likelihood of having extrapulmonary TB among patients with advanced HIV disease, recognizing these limitations, the WHO recommended the use of more sensitive rapid molecular diagnostic tests such as lateral flow urine lipoarabinomannan (LF-LAM) or sputum-Xpert as they offer greater sensitivity than the traditional smear microscopy for TB diagnosis. The United Nation targets requires that by 2027 the coverage of rapid diagnostic testing for TB to be 100%[19]. Overtime, there has been variation on the uptake of LF-LAM or sputum Xpert from different studies with the reported testing uptake being between 5% to 98%[20,21]. The variation in the uptake of these tests could be attributed but not limited to the availability of testing kits, reagents and technical capacity[13,22]. In addition, unawareness of WHO guidelines, data sources from published literature [(single vs multi sites studies); types of healthcare facilities (primary, secondary and tertiary)] could explain differences in the reported TB testing uptake using these rapid tests as previously noted by other researchers[23]. Data on the overall magnitude and compliance of WHO guidelines on the uptake of TB testing using LF-LAM is limited. Given these inconsistencies, a systematic review and meta-analysis is warranted to provide pooled estimates and identify disparities by age and study settings. In this review, we are addressing the following questions for studies conducted in Africa. Among patients with advanced HIV disease, what percent were tested for TB using WHO recommended rapid TB diagnostic molecular test of LF-LAM? (TB uptake): (1) Among those tested, what percentage tested positive? (2) Among those who tested positive, what percentage-initiated TB treatment? and (3) What is mortality rate among patients diagnosed with advanced HIV disease who are coinfected with TB?
The protocol for this systematic review has been registered in the International Prospective Registry of Systematic Review on October 31, 2024 with registration number CRD42024604091.
Because this was a systematic review of published manuscripts, ethical approval was not sought.
We searched PubMed, Cochrane CENTRAL, EMBASE and clinicaltrials.gov for the articles published between January 2011 and December 2024. The search period represents the time at which WHO guidelines for the management of patients with advanced HIV disease was operational. Search terms were used to capture information on TB testing using urine LF-LAM among people living with HIV who presented with advanced HIV disease in Africa. The search was restricted to papers written in English. Search results were uploaded to Covidence Systematic Review Software (Melbourne, Australia) for deduplication and screening.
Studies were included based on the guidelines of systematic reviews and meta-analysis with prevalence approach (CoCoPop) where the first and second ‘Co’ indicates condition/problem and context respectively and ‘Pop’ indicates study population as previously described[24]. In this review, under condition, we included studies that reported prevalence of TB testing uptake using LF-LAM among people presenting with advanced HIV disease; Context, we explored components of different studies that could explain variation on the reported prevalence of TB testing uptake such as study designs, number of study sites (single vs multiple sites), type of health care facilities (primary, secondary or tertiary); Population, we included people with advanced HIV disease.
Observational studies that involved people with advanced HIV disease in Africa, reported number and percentage of individuals who tested for TB using LF-LAM and written in English were eligible for inclusion.
Studies that reported the percentage of people who tested for TB without actual numerators and denominators used to compute those percentages. Studies that were not written in English.
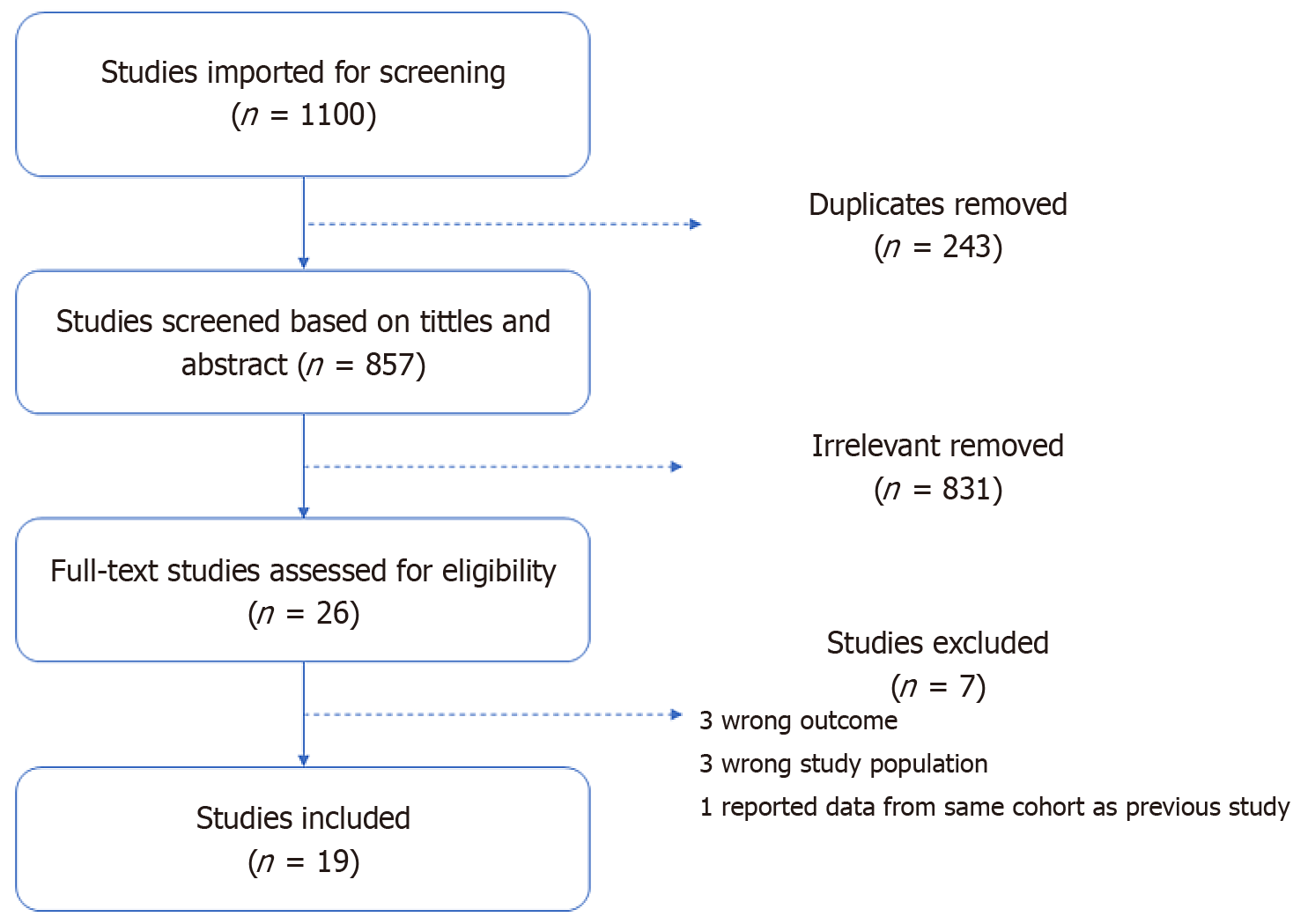
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Figure 1) describing the literature search process and included studies is presented below. This systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[25].
The manuscripts searched from outlined databases were managed by Covidence software from which the final list of manuscripts was deduplicated. Two pairs of authors (Haji Mbwana Ally, Habib Omari Ramadhani and Zuhura Mbwana Ally, Hassan Fredrick Fussi) independently completed the study selection for inclusion in the appraisal process. Disagreement between two independent pairs of authors for the inclusion of the manuscripts was handled by the third pair of authors (Mariam Salim Mbwana and Ibrahim Ahmed El-lmam).
Using a pre specified excel spread sheet template, two pairs of authors (Haji Mbwana Ally, Habib Omari Ramadhani and Zuhura Mbwana Ally, Hassan Fredrick Fussi) independently extracted the following data elements from the included studies: Authors, year of publication, the country in which the study was conducted, the year(s) at which data was collected, study design, study sites (single vs multi sites), TB test used (LF-LAM), number of people with advanced HIV disease, number of people eligible for TB testing, number of people tested for TB, number of people with positive TB test, number of people with positive TB test who initiated treatment, number of people with positive TB test and died. The data were then compared, and any disagreements between the two pairs of reviewers were resolved by consensus; the third pair of reviewers (Mariam Salim Mbwana and Ibrahim Ahmed El-lmam) was consulted when necessary. Strategies in the Cochrane Handbook for Systematic Reviews of Interventions for data management were followed[26].
The Joanna Briggs Institute (JBI) tools for cross sectional and cohort studies were used to assess quality of studies[27]. Two pairs of reviewers (Habib Omari Ramadhani and Haji Mbwana Ally) and (Hassan Fredrick Fussi and Lynn Moshi) independently performed and rated the quality of the studies using the JBI tools. The tool encompassed nine questions with four responses: (Yes, No, Not clear, Not applicable). A score of 1 was assigned to a “Yes” response and 0 to a “No” response. Total score was summed up and categorized into three groups, with (0-3), (4-6) and (7-9) scores indicating low, medium and high quality.
Outcome variables: The main outcome of interest was the TB testing uptake defined as percentage of individuals who tested for TB using LF-LAM among those who presented with advanced HIV disease. The secondary outcome were: (1) The prevalence of TB defined as percentage of individuals with positive LF-LAM among those tested; (2) Percentage of people who initiated treatment among those with positive TB test; and (3) Mortality among those diagnosed with TB.
Exposure variables: There was no main exposure variable, however, TB testing uptake and TB prevalence were compared between children and adults and between studies that involved multiple sites vs single site.
Using random effects model, we computed pooled prevalence of TB testing uptake, TB prevalence, treatment initiations and mortality. Since there was no extreme proportions (as low as ‘0’ or as high as ‘1’) requiring variance stabilization, all analyses were conducted using raw proportions, allowing for direct clinical interpretation of the pooled estimates. Subgroup analysis on the pooled estimates of TB testing uptake and TB prevalence were performed to compare children vs adults and between studies that involved multiple sites vs single using χ2 tests. We evaluated heterogeneity across studies using the I2 statistic and Cochran’s Q test. The I2 statistics explain the variance attributable to study heterogeneity with scores of 75%, 50% and 25% indicated high, moderate and low heterogeneity, respectively[28]. The publication bias was assessed using the Egger regression asymmetry test. For both heterogeneity and publication test, a P < 0.05 indicated the presence of heterogeneity and publication bias respectively. To explore the source of heterogeneity for outcome with moderate or higher degree of heterogeneity, an influential analysis using the leave-one-out method was performed[29]. Furthermore, a meta regression analysis was done to assess variation of the uptake of TB screening across studies and a sensitivity analysis to assess potential small-study effects was conducted using a trim-and-fill method. Studies with missing information, such as those that reported proportions of outcomes without actual numerators and/or deno
Our search resulted in 1100 articles. Of these, 243 were duplicates and deleted. The remaining 857 articles were eligible for title and abstracts screening. Of the twenty-six articles eligible for full text review, nineteen met inclusion criteria and were finally included in our analysis (Figure 1).
A total of nineteen studies with 16065 people with advanced HIV disease were analyzed. The review included studies conducted by thirteen countries in Africa. Sample size of included studies ranged from 97 to 5487 (Table 1). All studies reported TB testing uptake, and results of TB testing, eight reported TB treatment initiations and seven reported mortality data. Two studies reported TB testing uptake among children[30,31] and thirteen among adults[12,20,21,32-41] and four among both children and adults[7,13,42,43]. A total of fourteen studies involved multiple sites and three involved single sites and two not specified.
| Ref. | Country | Number of study sites | Study design | Type of population | AHD defined by | AHD | LF-LAM testing uptake | Prevalence of TB | TB treatment initiation | Prevalence of mortality | Quality scores |
| Benzekri et al[7], 2019 | Senegal | Multiple | X-sect | Both | Both | 123 | 39.3 | 28.6 | 6 | ||
| Hassan et al[20], 2022 | Tanzania | Multiple | Cohort | Adults | Both | 728 | 5.0 | 5.9 | 8 | ||
| Heller et al[12], 2022 | Malawi | Single | X-sect | Adults | CD4 | 245 | 89.4 | 28.3 | 62.3 | 9 | |
| Mbewe et al[32], 2022 | Zambia | Single | X-sect | Adults | Both | 200 | 61.1 | 13.3 | 6 | ||
| Kanyama et al[13], 2022 | Zambia | Single | Cohort | Both | CD4 | 221 | 96.8 | 23.8 | 6 | ||
| Lukoye et al[33], 2020 | Uganda | Multiple | X-sect | Adults | Both | 2025 | 55.1 | 12.4 | 97.1 | 7 | |
| Mathabire et al[34], 2019 | Multiple1 | Multiple | X-sect | Adults | Both | 1555 | 96.8 | 9 | |||
| Wake et al[21], 2022 | South Africa | Multiple | Cohort | Adults | CD4 | 181 | 97.8 | 17.5 | 90.3 | 16.1 | 7 |
| Kalyango et al[35], 2022 | Uganda | Multiple | X-sect | Adults | Both | 301 | 90.0 | 67.2 | 91.8 | 8 | |
| Åhsberg et al[36], 2023 | Ghana | Multiple | Cohort | Adults | CD4 | 248 | 45.2 | 13.4 | 93.3 | 13.3 | 9 |
| Nyukuri et al[38], 2024 | Kenya | Multiple | Cohort | Adults | Staging | 214 | 49.1 | 60.0 | 7.9 | 8 | |
| Levy-Braide et al[39], 2023 | Nigeria | Multiple | X-sect | Adults | CD4 | 5487 | 78.0 | 34.1 | 67.8 | 7 | |
| Nakanwagi et al[40], 2022 | Uganda | Multiple | X-sect | Children | CD4 | 1310 | 11.0 | 30.5 | 85.5 | 8 | |
| Onwah et al[31], 2024 | Nigeria | Multiple | Cohort | Children | Both | 215 | 45.6 | 18.4 | 8 | ||
| Kingbo et al[30], 2024 | Cote d‘Ivoire | Multiple | Cohort | Adults | CD4 | 114 | 40.4 | 7 | |||
| Matoga et al[41], 2021 | Multiple2 | Multiple | C-trial | Adults | CD4 | 658 | 61.8 | 7.9 | 7.1 | 8 | |
| Izco et al[42], 2021 | Mozambique | Cohort | Both | Both | 97 | 69.9 | 7 | ||||
| Åhsberg et al[37], 2022 | Ghana | Multiple | Cohort | Adults | CD4 | 174 | 93.1 | 25.3 | 46.3 | 9 | |
| Eigege et al[43], 2024 | Nigeria | Multiple | X-sect | Both | Both | 1969 | 75.8 | 25.0 | 47.2 | 46.3 | 7 |
Assessment of study quality showed that 16 (84.2%) were of high quality, and 3 (15.8%) were of moderate quality. No study was of low quality (Table 1).
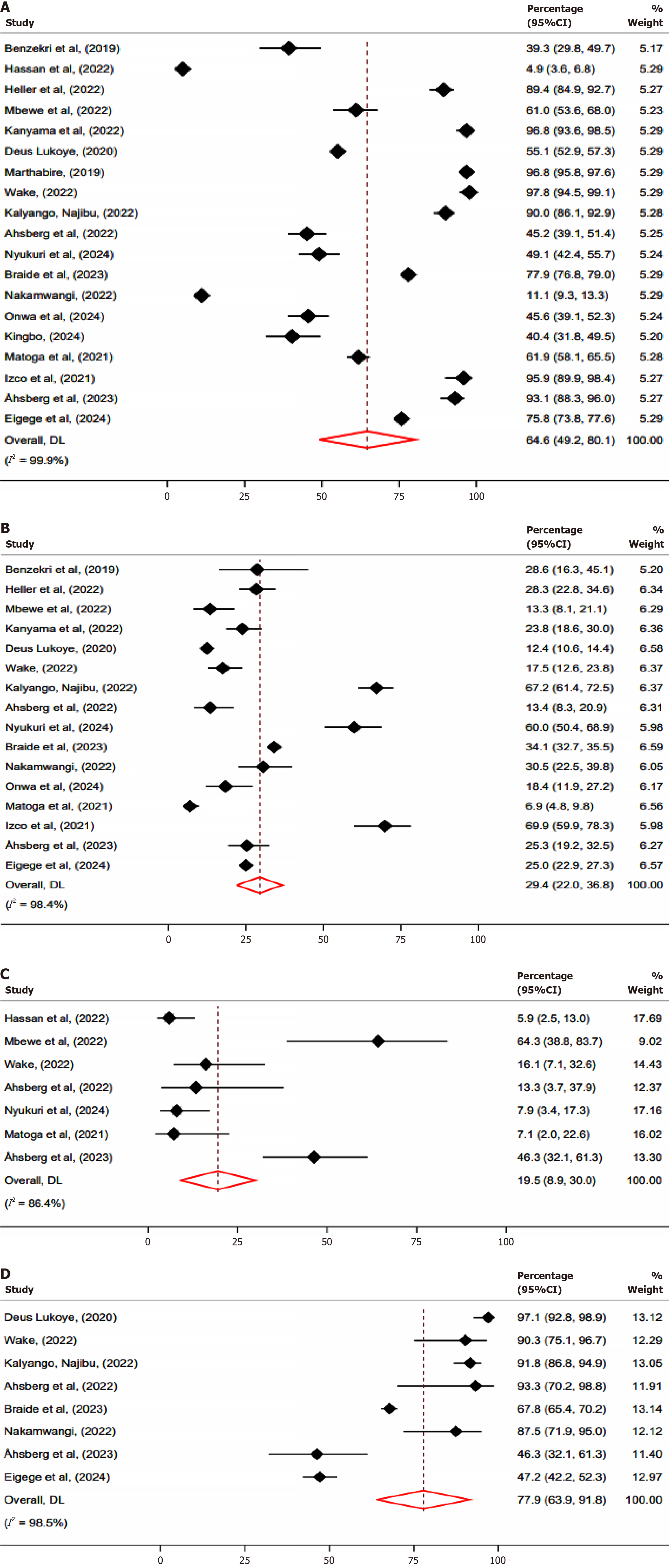
The meta-analysis assessed the prevalence of TB testing uptake, with the pooled prevalence of 64.6% (95%CI: 49.2–80.1) (Figure 2A), indicating that nearly only two-thirds of individuals eligible for TB testing were TB tested. A subgroup analysis based on population type revealed that children had a pooled testing uptake of 28.2% (95%CI: 5.6–62.0) compared to adults 61.3% (95%CI: 42.6–80.1), P value 0.003 (Supplementary Figure 1). Furthermore, testing uptake by study sites showed that multi-site studies had a pooled testing uptake of 58.8% (95%CI: 39.8–77.7) compared to 82.9% (95%CI: 67.6–98.1), among studies that involved single sites, (P = 0.002) (Supplementary Figure 2).
A meta-regression including year of publication, number of eligible participants, number of study site, study design, and criteria used to define advanced HIV disease did not identify any statistically significant predictors of screening uptake (all P > 0.05) (Supplementary Table 1), and residual heterogeneity remained high (I2 = 99.7%), suggesting that unmeasured contextual or implementation-related factors likely contribute to the variability in TB screening uptake.
Heterogeneity was observed in the meta-analysis (I2 = 99.9%), indicating substantial variability across studies. Egger’s test was conducted to assess potential publication bias, with results showing a beta coefficient of -6.64 (SE = 4.833), a z value of -1.37, and a P = 0.1698, suggesting no significant evidence of small-study effects. In addition, a trim-and-fill sensitivity analysis was performed to further assess potential publication bias. The analysis did not impute any missing studies, and the adjusted pooled effect size remained unchanged at 0.647 (95%CI: 0.515–0.778) (Supplementary Table 2), supporting the conclusion that small-study effects are unlikely to have influenced the findings.
Influential analysis was assessed using a leave-one-out sensitivity approach, where all studies had P values less than 0.001, indicating that no single study disproportionately influenced the pooled estimate of testing uptake (Supple
The meta-analysis estimated the pooled prevalence of TB at 29.4% (95%CI: 22.0–36.8) (Figure 2B), indicating slightly higher than a quarter of individuals were diagnosed with TB. A subgroup analysis by type of population showed that TB prevalence among children was 24.2% (95%CI: 12.4–36.1) compared to 27.6% (95%CI: 17.2–38.0) among adults, (P = 0.001) (Supplementary Figure 4). Furthermore, subgroup analysis by study site revealed that multi-site studies had a slightly higher pooled TB prevalence of 30.0% (95%CI: 21.6–38.5), compared to 21.9% (95%CI: 13.6–30.3) among studies that involved single-site (P = 0.001) (Supplementary Figure 5).
Heterogeneity was observed in the meta-analysis (I2 = 98.4%), indicating substantial variability across studies. Egger’s test was conducted to assess potential publication bias, yielding a beta coefficient of 3.82 (SE = 2.818), a z value of 1.36, and a
The meta-analysis estimated the pooled prevalence of mortality at 19.5% (95%CI: 8.9%–30.0%) (Figure 2C), indicating that nearly one in five individuals diagnosed with TB died. A subgroup analysis on mortality was not performed because there was only one study from a single site that reported mortality data.
Heterogeneity was observed (I2 = 86.4%), indicating variability across studies. Egger’s test was conducted to assess potential publication bias, yielding a beta coefficient of 5.08 (SE = 1.481), a z value of 3.43, and a P value of 0.0006, suggesting significant evidence of small-study effects. Influential analysis was assessed using a leave-one-out sensitivity approach, where all studies had P values less than 0.001, indicating that no single study disproportionately influenced the pooled estimate (Supplementary Figure 7).
The meta-analysis estimated that 77.9% (95%CI: 63.9%–91.8%) of those diagnosed with TB-had documentation of treatment initiations (Figure 2D). Heterogeneity was observed (I2 = 98.5%), reflecting considerable variability in the prevalence of treatment initiations across studies.
Generally, from the studies included in this analysis, there was no individual factors that were identified to be associated with the uptake of LF-LAM, however, several healthcare facility factors have been documented for the low uptake of LF-LAM. These included inconsistencies in measuring CD4 count to identify people with advanced HIV disease[32,34,38,40], lack of human resource for testing[13,36], and results interpretation[34], lack of testing kits and other commodities such as urine cups[38,39]. Increased awareness of systematic use of LF-LAM was associated with increased uptake of LF-LAM[30]
We conducted a systematic review and meta-analysis to understand compliance of the WHO guidelines in the management of patients with advanced HIV disease in Africa. Specifically, we summarized pooled estimate of those eligible for TB testing using LF-LAM. The pooled estimates of LF-LAM uptake were 65% with 28% among children and 66% among adults. Of those tested, 29% were diagnosed with TB and of these, 78% initiated treatment and 20% died. These data indicate gaps in the diagnosis of TB as well as significant disparities in TB testing for children compared to adults.
According to the 2024 Global TB report, number of deaths due to TB is nearly twice that caused by HIV/AIDS indicating how lethal is TB[44]. If diagnosed and treated accordingly, TB is a curable disease. Patients with HIV, particularly those with advanced disease, are at substantial risk of acquiring TB necessitating early diagnosis and treatment. Low uptake of TB testing among people with advanced HIV disease is concerning. Previous study showed that reasons for the low adoption of LF-LAM in the diagnosis of TB included country’s budgetary constraints, lack of country-specific data and piloting, delays of regulatory agency approval, lack of coordination between National TB and HIV programs, as well as perceived small population needing this service[45,46]. Other views of the low uptake of TB testing using urine LF-LAM included lack of personnel administering the tests[13], and concerns of test’s low sensitivity[21], restrictive eligibility criteria, reliance on CD4 testing, and lack of advocacy and awareness[22]. On the other hand, prior studies showed that LF-LAM is low cost, easy to use and its implementation is feasible[13,34] and useful in severely ill patients who cannot produce sputum or sputum scarce patients[21]. In addition, because TB testing using LF-LAM is a same day event, the technique reduces the number of clinic visits needed if the patient was to collect sputum that needs up to three clinic visits[47]. Collectively, the benefits of using LF-LAM for TB diagnosis outweigh its deficiencies and therefore investing on this diagnostic technology is critical for early detection and treatment of TB to reduce mortality associated with TB. We observed disparities in TB testing uptake using LF-LAM between studies involving children and adults, with lower uptake reported among children. These findings require additional studies to discern these disparities beyond the known general reasons for its low uptake. For example, children’s specific challenges such as difficulties of urine collection, performance variability may cause a low uptake of LF-LAM in children compared to adults. TB testing uptake was disproportionately higher in studies that involved single sites compared to those which involved multiple sites. As expected, most often, data from single site studies were from tertiary hospitals which are usually more resourceful compared to multiple sites studies that encompasses healthcare facilities of different tier[23].
Mortality among those diagnosed with TB is unacceptably high. Most of the patients presenting with advanced HIV are severely ill. Co-infection with TB further complicates their management. As previously narrated, one of the most common barriers in delaying seeking care among people living with HIV is stigma[1,3,4]. Because of these delays, patients present to healthcare facility with advanced HIV disease, often co-infected with opportunistic infections such as TB. In this era of universal access to antiretroviral therapy, addressing HIV related stigma will minimize proportion of people presenting with advanced HIV disease as well as mortality associated with these co-infections.
Nearly 78% of those diagnosed with TB have documentation of treatment initiations. Previous studies have docu
As stated previously, stigma against HIV impact people living with HIV and as a result some patients present late with advanced HIV disease. Poor immune status, advanced HIV-related disease, and the combined effects of both infections promote mortality and therefore, early HIV diagnosis would reduce mortality associated with TB in people living with HIV.
We acknowledge limitations of this systematic review. The study population involved people with advanced HIV disease based on the WHO definition. The variability of using either CD4 ≤ 200 or WHO stage III/IV to define advanced HIV disease potentially skewed pooled estimates, however, we could not exclusively distinguish which study used either criterion alone as most used both criteria. The overall uptake of LF-LAM may not be reflective of the actual uptake in Africa because of the use of data from small studies either from single or multiple sites. While using data from multiple sites encompasses healthcare facilities with different tiers, representing variations in resources and hence mimicking National data, the use of clinic auditing from the National databases would be an ideal to evaluate LF-LAM uptake. Adjusting for healthcare facility type would be more informative, however, inability to exclusively distinguish data from tertiary vs primary healthcare facilities limited the possibility to adjust for this factor in meta regression. Additionally, although meta-regression was conducted to explore sources of heterogeneity, the model explained only 5% of the between-study variance and none of the included variables were statistically significant. The high residual heterogeneity (I2 = 99.72%) suggests that unmeasured contextual or implementation-related factors likely contribute to the variability in TB screening uptake. Furthermore, only two studies reported uptake among children while thirteen studies reported uptake among adults. The small number of studies that reported children may under/overestimate the actual TB testing uptake among children. We reported TB treatment initiation, however, time to treatment initiation was not reported by majority of studies involved. Only 37% of the studies reported mortality data limiting our ability to get a clear overview of the mortality data for the entire population involved. In addition, we restricted our search to publications written in English, it is likely that other relevant publications from non-English journals were missed. The main strength of this research is the inclusion of 19 studies leadings to an overall large sample size to compute pooled estimates of LF-LAM testing uptake. This meta-analysis remains relevant as it is the first study that provides pooled estimates of the uptake of LF-LAM testing to understand the magnitude of compliance of WHO recommendations in the management of people presenting with advanced HIV disease in Africa.
We identified significant gaps in the diagnosis of TB using LF-LAM among people with advanced HIV disease in Africa. Our findings indicate suboptimal compliance of WHO guidelines in the management of people with advanced HIV disease. Mortality is high among those diagnosed with TB and to reduce mortality associated with TB, uptake of TB diagnosis should be heightened. As previously discussed, appropriate coordination between national TB and HIV programs must be strengthened. In country policies should consider investing in diagnostic tools, increase awareness, training of healthcare providers on the use of LF-LAM would improve its uptake, increase early detection and treatment and hence reduce mortality. Despite LF-LAM limitations, clinicians should have a high level of suspicion of TB diagnosis by considering factors such as prior history of TB and the presence of cough and night sweats in people living with HIV to increase TB diagnosis and treatment initiations. We recommend routine evaluation of national data on the compliance of these guidelines. These evaluations are critical to identify gaps and improve performance.
We acknowledged Emilie Ludeman for systematic search of the manuscripts and their compilation in the Covidence software.
| 1. | Belay GM, Endalamaw A, Ayele AD. Late presentation of HIV positive adults and its predictors to HIV/AIDS care in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Assen A, Molla F, Wondimu A, Abrha S, Melkam W, Tadesse E, Yilma Z, Eticha T, Abrha H, Workneh BD. Late presentation for diagnosis of HIV infection among HIV positive patients in South Tigray Zone, Ethiopia. BMC Public Health. 2016;16:558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Chone JS, Abecasis AB, Varandas L. Determinants of Late HIV Presentation at Ndlavela Health Center in Mozambique. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 4. | Lofgren SM, Tsui S, Natala N, Nakasujja N, Sebuliba R, Ndyetukira JF, Arinda A, Akinyange V, Hullsiek KH, Nalintya E, Sadiq A, Pastick KA, Stadleman A, Meya D, Boulware DR. Differences in Reasons for Late Presentation to HIV Care in Uganda Among Men and Women. AIDS Behav. 2023;27:303-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Drain PK, Losina E, Parker G, Giddy J, Ross D, Katz JN, Coleman SM, Bogart LM, Freedberg KA, Walensky RP, Bassett IV. Risk factors for late-stage HIV disease presentation at initial HIV diagnosis in Durban, South Africa. PLoS One. 2013;8:e55305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Kujawski SA, Lamb MR, Lahuerta M, McNairy ML, Ahoua L, Abacassamo F, Nuwagaba-Biribonwoha H, Gachuhi A, El-Sadr WM, Elul B. Advanced Human Immunodeficiency Virus Disease at Diagnosis in Mozambique and Swaziland. Open Forum Infect Dis. 2017;4:ofx156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Benzekri NA, Sambou JF, Ndong S, Tamba IT, Faye D, Diallo MB, Diatta JP, Faye K, Sall I, Sall F, Manga NM, Malomar JJ, Ndour CT, Hawes SE, Seydi M, Gottlieb GS. Prevalence, predictors, and management of advanced HIV disease among individuals initiating ART in Senegal, West Africa. BMC Infect Dis. 2019;19:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Lebelonyane R, Mills LA, Mogorosi C, Ussery F, Marukutira T, Theu J, Kapanda M, Matambo S, Block L, Raizes E, Makhema J, Lockman S, Bachanas P, Moore J, Jarvis JN. Advanced HIV disease in the Botswana combination prevention project: prevalence, risk factors, and outcomes. AIDS. 2020;34:2223-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Erjino E, Abera E, Lemma Tirore L. Time to Viral Load Suppression and Its Predictors Among Adult Patients on Antiretro Viral Therapy in Nigist Eleni Mohammed Memorial Comprehensive Specialized Hospital, Hossana, Southern Ethiopia. HIV AIDS (Auckl). 2023;15:157-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Palmer A, Gabler K, Rachlis B, Ding E, Chia J, Bacani N, Bayoumi AM, Closson K, Klein M, Cooper C, Burchell A, Walmsley S, Kaida A, Hogg R; Canadian Observational Cohort (CANOC) Collaboration. Viral suppression and viral rebound among young adults living with HIV in Canada. Medicine (Baltimore). 2018;97:e10562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Carmona S, Bor J, Nattey C, Maughan-Brown B, Maskew M, Fox MP, Glencross DK, Ford N, MacLeod WB. Persistent High Burden of Advanced HIV Disease Among Patients Seeking Care in South Africa's National HIV Program: Data From a Nationwide Laboratory Cohort. Clin Infect Dis. 2018;66:S111-S117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Heller T, Damba D, Kumwenda T, Huwa J, Kamamia C, Nhlema A, Wallrauch C, Chawinga C, Kanyama C, Gondwe-Chunda L, Ngoma J, Matanje B, Tweya H. Implementing Advanced HIV Disease Care for Inpatients in a Referral Hospital in Malawi - Demand, Results and Cost Implications. Ann Glob Health. 2022;88:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Kanyama C, Chagomerana MB, Chawinga C, Ngoma J, Shumba I, Kumwenda W, Armando B, Kumwenda T, Kumwenda E, Hosseinipour MC. Implementation of tuberculosis and cryptococcal meningitis rapid diagnostic tests amongst patients with advanced HIV at Kamuzu Central Hospital, Malawi, 2016-2017. BMC Infect Dis. 2022;22:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | The path that ends AIDS: UNAIDS Global AIDS Update 2023. Geneva: Joint United Nations Programme on HIV/AIDS. Available from: https://thepath.unaids.org/wp-content/themes/unaids2023/assets/files/2023_report.pdf. |
| 15. | Rajasingham R, Govender NP, Jordan A, Loyse A, Shroufi A, Denning DW, Meya DB, Chiller TM, Boulware DR. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis. 2022;22:1748-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 421] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 16. | World Health Organization. Tuberculosis and HIV. Available from: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/treatment/tuberculosis-hiv#:~:text=Tuberculosis%20(TB)%20remains%20the%20leading,million%20TB%20deaths%20that%20year. |
| 17. | World Health Organization. Global Tuberculosis report. Available from: https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf?sequence=1. |
| 18. | Lee-Rodriguez C, Wada PY, Hung YY, Skarbinski J. Association of Mortality and Years of Potential Life Lost With Active Tuberculosis in the United States. JAMA Netw Open. 2020;3:e2014481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Political declaration of the high-level meeting of the General Assembly on the fight against tuberculosis. Available from: https://www.un.org/pga/77/wp-content/uploads/sites/105/2023/09/TB-Final-Text.pdf. Accessed 6 - November. |
| 20. | Hassan FE, Senkoro M, Mnyambwa NP, Wilfred A, Molloy SF, Manisha H, Kivuyo S, Mfinanga SG. Implementation of WHO guidelines on management of advanced HIV disease and its impact among TB co-infected patients in Tanzania: a retrospective follow-up study. BMC Public Health. 2022;22:1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Wake RM, Govender NP, Omar SV, Ismail F, Tiemessen CT, Harrison TS, Jarvis JN. Rapid urine-based screening tests increase the yield of same-day tuberculosis diagnoses among patients living with advanced HIV disease. AIDS. 2022;36:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Mwaura M, Engel N. Constructing confidence: User perspectives on AlereLAM testing for tuberculosis. Int J Infect Dis. 2021;112:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Ally ZM, Mbishi JV, Mbwana MS, Bakari HM, Salim SM, Rodoshi ZN, Hundisa MI, Sileshi RM, Ayalew BD, Musoke R, Moshi L, Fakhoury YE, Ally HM, Ramadhani HO. Systematic review on the compliance of WHO guidelines in the management of patients with advanced HIV disease in Africa: The case of cryptococcal antigen screening. PLoS One. 2025;20:e0313453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 629] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 25. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 50909] [Article Influence: 10181.8] [Reference Citation Analysis (2)] |
| 26. | Higgins J, Thomas J, Chandler J, Cumpston M, Li TJ, Page M, Welch V. Cochrane Handbook for Systematic Reviews of Interventions. Available from: https://training.cochrane.org/handbook/current. |
| 27. | Munn Z, Moola S; Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. In J Evid-Based Healthc. 2015;13:147-153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 2018] [Article Influence: 183.5] [Reference Citation Analysis (0)] |
| 28. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48475] [Article Influence: 2107.6] [Reference Citation Analysis (4)] |
| 29. | Bakari HM, Alo O, Mbwana MS, Salim SM, Ludeman E, Lascko T, Ramadhani HO. Same-day ART initiation, loss to follow-up and viral load suppression among people living with HIV in low- and middle-income countries: systematic review and meta-analysis. Pan Afr Med J. 2023;46:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 30. | Kingbo MH, Bitty-Anderson A, Denoeud-Ndam L, Eboumou F, Mayi A, Nolna S, Kouadio MN, Diby CJ, Tiam A. Tiam. Improving identification and management of advanced HIV disease among people living with HIV in Cote d'Ivoire. Available from: https://www.iasociety.org/sites/default/files/AIDS2024/abstract-book/AIDS-2024_Abstracts.pdf. |
| 31. | Onwah O, Nwanja E, Toyo O, Akpan U, Unimuke M, Ezieke E, Okolo C, Nwangeneh C, Anika O, Onwuzuruigbo U, Oyawola B, Omo-Emmanuel K, Ogundehin D, James E, Obiora-Okafo C, Idemudia A, Nwadike C, Kakanfo K, Pius B, Onimode B, Asaolu O, Bashorun A, Gambo A, Pius J, Oyelaran O, Goldstein R, Onyedinachi O, Adegboye A, Eyo A. Improving uptake of tuberculosis testing using urine Lipoarabinomannan among children with advanced HIV disease: outcomes of a quality improvement initiative in southern Nigeria. Available from: https://www.iasociety.org/sites/default/files/AIDS2024/abstract-book/AIDS-2024_Abstracts.pdf. |
| 32. | Mbewe N, Vinikoor MJ, Fwoloshi S, Mwitumwa M, Lakhi S, Sivile S, Yavatkar M, Lindsay B, Stafford K, Hachaambwa L, Mulenga L, Claassen CW. Advanced HIV disease management practices within inpatient medicine units at a referral hospital in Zambia: a retrospective chart review. AIDS Res Ther. 2022;19:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Lukoye D. Scaling up TB LAM testing and linkage to TB treatment among ART Naïve HIV patients by the IDI in urban and rural Uganda. Available from: https://ug.usembassy.gov/wp-content/uploads/sites/42/Scaling-up-TB-LAM-testing-and-linkage-to-TB-treatment-among-ART-Na%C3%AFve-HIV-patients-by-the-IDI-in-urban-and-rural-Uganda_Dr.-Deus-Lukoye_CDC-Uganda.pdf. |
| 34. | Mathabire Rucker SC, Cossa L, Harrison RE, Mpunga J, Lobo S, Kisaka Kimupelenge P, Mandar Kol'Ampwe F, Amoros Quiles I, Molfino L, Szumilin E, Telnov O, Ndlovu Z, Huerga H. Feasibility of using Determine TB-LAM to diagnose tuberculosis in HIV-positive patients in programmatic conditions: a multisite study. Glob Health Action. 2019;12:1672366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Kalyango N. Uptake of urine lipoarabinomannan and associated factors in diagnosis of tuberculosis among patients with advanced HIV disease in selected Kampala Capital City Authority HIV clinic. Available from: http://makir.mak.ac.ug/handle/10570/11348. |
| 36. | Åhsberg J, Bjerrum S, Ganu VJ, Kwashie A, Commey JO, Adusi-Poku Y, Puplampu P, Andersen ÅB, Kenu E, Lartey M, Johansen IS. The in-hospital tuberculosis diagnostic cascade and early clinical outcomes among people living with HIV before and during the COVID-19 pandemic - a prospective multisite cohort study from Ghana. Int J Infect Dis. 2023;128:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Åhsberg J, Puplampu P, Kwashie A, Commey JO, Ganu VJ, Omari MA, Adusi-Poku Y, Andersen ÅB, Kenu E, Lartey M, Johansen IS, Bjerrum S. Point-of-Care Urine Lipoarabinomannan Testing to Guide Tuberculosis Treatment Among Severely Ill Inpatients With Human Immunodeficiency Virus in Real-World Practice: A Multicenter Stepped Wedge Cluster-Randomized Trial From Ghana. Clin Infect Dis. 2023;77:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 38. | Nyukuri L, Walata S, Alwang'a H, Muinde R, Canagasabey D, Thior I, Thoya J. Use of tuberculosis lipoarabinomannan to improve management of and avert mortalities among newly diagnosed people living with HIV with advanced HIV disease: Findings from Kakamega County, Kenya. Available from: https://plus.iasociety.org/e-posters/use-tuberculosis-lipoarabinomannan-tb-lam-improve-management-and-avert-mortalities-among. |
| 39. | Levy-Braide B, Otubu N, Abudiore O, Eigege W, Sowale O, Inyang A, Amamilo I, Rathakrishnan D, Moore A, Conroy J, Amole C, Lufadeju F, Wiwa O, Adesigbin C, Nwaokenneya P, Atu U, Patiko MI, A Ikpeazu A, Oladele R, Agbaji O, Akanmu S. Lessons Learned from the introduction of advanced HIV disease package of care in Nigeria. Available from: https://www.iasociety.org/sites/default/files/IAS2023/abstract-book/IAS_2023__Abstracts.pdf. |
| 40. | Nakanwagi M, Namuwenge P, Arinaitwe I, Mulowoza C, Ochora EN, Nazziwa E, Magongo EN. Namusoke Magongo. Low utilization of TB-LAM for TB screening among children under five with advanced HIV disease in Uganda: a descriptive analysis of surveillance data. Available from: https://www.aids2022.org/wp-content/uploads/2022/08/AIDS2022_abstract_book.pdf. |
| 41. | Matoga MM, Bisson GP, Gupta A, Miyahara S, Sun X, Fry C, Manabe YC, Kumwenda J, Cecilia K, Nyirenda M, Ngongondo M, Mbewe A, Lagat D, Wallis C, Mugerwa H, Hosseinipour MC. Urine Lipoarabinomannan Testing in Adults With Advanced Human Immunodeficiency Virus in a Trial of Empiric Tuberculosis Therapy. Clin Infect Dis. 2021;73:e870-e877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Izco S, Murias-Closas A, Jordan AM, Greene G, Catorze N, Chiconela H, Garcia JI, Blanco-Arevalo A, Febrer A, Casellas A, Saavedra B, Chiller T, Nhampossa T, Garcia-Basteiro A, Letang E. Improved detection and management of advanced HIV disease through a community adult TB-contact tracing intervention with same-day provision of the WHO-recommended package of care including ART initiation in a rural district of Mozambique. J Int AIDS Soc. 2021;24:e25775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Eigege W, LevyBraideB, Otubu N, Abudiore O, Sowale O, Inyang A, Rathakrishnan D, Conroy J, Amamilo I, Lufadeju F, Amole C, Wiwa O, Adesigbin C, Nwaokenneya P, Ikpeazu A, Oladele R, Agbaji O, Akanmu S. Improved tuberculosis case finding among patients with Advanced HIV Disease in Nigeria through the deployment of a point of care tuberculosis diagnostic test. Available from: https://www.aids2022.org/wp-content/uploads/2022/08/AIDS2022_abstract_book.pdf. |
| 44. | World Health Organization. Global tuberculosis report. Available from: https://iris.who.int/bitstream/handle/10665/379339/9789240101531-eng.pdf?sequence=1. |
| 45. | Singhroy DN, MacLean E, Kohli M, Lessem E, Branigan D, England K, Suleiman K, Drain PK, Ruhwald M, Schumacher S, Denkinger CM, Waning B, Van Gemert W, Pai M. Adoption and uptake of the lateral flow urine LAM test in countries with high tuberculosis and HIV/AIDS burden: current landscape and barriers. Gates Open Res. 2020;4:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Nathavitharana RR, Pai M. New strategies for inpatients with HIV and tuberculosis. Lancet. 2018;392:256-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Blok L, van den Hof S, Mfinanga SG, Kahwa A, Ngadaya E, Oey L, Dieleman M. Measuring workload for tuberculosis service provision at primary care level: a methodology. Hum Resour Health. 2012;10:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Floridia M, Ciccacci F, Andreotti M, Hassane A, Sidumo Z, Magid NA, Sotomane H, David M, Mutemba E, Cebola J, Mugunhe RJ, Riccardi F, Marazzi MC, Giuliano M, Palombi L, Mancinelli S. Tuberculosis Case Finding With Combined Rapid Point-of-Care Assays (Xpert MTB/RIF and Determine TB LAM) in HIV-Positive Individuals Starting Antiretroviral Therapy in Mozambique. Clin Infect Dis. 2017;65:1878-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Gils T, Madonsela T, Kamele M, Ayakaka I, Van Heerden A, Vlieghe E, Bresser M, Decroo T, Lynen L, Reither K, Bosman S. Low tuberculosis treatment initiation after positive tuberculosis lipoarabinomannan results. ERJ Open Res. 2024;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/