Published online Sep 25, 2025. doi: 10.5501/wjv.v14.i3.108405
Revised: May 4, 2025
Accepted: July 18, 2025
Published online: September 25, 2025
Processing time: 164 Days and 18.3 Hours
Lassa fever (LF) is a serious acute viral hemorrhagic illness that is endemic to West Africa where it affects an estimated two million people and results in up to 10000 deaths each year. The disease is caused by the Lassa virus (LASV), part of the Arenaviridae family, and is primarily transmitted through contact with urine or feces of infected Mastomys natalensis rodents. Human-to-human transmission, particularly in healthcare and community settings, further amplifies the risk of spread. Since its discovery in 1969, LF continues to be a neglected tropical disease with significant health impacts, especially in vulnerable populations such as pregnant females and those with weakened immune systems. The clinical spectrum of LF varies from mild, flu-like symptoms to severe complications including bleeding, brain inflammation, and multiple organ dysfunction with neonates and pregnant female showing the highest fatality rates. Accurate diagnosis is hindered by symptom overlap with common regional illnesses such as malaria and typhoid, underlining the urgent need for strengthened diagnostic infrastructure and rapid testing methods. While ribavirin remains the main antiviral treatment, its effectiveness depends heavily on early administration. Currently, no approved vaccine exists; however, promising candidates like vesicular stomatitis virus (VSV)ΔG-LASVGPC, INO-4500, and measles virus-based (MV)-LASV are undergoing preclinical and early-phase clinical evaluation, exhibiting encouraging immune responses in animal and human studies. A comprehensive strategy combining public health education, rodent control measures, robust infection prevention in clinical settings, and international cooperation in vaccine and drug research is essential to curb the impact of LF.
Core Tip: This minireview comprehensively examined Lassa fever, a neglected viral hemorrhagic disease endemic to West Africa and emphasized its virology, transmission dynamics, diagnostic challenges, and clinical management. We highlighted recent advances in vaccine development, including DNA-based and viral vector platforms, and called for integrated public health strategies, improved diagnostics, and international collaboration to reduce its growing health burden.
- Citation: Uppala PK, Karanam SK, Kandra NV, Edhi S. Lassa fever: A comprehensive review of virology, clinical management, and global health implications. World J Virol 2025; 14(3): 108405
- URL: https://www.wjgnet.com/2220-3249/full/v14/i3/108405.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i3.108405
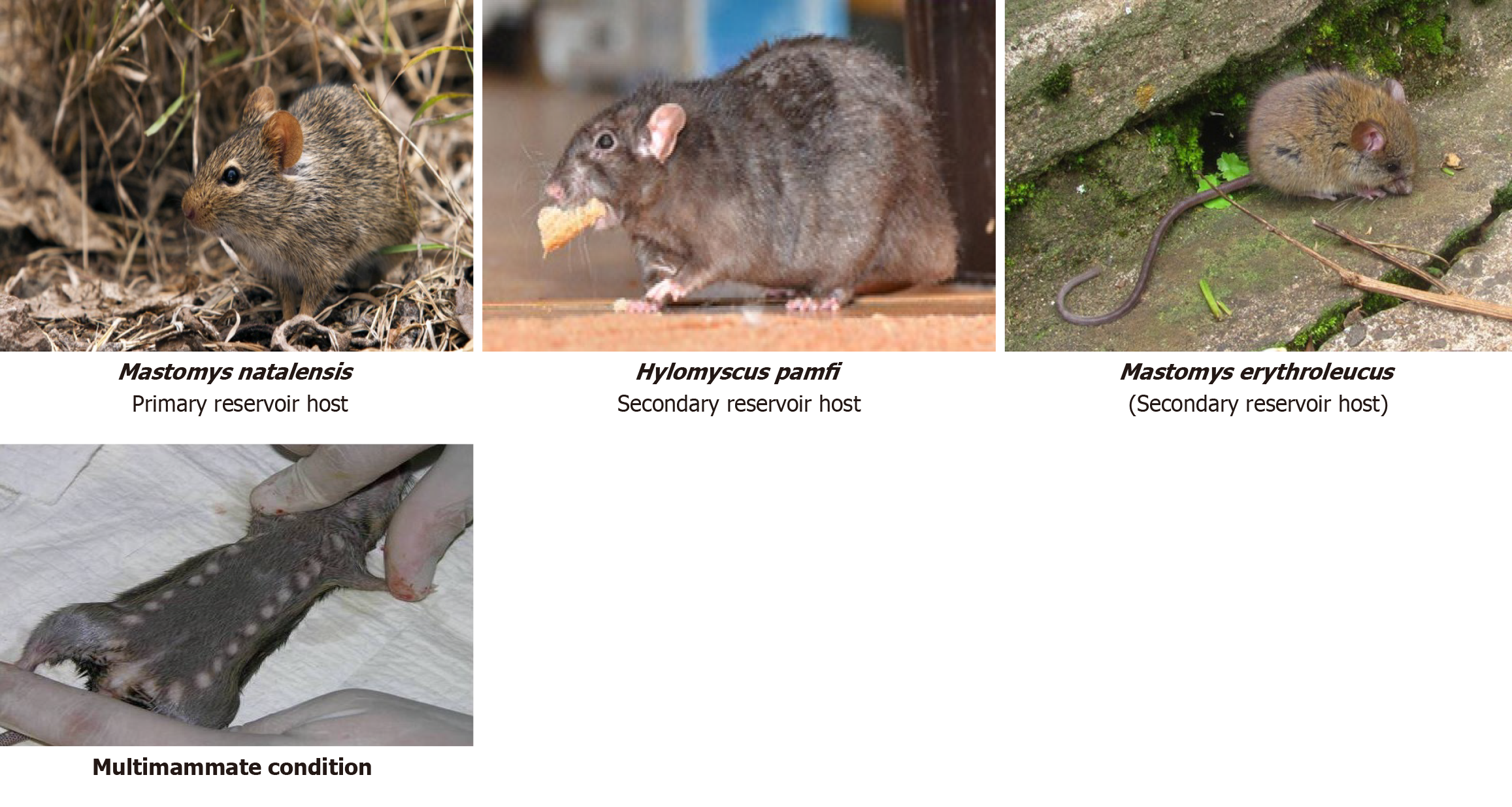
Lassa fever (LF) is a rodent-borne viral illness that remains a major public health concern, especially in West African regions where environmental conditions, poor infrastructure, and socioeconomic challenges support its ongoing transmission. The infection is caused by the Lassa virus (LASV), classified under old world arenaviruses, and is primarily acquired through exposure to food or household items tainted with the urine or feces of infected rodents. The principal animal host is Mastomys natalensis while Hylomyscus pamfi and Mastomys erythroleucus serve as secondary carriers[1]. These rodent species often inhabit human dwellings, increasing the likelihood of zoonotic transmission (Figure 1)[2].
These rodents play a critical role as the natural reservoirs of the LASV and have been utilized in scientific research since 1939. Mastomys natalensis, often referred to as the “multimammate rat”, gets its name from the female's numerous and visibly prominent mammary glands.
LASV was first detected in 1969 following an outbreak in the town of Lassa in Nigeria and has since become endemic in multiple West African nations, such as Sierra Leone, Liberia, Guinea, Benin, Togo, and Nigeria. Outbreaks tend to surge during the dry season from November to May, coinciding with heightened rodent activity and greater human exposure. However, the actual prevalence of the disease is likely underestimated due to diagnostic limitations, frequent misidentification, and the general similarity of symptoms to other febrile illnesses[3].
The World Health Organization (WHO) classifies LF as a high-priority pathogen because of its potential to cause outbreaks, significant mortality rates among hospitalized individuals, and the current lack of an approved vaccine for prevention. Sporadic exportation of cases to Europe and North America has raised global awareness of its potential for international spread[4]. This minireview synthesized current knowledge on the virology, epidemiology, clinical spectrum, diagnostics, management, prophylaxis, vaccine development pipeline, and global impact of LF, highlighting both progress and persisting gaps in addressing this neglected tropical disease, and emphasized the need for coordinated action and research.
LF presents a serious health risk to vulnerable groups such as newborns, young children, and pregnant females in West Africa with fatality rates reported as high as 75% in neonates and over 33% in expectant mothers. Ensuring the development of a safe, single-dose vaccine and the inclusion of these populations in clinical trials with strong safety protocols is essential for achieving effective disease prevention and management[5].
Emerging evidence indicates that environmental factors such as climate change, urbanization, and ecosystem degradation significantly influence LF transmission dynamics. Changes in temperature, rainfall patterns, and land use can expand the habitats of Mastomys natalensis and increase human-rodent interactions. Urban sprawl, deforestation, and agricultural expansion often drive rodents into closer proximity with human dwellings, heightening the risk of zoonotic spillover events. In addition poorly planned urbanization without adequate sanitation infrastructure facilitates rodent infestations in densely populated areas. Recognizing the role of environmental determinants is crucial for designing sustainable rodent control strategies and for forecasting future outbreaks. Integrating ecological surveillance and promoting environmentally sustainable urban planning should therefore form part of comprehensive LF control programs.
Understanding the ecology of LF requires a One Health approach that integrates environmental, animal, and human health data. Since Mastomys natalensis rodents serve as the natural reservoir for the LASV, environmental factors such as climate change, land use patterns, and agricultural practices directly influence disease transmission dynamics. Human encroachment into rodent habitats, poor sanitation, and food storage practices increase the likelihood of zoonotic spillover events. Incorporating surveillance data from rodent populations, environmental monitoring, and human health indicators would provide a more comprehensive epidemiological framework. Strengthening cross-sectoral collaboration between veterinary services, environmental agencies, and public health institutions is vital for the sustainable mana
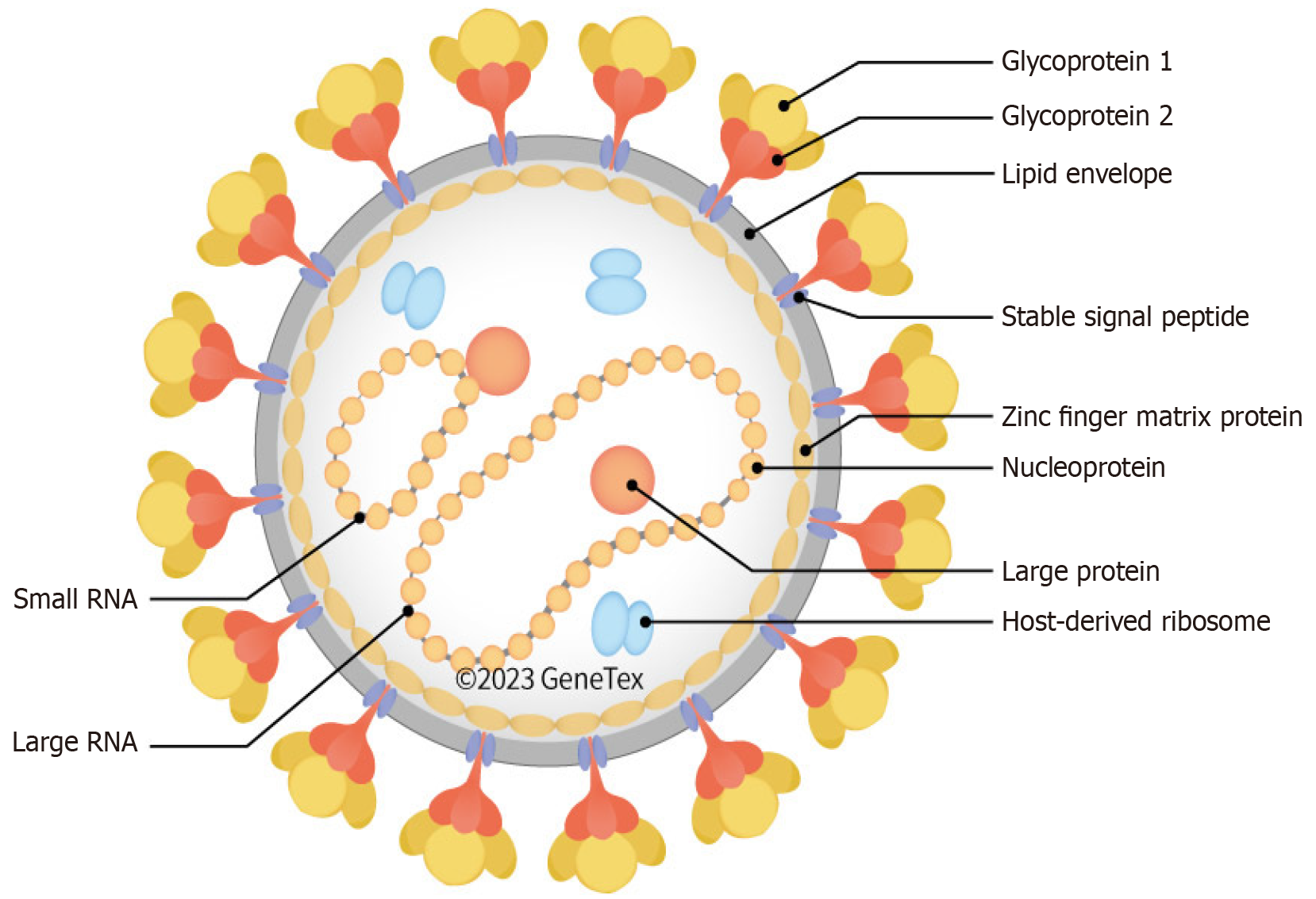
LASV possesses a single-stranded RNA genome divided into two segments, small and large, that utilize an ambisense coding mechanism to produce four essential proteins: (1) Nucleoprotein (NP); (2) Glycoprotein precursor (GPC); (3) RNA-dependent RNA polymerase; and (4) The matrix protein (Z) (Figure 2).
The NP plays a vital role in packaging the viral genome and helps the virus evade the host immune response by breaking down double-stranded RNA. The GPC is processed into GP1, GP2, and a stable signal peptide, which collectively enable the virus to enter host cells and mediate membrane fusion. The RNA-dependent RNA polymerase protein functions as the RNA polymerase, driving viral RNA replication, while the Z protein regulates virus assembly and release from the host cell[6].
LASV suppresses the host immune response through multiple mechanisms. NP inhibits interferon signaling by degrading viral RNA intermediates. The Z protein disrupts the RIG-I and mitochondrial antiviral signaling pathways. Additionally, LASV impairs dendritic cell maturation and antigen presentation, weakening T cell activation[7].
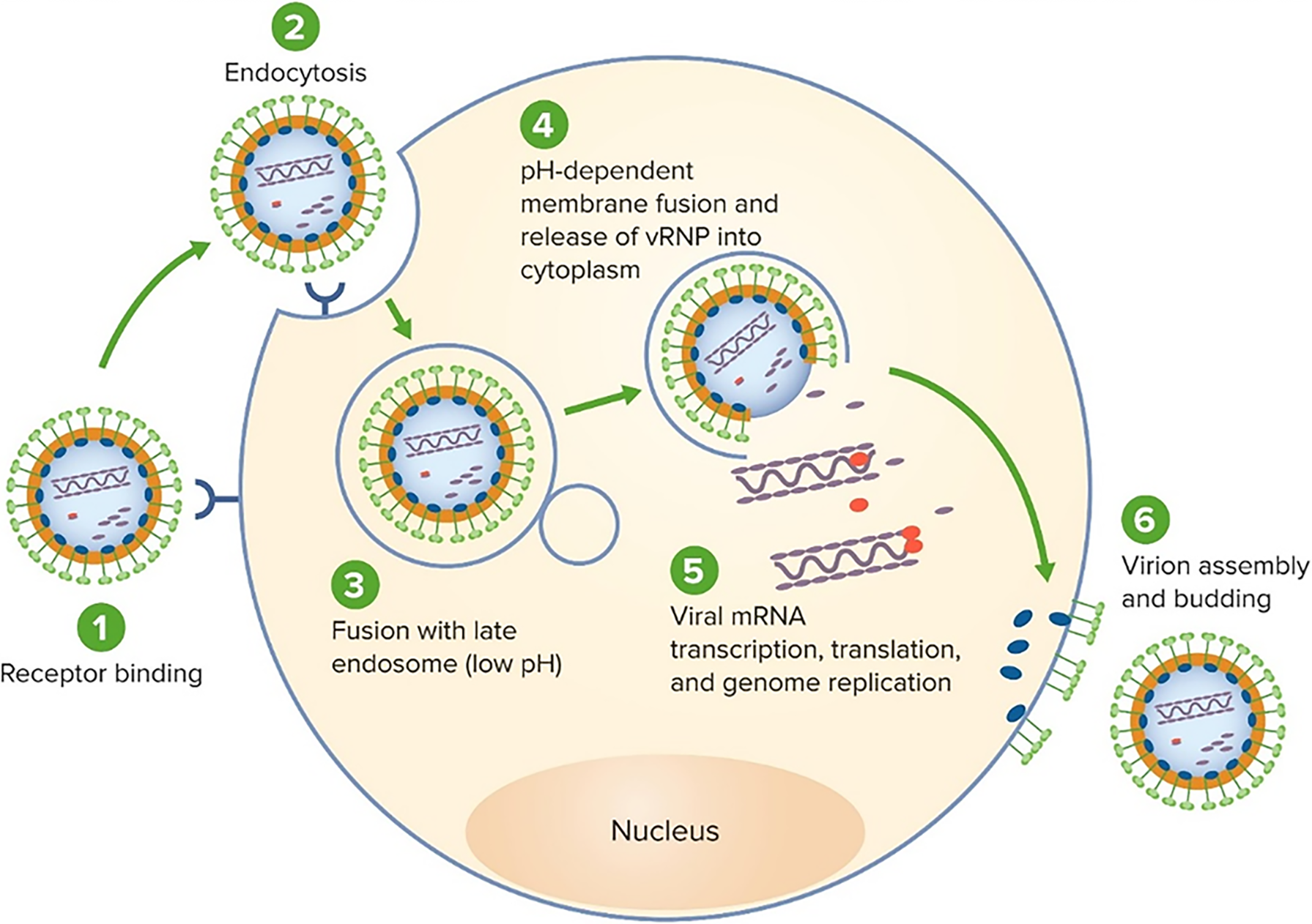
LASV enters host cells by binding to α-dystroglycan with LAMP1 enhancing viral fusion in endosomal compartments. The virus exhibits tropism for endothelial cells, hepatocytes, and macrophages, contributing to systemic inflammation, vascular leakage, and immune suppression (Figure 3).
LASV modulates host gene expression, inhibits apoptosis, and alters cellular pathways to favor viral replication. These interactions ensure viral persistence and facilitate immune evasion during infection[8].
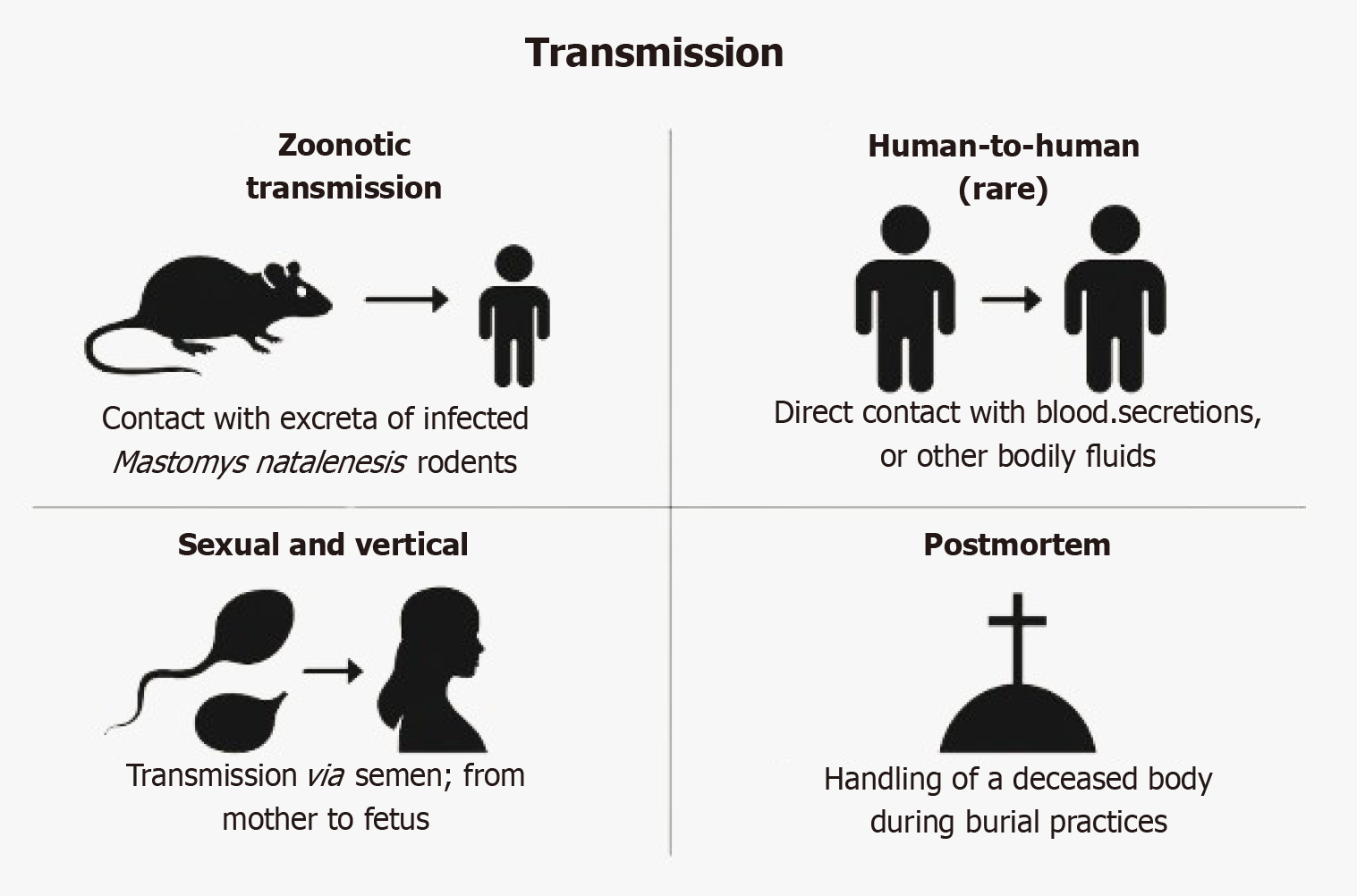
Initial transmission primarily results from contact with urine, feces, or saliva of Mastomys natalensis rodents, which frequently contaminate food and household surfaces particularly in rural settings.
LF can spread through direct exposure to the blood, bodily fluids, or secretions of infected individuals. This mode of transmission poses a serious risk in medical environments, especially where infection control measures are insufficient. The reuse of unsterilized medical equipment, such as needles, can further contribute to the spread of the virus.
While nosocomial transmission remains a critical concern, community-based spread of LF also significantly contributes to outbreak persistence. Household clusters often emerge when family members care for infected individuals without proper protective measures, unknowingly facilitating virus transmission through direct contact with body fluids. Informal caregiving practices, common in rural and underserved areas, usually lack awareness of infection control protocols such as hand hygiene, disinfection, and use of basic barriers. Additionally, shared living spaces, limited sanitation infrastructure, and cultural norms promoting physical caregiving during illness further amplify the risk of secondary infections within families and close-knit communities. Addressing these community-level transmission dynamics requires targeted health education campaigns, distribution of basic hygiene supplies, and empowering households with practical knowledge on infection prevention and early isolation practices.
Sexual transmission has been reported due to virus persistence in semen. Vertical transmission can result in miscarriage or stillbirth. Cultural burial practices and environmental conditions also contribute to sustained transmission (Figure 4). Understanding these transmission routes is key for prevention, particularly in community education and healthcare protocols[9,10].
LF exhibits a broad spectrum of clinical symptoms, which progress through distinct stages, ranging from mild flu-like illness to life-threatening complications[11,12]. Table 1 summarizes the major clinical phases, symptoms, and risk considerations.
| Stage | Duration | Symptoms | Description |
| Incubation period | Days 2-21 | Typical asymptomatic | Virus is replicating; no external signs |
| Early stage | Days 1-6 | Fever, sore throat, muscle aches, chest pain, vomiting, diarrhea | Resembles malaria or typhoid; diagnostic confusion common |
| Progression to severe | Days 7-14 | Facial swelling, mucosal bleeding, pleural/pericardial effusion, hypotension, respiratory distress | Indicates systemic involvement and vascular leakage |
| Neurological phase | Advanced (≥ day 10) | Confusion, seizures, encephalopathy | May progress to multiorgan failure |
| Complications | Variable | Hearing loss (up to 30% of survivors), myocarditis, chronic fatigue, spontaneous abortion (pregnancy) | Hearing loss may be permanent; pregnant females are at very high risk |
| Case fatality risk | Severe cases | 15%-20% in hospitalized patients | Higher in late-presenting cases and pregnant females |
| Recovery phase | After 2-3 weeks | Weakness, fatigue, dizziness, depression | Long recovery; some may need audiological or psychological follow-up |
Prompt recognition of LF significantly improves patient outcomes and limits the potential for community or nosocomial spread. Given the nonspecific nature of early symptoms, especially in endemic areas, healthcare providers must maintain a high index of suspicion. Early laboratory diagnosis and timely antiviral therapy are crucial to reduce mortality. Con
Diagnosing LF[14,15] remains a significant challenge due to its nonspecific symptoms, which often resemble those of malaria, typhoid, or other viral hemorrhagic fevers[16-19]. As a result, laboratory confirmation is essential to ensure accurate diagnosis, initiate appropriate treatment, and contain outbreaks. Various diagnostic modalities have been developed, each with unique advantages and limitations as summarized below (Table 2).
| Method | Type | Sample | Turnaround time | Advantages | Limitations |
| Reverse transcription PCR | Molecular | Blood or tissue | Few hours | Highly sensitive and specific; early detection | Requires specialized labs and trained personnel |
| ELISA (IgM, IgG, antigen) | Serological | Serum or plasma | 1-2 days | Useful for acute and retrospective diagnosis | Limited sensitivity in early stages |
| Virus isolation | Culture (definitive) | Blood or tissue | Several days | Confirmatory; research reference standard | Requires Biosafety Laboratory-4 facility; high biosafety risk |
| Next-generation sequencing | Genomic surveillance | Blood or tissue | Variable | Tracks evolution and strain diversity | Expensive and technology-intensive |
| Rapid diagnostic tests | Immunochromatography | Blood sample | 15-30 min | Fast, suitable for remote settings | Still under development and validation |
| Supportive laboratory indicators | Clinical chemistry | Blood, urine | Few hours | Suggestive (e.g., elevated aspartate aminotransferase, thrombocytopenia) | Not confirmatory; supportive only |
In addition to deploying accurate diagnostic tools, strengthening laboratory infrastructure and enhancing training for healthcare personnel are critical components of effective disease management and outbreak control in endemic regions.
Accurate and timely diagnosis of LF remains a substantial challenge, particularly in endemic and resource-limited settings. Although several diagnostic methods are available, each has practical limitations affecting their real-world effectiveness.
Reverse transcription PCR is the gold standard for early detection due to its high sensitivity and specificity; however, it demands well-equipped laboratories, cold-chain storage for reagents, and trained personnel and is largely inaccessible in rural areas where LF is most prevalent. In addition logistical barriers like sample transport delays may compromise results.
Serological assays such as ELISA (IgG, IgM, and antigen detection) are useful for both acute and retrospective diagnosis. However, ELISA tests may yield false negatives during early infection phases when antibodies have not yet formed or false positives due to cross-reactivity with other endemic viruses.
Rapid diagnostic tests, though promising for point-of-care screening, currently suffer from limited sensitivity and specificity. High rates of false positives or negatives can lead to misdiagnosis, unnecessary treatments, or missed cases, particularly during outbreaks. Moreover, the acceptance of rapid diagnostic tests in local communities may be hindered by distrust, cost concerns, and lack of awareness about the importance of early diagnosis.
In terms of logistics many diagnostic platforms still require cold-chain maintenance, a significant hurdle in remote regions lacking stable electricity. The cost of advanced diagnostic methods is often prohibitive for widespread deploy
Addressing these diagnostic challenges is vital to strengthening surveillance, improving outbreak response, and ultimately reducing LF morbidity and mortality. Investment in decentralized, low-cost, and community-accepted diagnostic solutions remains a critical priority.
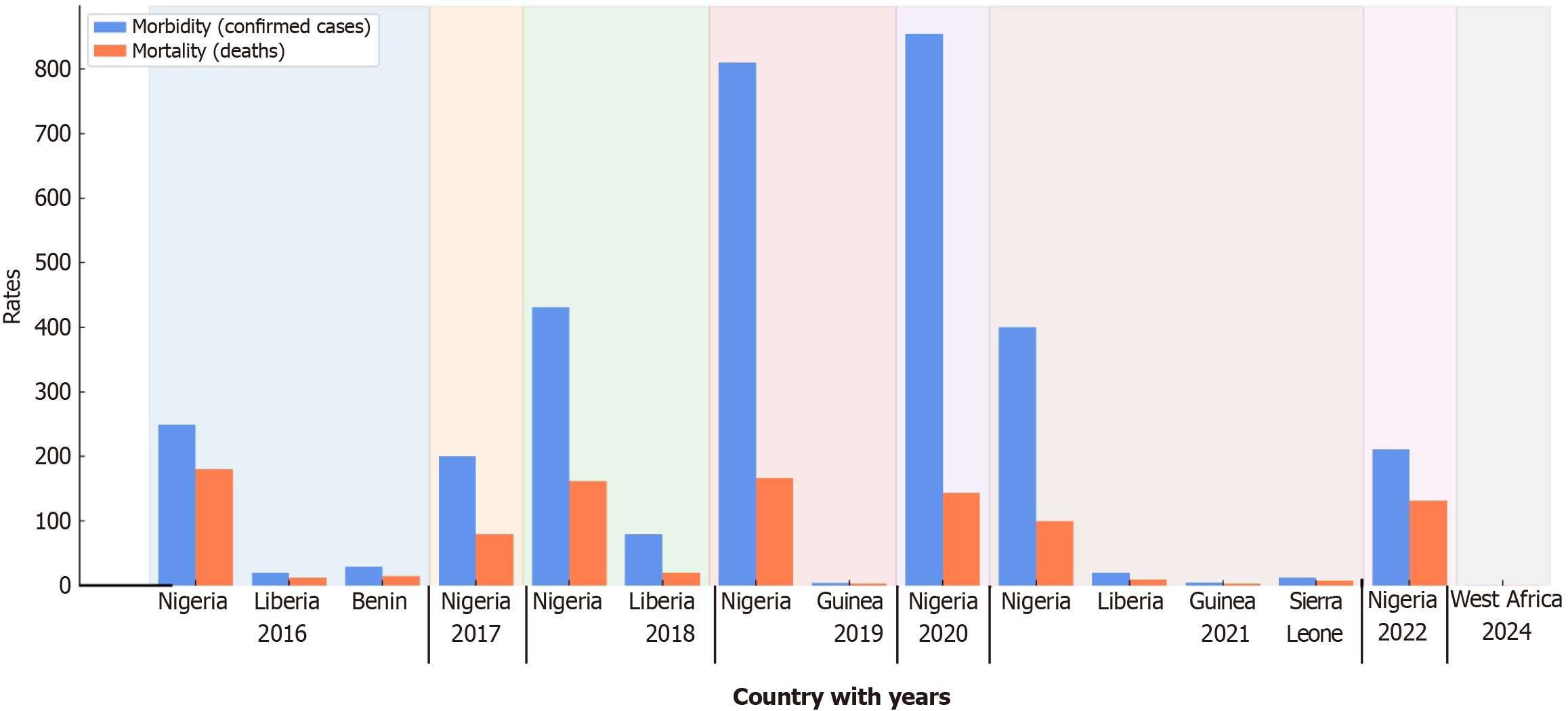
Visual representation of LF endemic regions through epidemiological maps provides critical insights into the distribution and intensity of outbreaks. Geospatial analyses have identified consistent hotspots across Nigeria, Sierra Leone, Liberia, and Guinea, with emerging risks noted in neighboring countries like Benin, Togo, and Côte d’Ivoire. Mapping rodent reservoir distributions alongside human case data allows for the prediction of at-risk zones, especially as environmental and socioeconomic factors evolve. Incorporating geospatial tools in surveillance can improve early outbreak detection, optimize resource allocation, and guide targeted public health interventions. Future research should prioritize high-resolution mapping and cross-border data sharing to enhance preparedness strategies in both endemic and non-endemic regions (Figure 5)[20].
The management of LF requires a comprehensive approach combining antiviral therapy, supportive care, management of complications, specialized care for high-risk groups, and long-term follow-up. Early treatment, especially within 6 days of symptom onset, significantly reduces mortality (Table 3).
| Component | Objective | Key interventions |
| Antiviral therapy | Inhibit viral replication | Intravenous ribavirin (ideally within first 6 days); oral ribavirin for mild or remote settings |
| Supportive care | Stabilize and support vital functions | Intravenous fluids and electrolyte balance; nutritional support; oxygen therapy; antipyretics; blood transfusions |
| Severe case management | Manage complications and prevent multiorgan failure | Antibiotics for secondary infections; dialysis for renal failure; ventilation support; seizure control |
| Pregnancy-specific care | Reduce maternal and fetal mortality | Specialized obstetric care; consider therapeutic abortion in advanced pregnancy with severe infection |
| Post-recovery care | Promote long-term recovery and quality of life | Audiology screening and support; psychological counseling; physical rehabilitation and follow-up |
Ribavirin remains the primary antiviral but is time-sensitive with reduced effectiveness if started late. Recent advances include favipiravir (an RNA polymerase inhibitor) and promising monoclonal antibody therapies (e.g., LHF-535, LASV-specific cocktails), which have shown significant protective efficacy in preclinical studies even at later disease stages. Combination therapies are under investigation to enhance outcomes further.
Supportive care focuses on maintaining hydration, oxygenation, nutrition, and controlling fever. In severe cases, antibiotics for secondary infections, dialysis for renal failure, and ventilatory support are crucial. Pregnant females require urgent and specialized care due to the high maternal and fetal fatality; therapeutic abortion may be considered in severe infections.
Post-recovery care is essential to address complications such as hearing loss, neurological sequelae, and psychological distress. Rehabilitation services, such as audiology, counseling, and physiotherapy, help survivors regain quality of life[21,22].
Accurate media communication during LF outbreaks is vital to reduce stigma and encourage early treatment-seeking. Poor reporting can fuel fear and discrimination. Survivors often face socioeconomic challenges such as job loss and social exclusion, highlighting the need for psychosocial support and reintegration programs alongside medical care.
Ongoing research into novel antivirals and immunotherapies remains vital for improving treatment accessibility and outcomes in endemic regions. Although ribavirin remains the mainstay treatment for LF, its efficacy is time-dependent and often limited. Alternative therapeutics are gaining attention with favipiravir demonstrating superior protection compared with ribavirin in animal models even with delayed administration. Combination therapies, such as ribavirin with favipiravir, have shown synergistic effects, enhancing survival outcomes.
Monoclonal antibody therapies targeting LASV glycoproteins have also emerged as promising interventions, offering broad cross-lineage protection and effectiveness even when administered during advanced disease stages. Additionally, host-targeted therapies like CC-90009, which degrades the cellular protein GSPT1, have shown potent antiviral effects in preclinical studies. Other repurposed entry inhibitors such as lacidipine and phenothrin further expand the therapeutic landscape.
These advances highlight a shift toward combination regimens and host-directed strategies, underscoring the need for diversified and effective treatment options beyond conventional antivirals.
LF disproportionately impacts pregnant females with maternal and fetal mortality rates significantly higher compared with the general population. Despite this heightened vulnerability healthcare interventions often fail to adequately address the specific needs of females, particularly during pregnancy. Specialized obstetric care, early diagnosis, and tailored antiviral treatment protocols are crucial to improving outcomes. Moreover, females in endemic regions often serve as primary caregivers, further increasing their exposure risk within households and communities. Gender-sensitive health policies, including prioritization of pregnant females in clinical trials, expanded maternal health services during outbreaks, and culturally appropriate health education programs, are essential components for an effective and equitable response to LF.
Effective prevention of LF relies on a coordinated approach combining public health education, environmental mana
Rodent control remains a cornerstone strategy. Efforts should focus on reducing Mastomys natalensis populations by eliminating food sources and hiding places, maintaining structural barriers in homes, promoting safe trapping methods, and discouraging bush burning and rodent consumption. At the household and community levels, hygiene practices such as secure food storage, clean environments, and proper waste disposal are critical[23,24].
Traditional rodent control strategies, such as chemical rodenticides, pose significant environmental and human health risks, particularly in fragile ecosystems common to LF-endemic regions. Therefore, innovative, community-driven, and sustainable approaches are needed. Community engagement programs promoting household hygiene, use of rodent-proof storage containers, safe food handling practices, and environmental sanitation can reduce rodent-human interactions without chemical dependence. Ecological rodent control methods, such as habitat modification (clearing vegetation around homes, sealing entry points), and encouraging natural predators like owls, have shown promise. Integrating rodent surveillance programs into local public health initiatives can also help monitor population dynamics and identify hotspots for targeted interventions. These participatory, low-cost, and environmentally safe strategies can empower communities and provide a longer-term, sustainable solution to reducing LASV transmission risk.
In healthcare settings strict infection control measures must be implemented, including patient isolation, the use of personal protective equipment (PPE), sterilization of medical equipment, and specialized care for high-risk groups like pregnant females. Strengthening diagnostic infrastructure, enhancing surveillance systems, and investing in vaccine and therapeutic development are vital for long-term control.
However, the successful implementation of these strategies faces significant barriers. Entrenched poverty limits access to safe housing and sanitation while low literacy rates hinder public understanding of preventive measures. In conflict-affected or politically unstable regions, healthcare access and community outreach are further compromised, making routine surveillance and response activities difficult. Addressing these challenges requires tailored community engagement, inclusive education strategies, resource allocation, and the involvement of local leaders to build trust and ensure intervention success.
Sociocultural practices in endemic regions play a significant role in sustaining LF transmission and often pose challenges to control measures. Traditional healing practices, which may involve close contact with bodily fluids of symptomatic individuals, can accelerate person-to-person transmission in community settings. Additionally, customary funeral rites that involve washing, touching, or preparing the bodies of deceased individuals without protective measures further heighten the risk of virus spread. In many rural areas strong cultural reliance on traditional healers over formal healthcare facilities delays timely diagnosis and treatment. Low literacy rates, distrust of public health interventions, and deeply rooted beliefs about disease causation can also hinder vaccination campaigns and outbreak response efforts. Therefore, integrating culturally sensitive health education, involving community leaders, and respecting local traditions while promoting safe practices are essential strategies to improve the acceptance and effectiveness of public health measures against LF (Table 4).
| Level | Key strategies |
| Community level | Promote hygiene and rodent-proof food storage; educate communities through culturally appropriate materials; improve waste management practices; discourage handling and consumption of rodents |
| Healthcare level | Mandate personal protective equipment use; ensure patient isolation; train healthcare workers in infection prevention; sterilize equipment; provide obstetric care for pregnant females |
| National level | Strengthen disease surveillance; expand laboratory networks; integrate Lassa fever control into national health policies; ensure emergency preparedness |
| International level | Support cross-border data sharing; invest in vaccine and therapeutic research; collaborate with global health organizations (e.g., World Health Organization, Coalition for Epidemic Preparedness Innovations); improve access to rapid diagnostics and antivirals |
Although LF remains predominantly endemic to West Africa, its global health significance is steadily increasing due to expanding travel, migration, and international trade. Cases of LF have been sporadically reported in non-endemic countries, including the United States, United Kingdom, Germany, and the Netherlands, often through travelers returning from endemic regions. These imported cases highlight the potential for international transmission and the need for heightened global surveillance.
In non-endemic regions, the rarity of LF poses diagnostic challenges, often resulting in delayed identification and management. Healthcare systems outside West Africa must maintain awareness and preparedness to recognize potential cases promptly, especially in travelers presenting with febrile illness. Early isolation, strict infection control measures, and laboratory diagnostic capabilities are critical to prevent secondary transmission in healthcare and community settings.
The WHO classifies LF as a priority disease requiring urgent research and development of countermeasures, including vaccines, therapeutics, and rapid diagnostic tools. Strengthening international collaboration is vital, particularly for resource-sharing, capacity building in diagnostics, and response preparedness in non-endemic regions.
Additionally, global public health efforts should focus on educating travelers, improving screening protocols at points of entry, and integrating LF into broader pandemic preparedness frameworks. Research into the survivability of LASV under varying environmental conditions and modeling potential outbreak scenarios outside Africa are also essential to anticipate and mitigate future risks.
The global relevance of LF is growing with increased travel and trade. Imported cases have been documented in non-endemic countries, emphasizing the need for awareness among healthcare professionals worldwide.
Although no vaccine is currently available, several candidates are in clinical development. These include recombinant VSV-based and DNA-based platforms. International partnerships are vital to accelerate vaccine readiness and therapeutic innovations[24].
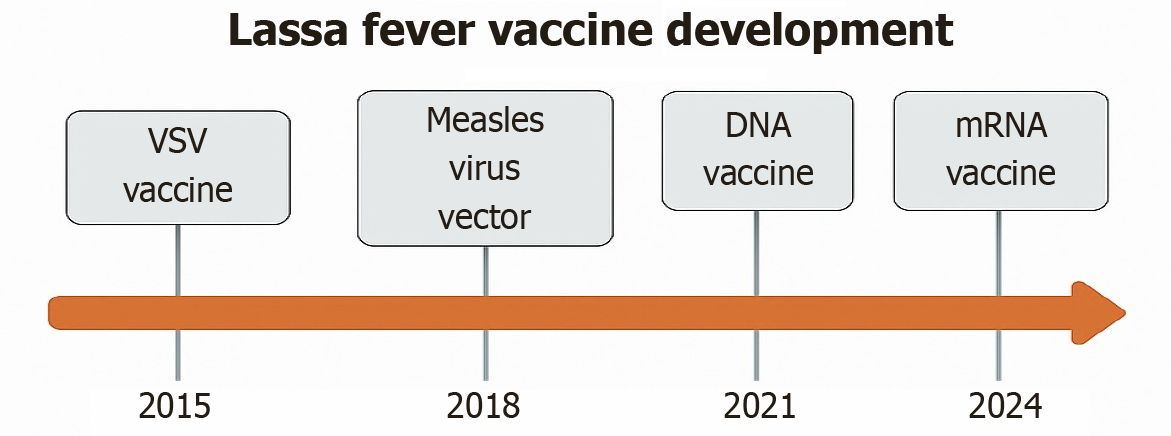
LF vaccine research has witnessed notable advancements over recent years, leveraging diverse platforms including DNA-based, viral vector, live-attenuated, mRNA-based, and nanoparticle-based technologies. Among the leading candidates are the VSV-based (VSVΔG-LASVGPC) vaccine and the INO-4500 DNA vaccine, which have progressed to phase I/II clinical trials and demonstrated strong humoral and cellular immune responses in animal models and early human studies[25]. Additionally, the MV-LASV and adenovirus-vectored (known as ChAdOx1-LASV-GPC) vaccines have shown promise, particularly in offering cross-clade protection critical for the genetically diverse LASV strains circulating across West Africa.
Emerging platforms such as mRNA and nanoparticle-based vaccines, while scientifically promising, face practical challenges for large-scale deployment in endemic settings. These include the need for ultra-cold storage conditions, high production costs, and the requirement for trained personnel for vaccine delivery, limiting immediate feasibility in low-resource environments. Addressing these barriers through thermostable formulations, simplified dosing schedules, and context-specific delivery models is essential to ensure equitable vaccine access and acceptance in affected communities.
Moreover, ethical considerations in vaccine trial designs have become increasingly important, particularly for vulnerable populations such as pregnant females, neonates, and children who experience disproportionately high fatality rates from LF. Historically excluded from early-phase studies, these populations must now be prioritized through ethically sound, carefully monitored trials that balance risk mitigation with the urgent need for protection. Tailored informed consent processes, rigorous safety surveillance, and long-term follow-up mechanisms must be integral components of trial designs to uphold ethical standards while closing critical gaps in vaccine coverage.
Continued investment, collaborative partnerships, and a One Health-centered approach that integrates human, animal, and environmental health perspectives are vital to advance vaccine development and deployment efforts effectively (Figure 6). Table 5 summarizes key vaccine candidates, highlighting their platforms, clinical stages, immune responses, and model-based protection efficacy.
| Vaccine platform | Stage | Immune response | Protection (models) |
| VSV-based (VSVΔG -LASVGPC) | Phase I/II | Strong humoral and T cell | Guinea pigs, NHPs |
| Reassortant ML29 | Preclinical | IgG, IFN-γ T cells | Guinea pigs, NHPs |
| DNA-based (INO-4500) | Phase I | IgG, neutralizing Abs | Guinea pigs, NHPs |
| Measles virus vector (MV-LASV) | Phase I | CD4/CD8 T cells, IgG | NHPs |
| Vaccinia virus vector | Preclinical | Mixed | Guinea pigs, NHPs |
| ChAdOx1 adenovirus vector | Preclinical | T cell and IgG | Mice, Guinea pigs |
| mRNA-based | Preclinical | Fc-mediated + T cell | Guinea pigs, mice |
| Yellow fever 17D vector | Preclinical | IgG | Guinea pigs |
| VEEV RNA replicon | Preclinical | T cell mediated | Guinea pigs |
| Rabies virus vector | Preclinical | ADCC, IgG | Guinea pigs, NHPs |
| Protein nanoparticle | Preclinical | Neutralizing Abs | Guinea pigs |
| Virus-like particles | Preclinical | Neutralizing Abs | Rabbits |
The management and control of LF continue to be hindered by a combination of clinical, infrastructural, epidemiological, and socioenvironmental challenges. One of the foremost issues is the nonspecific early presentation of the disease, often resembling other febrile illnesses like malaria, typhoid, and dengue, leading to frequent misdiagnosis, particularly in rural and remote settings with limited diagnostic capabilities[26]. This diagnostic delay not only worsens individual outcomes but also sustains community transmission.
The high virulence and case fatality rates, especially among pregnant females and neonates, pose significant chal
Efforts to control the rodent vector, Mastomys natalensis, are complicated by the proximity of the rodents to human dwellings, particularly in impoverished areas. Biological control measures, such as using natural predators like cats, carry ecological risks, while chemical control strategies face logistical and safety challenges.
Survivors of LF often suffer long-term sequelae including sensorineural hearing loss, chronic fatigue, and psychological trauma, yet rehabilitation services in endemic regions are severely limited[28]. Public health preparedness remains constrained by a lack of approved vaccines, insufficient availability of antivirals like ribavirin, and limited access to rapid diagnostic tools, especially in rural areas.
Community stigma, fear, and insufficient awareness among healthcare providers further delay diagnosis and care, compounding the impact of outbreaks. With increasing global travel imported cases in non-endemic countries highlight the urgency for international disease surveillance, rapid diagnostic capacity, and clinical preparedness[29].
Short-term priorities (immediate action 0-2 years): (1) Enhance diagnostic capabilities. Equip primary healthcare centers in endemic areas with rapid diagnostic kits and train personnel in early recognition and management; (2) Strengthen infection control. Mandate the consistent use of PPE, implement strict infection control guidelines, and ensure supply chain resilience during outbreaks; (3) Community education. Launch widespread public health campaigns tailored to low-literacy populations, addressing myths, stigma, and the importance of early medical intervention; and (4) Support for HCWs. Provide ongoing training, psychological support, and hazard pay to HCWs involved in LF care.
Mid-term priorities (2-5 years): (1) Rodent control programs. Implement sustainable rodent control interventions combined with housing improvement initiatives to reduce human-rodent interactions; (2) Survivor rehabilitation programs. Establish dedicated centers for audiological rehabilitation, mental health support, and long-term follow-up of survivors of LF; and (3) Clinical trials acceleration. Expand ethically sound vaccine and therapeutic trials to include high-risk populations like pregnant females and children.
Long-term priorities (5-10 years): (1) Integrated One Health surveillance. Develop multisectoral surveillance systems that integrate human, animal, and environmental health data for early outbreak detection and control; (2) Vaccine deployment infrastructure. Invest in cold-chain systems, community vaccine education programs, and equitable distribution models to ensure successful vaccine rollout; and (3) Global coordination. Foster regional and international partnerships for cross-border outbreak management, data sharing, and funding for vaccine research and public health infrastructure.
By following a layered strategy combining urgent clinical interventions, public health education, infrastructure strengthening, and global collaboration, stakeholders can move towards the sustainable control and eventual elimination of LF as a major public health threat. While LF continues to exert its heaviest toll in West Africa, its implications extend far beyond regional borders. A coordinated, global approach involving surveillance, education, research, and vaccine development is critical to addressing both the current burden and the emerging threats posed by LF worldwide. Without a coordinated and sustained effort across all levels, from household to global health governance, LF will remain a persistent and expanding threat.
Post-recovery complications and socioeconomic impact: LF survivors often suffer long-term effects such as hearing loss, neurological issues, and psychological trauma that significantly impact their quality of life. These complications place additional strain on under-resourced health systems where rehabilitation services are scarce, especially in rural areas. Survivors frequently face high medical costs, loss of income, and social stigma, leading to long-term socioeconomic hardship. The lack of structured rehabilitation programs further worsens these outcomes. Strengthening healthcare infrastructure and providing social support are essential to reduce the long-term burden on individuals and communities affected by LF.
HCWs remain particularly vulnerable during LF outbreaks, facing not only a high risk of infection due to frequent exposure to infectious fluids but also substantial psychological stress. The lack of PPE in early outbreak phases, fear of contracting the disease, stigma associated with infection, and witnessing high patient mortality contribute significantly to anxiety, burnout, and emotional exhaustion among HCWs. Inadequate infection control measures further amplify occupational hazards, leading to nosocomial transmission. Strengthening mental health support systems, ensuring adequate training in infection prevention, and providing consistent access to protective gear are critical steps to safeguard the physical and psychological well-being of frontline workers during outbreaks.
Global frameworks like the WHO R and D Blueprint and Coalition for Epidemic Preparedness Innovations have significantly advanced LF research, particularly in vaccine development. However, challenges remain in ensuring equitable access, integrating innovations into local healthcare systems and addressing logistical barriers such as cold-chain requirements. Strengthening collaboration with endemic-country institutions and improving regulatory pathways are essential for translating these global efforts into meaningful, on-ground impact (Table 6)[30-33].
| Feature | MARV | EBOV | Lassa virus |
| Virus family | Filoviridae | Filoviridae | Arenaviridae |
| First identified outbreak | 1967: Marburg and Frankfurt, Germany and Belgrade, Serbia | 1976: Yambuku, Democratic Republic of the Congo and Nzara, Sudan | 1969: Lassa, Nigeria |
| Origin of outbreaks | African green monkeys imported from Uganda | Suspected zoonotic transmission with bats as reservoirs and transmission to humans or other primates | Contact with Mastomys natalensis rodents |
| Reservoir hosts | Egyptian fruit bat (Rousettus aegyptiacus) suspected | Fruit bats (Pteropodidae family), particularly Eidolon helvum | Multimammate rats (Mastomys natalensis) |
| Case fatality rate | 24%-90%, depending on outbreak and case management | 25%-90%, depending on outbreak and case management | 1% overall; up to 15%-20% in hospitalized patients; higher in pregnant females |
| Geographic distribution | Primarily sub-Saharan Africa | Primarily sub-Saharan Africa | West Africa: Nigeria, Sierra Leone, Liberia, Guinea, others |
| Symptoms | Hemorrhagic fever, severe malaise, high fever, vomiting, diarrhea, organ dysfunction | Similar to MARV: Hemorrhagic fever, malaise, vomiting, diarrhea, multiorgan failure | Fever, sore throat, vomiting, bleeding, neurological complications |
| Transmission | Direct contact with bodily fluids (e.g., blood, saliva, urine) of infected persons or animals | Direct contact with bodily fluids of infected persons or animals, contaminated surfaces | Exposure to rodent excreta; human-to-human via body fluids |
| Laboratory diagnosis | PCR, ELISA, virus isolation | PCR, ELISA, virus isolation | PCR, ELISA (IgM, IgG), virus isolation |
| Vaccines | No approved vaccine (research ongoing) | Approved vaccines available (e.g., recombinant vesicular stomatitis virus-ZEBOV for Zaire strain) | No approved vaccine (clinical trials ongoing) |
| Notable outbreaks | Angola (2004-2005), Democratic Republic of the Congo (1998-2000) | West Africa (2014-2016), Democratic Republic of the Congo (multiple outbreaks) | Nigeria (multiple outbreaks annually), Sierra Leone epidemics |
LF continues to be a significant and escalating health concern in West Africa, especially among high-risk groups like newborns, children, and expectant mothers. Although discovered more than 50 years ago, it remains difficult to manage and diagnose due to vague clinical presentation, limited reporting, and inadequate healthcare systems in endemic areas. The notably high mortality rates in critical cases, particularly among hospitalized individuals and pregnant females, highlight the pressing need for improved early diagnosis, effective treatment protocols, and comprehensive prevention measures. Enhancing laboratory capabilities, enforcing stringent infection control measures, and increasing public education are crucial steps in curbing its spread both in communities and healthcare environments. Promising advan
I offer my sincere thanks to Maharajah’s College of Pharmacy, Vizianagaram for continuous support and cooperation for the completion of this work.
| 1. | Garry RF. Lassa fever - the road ahead. Nat Rev Microbiol. 2023;21:87-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 2. | Kelly JD, Barrie MB, Ross RA, Temple BA, Moses LM, Bausch DG. Housing equity for health equity: a rights-based approach to the control of Lassa fever in post-war Sierra Leone. BMC Int Health Hum Rights. 2013;13:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 204] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Sulis G, Peebles A, Basta NE. Lassa fever vaccine candidates: A scoping review of vaccine clinical trials. Trop Med Int Health. 2023;28:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Chaudhary M, Cutland CL, Bonet M, Gentile A, Jones CE, Marshall HS, Stergachis A, Voss G, Darko DM, Sevene E, Hyde T, Fairlie L, Kampmann B, Everett D, Munoz FM. Burden of Lassa fever disease in pregnant women and children and options for prevention. Vaccine. 2025;43:126479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Ibekwe T. Lassa fever: the challenges of curtailing a deadly disease. Pan Afr Med J. 2012;11:55. [PubMed] |
| 7. | Murphy H, Ly H. Understanding Immune Responses to Lassa Virus Infection and to Its Candidate Vaccines. Vaccines (Basel). 2022;10:1668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Raabe V, Mehta AK, Evans JD; Science Working Group of the National Emerging Special Pathogens Training and Education Center (NETEC) Special Pathogens Research Network (SPRN). Lassa Virus Infection: a Summary for Clinicians. Int J Infect Dis. 2022;119:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | Jamil S, Chaudhary N, Muhammad F, Chowdhury AA, Akhter A. The Lassa fever outbreak in Africa: Correspondence. Microbes Infect Dis. 2024. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Holy R, Navara M, Dosel P, Fundova P, Prazenica P, Hahn A. Hyperbaric oxygen therapy in idiopathic sudden sensorineural hearing loss (ISSNHL) in association with combined treatment. Undersea Hyperb Med. 2011;38:137-142. [PubMed] |
| 11. | Sano H, Kamijo T, Ino T, Okamoto M. Edaravone, a free radical scavenger, in the treatment of idiopathic sudden sensorineural hearing loss with profound hearing loss. Auris Nasus Larynx. 2010;37:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Besson ME, Pépin M, Metral PA. Lassa Fever: Critical Review and Prospects for Control. Trop Med Infect Dis. 2024;9:178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Mazzola LT, Kelly-Cirino C. Diagnostics for Lassa fever virus: a genetically diverse pathogen found in low-resource settings. BMJ Glob Health. 2019;4:e001116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Happi AN, Happi CT, Schoepp RJ. Lassa fever diagnostics: past, present, and future. Curr Opin Virol. 2019;37:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Doohan P, Jorgensen D, Naidoo TM, McCain K, Hicks JT, McCabe R, Bhatia S, Charniga K, Cuomo-Dannenburg G, Hamlet A, Nash RK, Nikitin D, Rawson T, Sheppard RJ, Unwin HJT, van Elsland S, Cori A, Morgenstern C, Imai-Eaton N; Pathogen Epidemiology Review Group. Lassa fever outbreaks, mathematical models, and disease parameters: a systematic review and meta-analysis. Lancet Glob Health. 2024;12:e1962-e1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Boisen ML, Uyigue E, Aiyepada J, Siddle KJ, Oestereich L, Nelson DKS, Bush DJ, Rowland MM, Heinrich ML, Eromon P, Kayode AT, Odia I, Adomeh DI, Muoebonam EB, Akhilomen P, Okonofua G, Osiemi B, Omoregie O, Airende M, Agbukor J, Ehikhametalor S, Aire CO, Duraffour S, Pahlmann M, Böhm W, Barnes KG, Mehta S, Momoh M, Sandi JD, Goba A, Folarin OA, Ogbaini-Emovan E, Asogun DA, Tobin EA, Akpede GO, Okogbenin SA, Okokhere PO, Grant DS, Schieffelin JS, Sabeti PC, Günther S, Happi CT, Branco LM, Garry RF. Field evaluation of a Pan-Lassa rapid diagnostic test during the 2018 Nigerian Lassa fever outbreak. Sci Rep. 2020;10:8724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Aloke C, Obasi NA, Aja PM, Emelike CU, Egwu CO, Jeje O, Edeogu CO, Onisuru OO, Orji OU, Achilonu I. Combating Lassa Fever in West African Sub-Region: Progress, Challenges, and Future Perspectives. Viruses. 2023;15:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Warner BM, Safronetz D, Stein DR. Current perspectives on vaccines and therapeutics for Lassa Fever. Virol J. 2024;21:320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Isaac AB, Karolina W, Temitope AA, Anuska R, Joanne E, Deborah A, Bianca OC, Filip T, Zofia P, Oluwasegun OI, Oluwaferanmi O, Grace BT. Prospects of Lassa Fever Candidate Vaccines. Afr J Infect Dis. 2022;16:46-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Balogun OO, Akande OW, Hamer DH. Lassa Fever: An Evolving Emergency in West Africa. Am J Trop Med Hyg. 2020;104:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. 2003;327:1271-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Garnett LE, Strong JE. Lassa fever: With 50 years of study, hundreds of thousands of patients and an extremely high disease burden, what have we learned? Curr Opin Virol. 2019;37:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Smith DRM, Turner J, Fahr P, Attfield LA, Bessell PR, Donnelly CA, Gibb R, Jones KE, Redding DW, Asogun D, Ayodeji OO, Azuogu BN, Fischer WA 2nd, Jan K, Olayinka AT, Wohl DA, Torkelson AA, Dinkel KA, Nixon EJ, Pouwels KB, Hollingsworth TD. Health and economic impacts of Lassa vaccination campaigns in West Africa. Nat Med. 2024;30:3568-3577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Duvignaud A, Etafo IC, Jaspard M, Salau Q, Serra B, Kareem AJ, Juchet S, Jegede TO, Gabillard D, Abidoye AT, Le Gal C, Abejegah C, Owhin S, Okwaraeke K, Doutchi M, Katembo Vihundira J, Besong-Lache RM, Seri B, Bérerd-Camara M, Salam APA, Olayinka A, Horby P, Ogbaini-Emovon E, Duraffour S, Ahmed LA, Günther S, Adedosu AN, Anglaret X, Malvy D, Lang HJ, Ayodeji OO. Presentation and Outcomes of Lassa Fever in Children in Nigeria: A Prospective Cohort Study (LASCOPE). J Pediatric Infect Dis Soc. 2024;13:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Arruda LB, Haider N, Olayemi A, Simons D, Ehichioya D, Yinka-Ogunleye A, Ansumana R, Thomason MJ, Asogun D, Ihekweazu C, Fichet-Calvet E, Kock RA. The niche of One Health approaches in Lassa fever surveillance and control. Ann Clin Microbiol Antimicrob. 2021;20:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Efe UO, Edward EO, Chinaza AA, Emmanuella OC, Chidinma NJ. Lassa Fever: A Mini Review of Clinical Features, Diagnosis and Treatment. Asian J Res Infect Dis. 2024;15:7-13. [DOI] [Full Text] |
| 27. | Okwuraiwe AP, Faye O, Ige FA, James AB, Shaibu JO, Faye M, Amoo OS, Ndiaye O, Salu OB, Omilabu SA, Audu RA. Surveillance of Viral Hemorrhagic Fever Viruses in Lassa Fever Suspects in Ondo State, Nigeria. Eur J Med Health Sci. 2022;4:78-81. [DOI] [Full Text] |
| 28. | Naeem A, Zahid S, Hafeez MH, Bibi A, Tabassum S, Akilimali A. Re-emergence of Lassa fever in Nigeria: A new challenge for public health authorities. Health Sci Rep. 2023;6:e1628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Karanam SK, Nagvishnu K, Uppala PK, Edhi S, Varri SR. Crimean-Congo hemorrhagic fever: Pathogenesis, transmission and public health challenges. World J Virol. 2025;14:100003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Luby JP, Sanders CV. Green monkey disease ("Marburg virus" disease): a new zoonosis. Ann Intern Med. 1969;71:657-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Rivera A, Messaoudi I. Pathophysiology of Ebola Virus Infection: Current Challenges and Future Hopes. ACS Infect Dis. 2015;1:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Jacob ST, Crozier I, Fischer WA 2nd, Hewlett A, Kraft CS, Vega MA, Soka MJ, Wahl V, Griffiths A, Bollinger L, Kuhn JH. Ebola virus disease. Nat Rev Dis Primers. 2020;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 404] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 33. | Asogun DA, Günther S, Akpede GO, Ihekweazu C, Zumla A. Lassa Fever: Epidemiology, Clinical Features, Diagnosis, Management and Prevention. Infect Dis Clin North Am. 2019;33:933-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/