Published online Jun 25, 2025. doi: 10.5501/wjv.v14.i2.106479
Revised: April 4, 2025
Accepted: May 7, 2025
Published online: June 25, 2025
Processing time: 116 Days and 12.6 Hours
Treatment response to direct-acting antivirals (DAAs) is a challenging issue and the identification of non-responders patients is very important.
To evaluate the relation between baseline serum levels of hyaluronic acid (HA) and type III procollagen N-peptide (PIIINP) with direct-acting antivirals treatment failure in Egyptian patients with chronic hepatitis C.
Hepatitis C patients (responders and non-responders to sofosbuvir/daclatasvir) were tested for HA and PIIINP using sensitive chemiluminescent immunoassay.
There were distinctly higher PIIINP (P = 0.0003) and HA (P < 0.0001) levels in non-responders than responders patients with a good ability for distinguishing non-responders from patients with sustained virological response (area under the curve = 0.766 for HA and 0.684 for PIIINP). Logistic regression analysis revealed that the HA × PIIINP is the model with the highest predictive ability (area under the curve = 0.809). Diagnostic performances were superior to each marker alone with good sensitivity (74.7%), specificity (74%), positive predictive (68.3%), negative predictive values (79.6%) and accuracy (74.3%). The multiplication of HA × PIIINP is correlated significantly (P < 0.05) with elevated liver enzymes (r = 0.212), decreased albumin (r = -0.26), elevated aspartate aminotransferase-platelet ratio index (r = 0.223) and elevated fibrosis-4 score (r = 0.216) scores.
These findings suggested the remarkable role of fibrogensis markers HA and PIIINP in the prediction of hepatitis C virus DAAs treatment response. Multiplying HA with PIIINP values increase the sensitivity to detect treatment success and thus may aim to improve treatment duration and the disease control.
Core Tip: In any medical therapy, treatment response proper prognosis is very important to reduce impacts of the medication and the disease as well. This study highlighted the significant role of hyaluronic acid and type III procollagen N-peptide in the prediction of hepatitis C virus direct-acting antivirals treatment response. Findings revealed that multiplying hyaluronic acid with type III procollagen N-peptide values increase the sensitivity to detect treatment success (74.7% sensitivity, 74% specificity and 74.3% accuracy).
- Citation: Abdelrazek MA, Elghwab AI, Tabll AA, Elsayed EH, El Behery M. Evaluation of hyaluronic acid and type III procollagen peptide as predictors for treatment response to direct-acting antivirals. World J Virol 2025; 14(2): 106479
- URL: https://www.wjgnet.com/2220-3249/full/v14/i2/106479.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i2.106479
Based on the World Health Organization, chronic hepatitis C (CHC) infection affects around 58 million people globally and, thus, still a major burden worldwide[1]. Annually, there are about 1.5 million new hepatitis C virus (HCV) cases and 290000 deaths related to HCV infection[2]. HCV related chronic hepatitis may causes inflammations ranging from minor ailments to stubborn, severe disorders involving liver fibrosis, cirrhosis and hepatocellular carcinoma[2].
For treating HCV infections, direct-acting antiviral (DAA) agents’ introduction not only dramatically improved the safety profile and increased therapy effectiveness, but also facilitated treatment monitoring worldwide[3]. These DAAs target several HCV replication cycle points, initiating RNA chain termination or directly binding replicase complex components[4]. HCV current therapy regimens that include DAAs have significantly elevated sustained virological response (SVR) rate (> 90%), shorter treatment duration, higher resistance barrier and good tolerability[5]. However, with DAA regimens, a small percentage of cases do not deliver SVR due to the upgrowth of resistance associated substitutions at the relapse time and at baseline that may be related to treatment failure[5,6]. Despite this relatively small percentage of therapy failure, as infected individuals treated with DAA regimens increases, the number of non-responder cases will also continue to increase[7]. Thus, studies regarding the identification of these patients are important so that treatment decision may be prescribed at the first visit to prevent patients remaining at risk of advanced liver diseases development and also the need for retreatment[7].
One of the extracellular matrix fundamental constituents is hyaluronic acid (HA), which is mainly released from hepatic stellate cells and degraded by sinusoidal endothelial cells[8]. In chronic liver diseases, several reports have shown that HA could associate with histological hepatic fibrosis stages[8]. Also, amino-terminal peptide of type III procollagen N-peptide (PIIINP) is a non-invasive fibrosis marker that is released from its precursor peptide during the deposition and synthesis of collagen type III[9,10]. It is a fibrillar collagen that is abundant in a variety of internal organs and particularly in the skin[9].
Previous study of Leroy et al[11] suggested that interferon alpha (IFN-α) treatment failure was associated with elevated fibrogenesis markers including HA and PIIINP. In virologic non-responder patients, IFN-α therapy was unable to improve the histological outcome and to reduce these proteins levels[11]. This study aimed to reveal the association between baseline serum levels of HA and PIIINP with DAA treatment failure in Egyptian patients with CHC. The second aim was to develop a combined use of these fibrogenesis proteins as a pretreatment predictor of DAA treatment failure.
This prospective study included Egyptian patients who treated for CHC infection at Sherbin Central Hospital, Dakahlia Governorate, Egypt. Diagnosis of HCV was based on anti-HCV detection and confirmed by real-time PCR. Inclusion criteria were all adult cases (> 18 years old) with compensated liver disease. The exclusion criteria were assessed based on the protocol of the Egyptian national treatment programme of HCV treatment[12]. These included human immunodeficiency virus patients, hepatocellular carcinoma except 6 months after intervention, pregnancy or inability to use effective contraception, extra-hepatic cancers, uncontrolled diabetes mellitus, platelet count < 50000/mm3, serum albumin < 2.8 g/dL, serum bilirubin > 3 mg/dL, international normalized ratio > 1.7, and cirrhotic patients with Child C stage.
In accordance with the Egyptian National Committee for Control of Viral Hepatitis, a total of 4300 cases were subjected to DAAs regimens therapy [sofosbuvir (SOF)/daclatasvir (DCV)] for HCV. PCR assay was performed 3 times for each case: (1) Prior to treatment initiation; (2) 12 weeks after treatment; and (3) 3 months (24 weeks) after treatment to determine SVR[13]. A total of 4225 cases were achieved SVR (response rate of 98.25%) and 75 cases were non-responders. All non-responders patients were included (n = 75), while only 100 cases were randomly selected from responders and were age- and gender- matched with non-responders cases.
Fresh serum was used for biochemical parameters investigations including liver enzymes [alkaline phosphatase (ALP) and alanine aminotransferase (ALT) and aspartate aminotransferase (AST)], albumin, total bilirubin and kidney function test (creatinine). These chemical parameters were all measured using automatic biochemistry analyzer (Rresponse 920, DiaSys, Germany) and commercial kits. Citrated-anticoagulated treated blood was used for prothrombin time measure
Both HA and PIIINP were measured using an automated chemiluminescent immunoassay (CLIA; Maglumi 800, Snibe, Shenzhen, China) and the kits were provided by their manufacturers.
All analyses were performed using GraphPad prism version 9.0 and SPSS version 20. Numerical/continuous and categorical variables were presented as mean ± SD and numbers, respectively. All data were normally distributed and were compared by unpaired student t test or χ2 test, appropriately. Both fibrosis-4 score (FIB-4)[14] and AST-platelet ratio index (APRI)[15] were measured for liver fibrosis assessment. For SVR prediction, diagnostic performance of each fibrogensis marker was expressed as sensitivity, specificity, positive predictive (PPV) and negative predictive values (NPV), and area under the receiver operating characteristic curve (AUC). Stepwise logistic regression was applied for all independent variables, then a smaller model was developed included only significant variables. Statistical significance was set at P < 0.05.
The study protocol was approved (No. MED (1/6/2023) s.no (96) BIO 004) by the Ethics Committee of Faculty of Medicine, Port Said University in accordance with the ethical guidelines of the “Helsinki Declaration”.
A total of 175 CHC patients who had received HCV DAA therapy were included. According to treatment response, patients were classified into non-responders who did not achieve SVR24, included 75 patients, 30 males and 45 females, with a mean age of 51.55 ± 6.99 years; and responders comprising 100 (40 males and 60 females) selected patients who achieved SVR24, with a mean age of 49.4 ± 7.0 years. As shown in Table 1, gender was matched among groups (P = 0.242). Although non-responders were older than responders patients, there was no significant difference (P = 0.071). Non-responders were associated (P < 0.05) with elevated liver enzymes (ALT, AST, and ALP) and bilirubin levels and decreased serum albumin levels. Also, platelets were distinctly lower in non-responders than responders group. With respect to liver fibrosis assessment, APRI score (P = 0.0001) and FIB-4 (P = 0.0001) were higher in non-responders than in responders participants (Table 1).
| Variables | Non-responder | Responder | P value |
| Gender (male/female) | 30/45 | 40/60 | 0.242 |
| Age (years) | 51.55 ± 6.99 | 49.4 ± 7.0 | 0.071 |
| Alanine aminotransaminase (U/L) | 70.2 ± 21.0 | 60.0± 15.8 | 0.017 |
| Aspartate aminotransaminase (U/L) | 68.9 ± 16.9 | 54.6 ± 14.9 | 0.004 |
| ALP (U/L) | 123.9 ± 32.8 | 98.9 ± 20.9 | 0.018 |
| Bilirubin (mg/dL) | 1.15 ± 0.28 | 0.96 ± 0.35 | 0.011 |
| Albumin (g/dL) | 3.35 ± 0.39 | 3.88 ± 0.51 | 0.0001 |
| Creatinine (mg/dL) | 0.87 ± 0.18 | 0.81 ± 0.16 | 0.082 |
| α-fetoprotein (ng/mL) | 3.62 ± 1.19 | 3.59 ± 0.88 | 0.769 |
| INR | 1.25 ± 0.15 | 1.13 ± 0.06 | 0.069 |
| Hemoglobin (g/dL) | 11.88 ± 2.60 | 13.10 ± 1.34 | 0.071 |
| Platelets (× 109/L) | 155.9 ± 43.9 | 191.2 ± 35.9 | 0.0001 |
| White blood cells (× 109/L) | 4.91 ± 1.67 | 5.49 ± 1.37 | 0.0620 |
| Red blood cells (× 1012/L) | 5.33 ± 1.88 | 4.98 ± 1.17 | 0.0840 |
| APRI | 1.20 ± 0.31 | 0.78 ± 0.22 | 0.0001 |
| FIB-4 | 3.11 ± 1.01 | 1.94 ± 0.56 | 0.0001 |
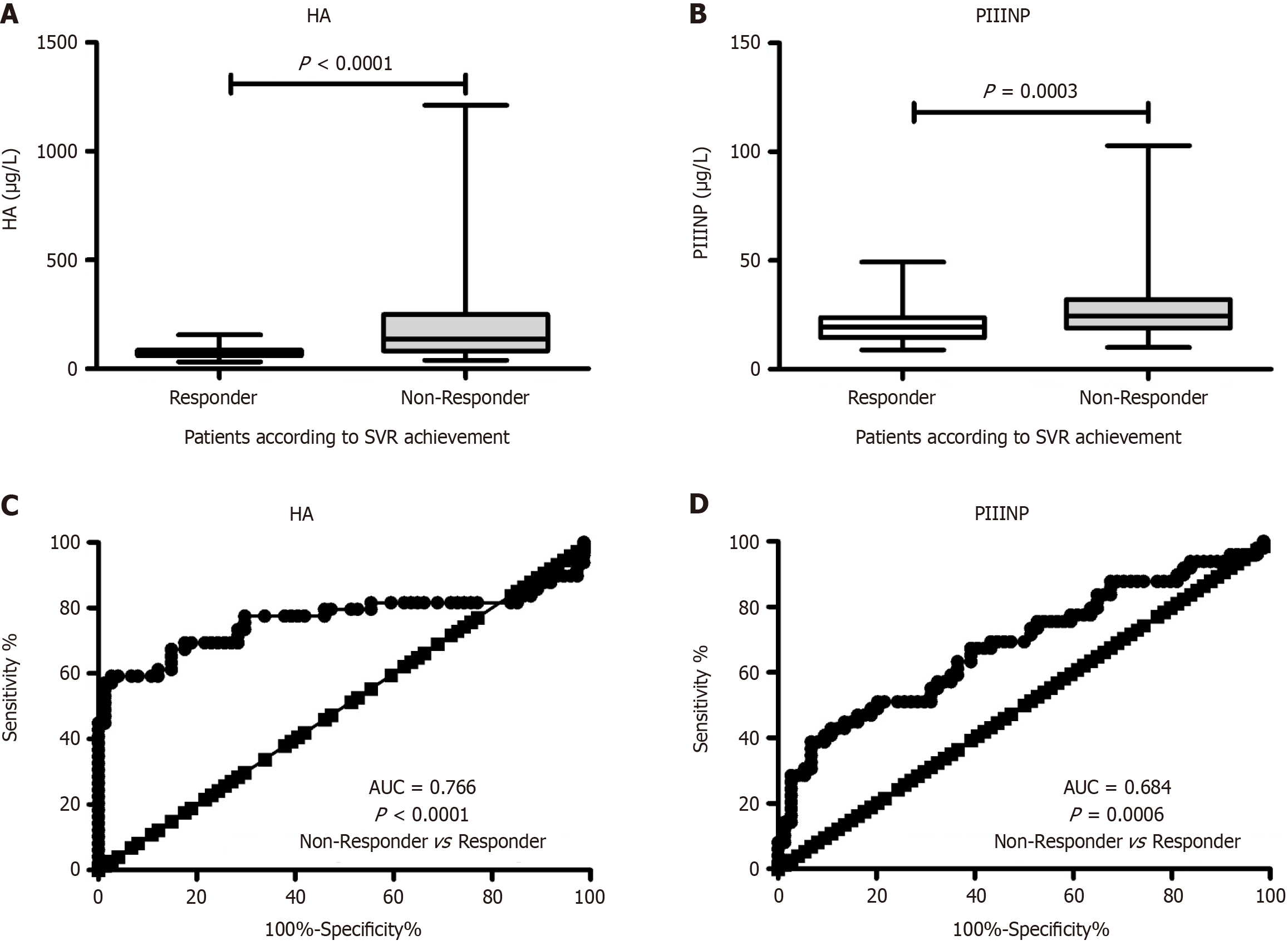
Regarding baseline serum levels, we reported marked variations among groups with distinctly higher HA (P < 0.0001, Figure 1A) and PIIINP (P = 0.0003; Figure 1B) levels in non-responders than responders patients. Moreover, HA (AUC = 0.766; P < 0.0001; Figure 1C) and PIIINP (AUC = 0.684; P = 0.0006; Figure 1D) had a good ability for distinguishing non-responders from patients with SVR24.
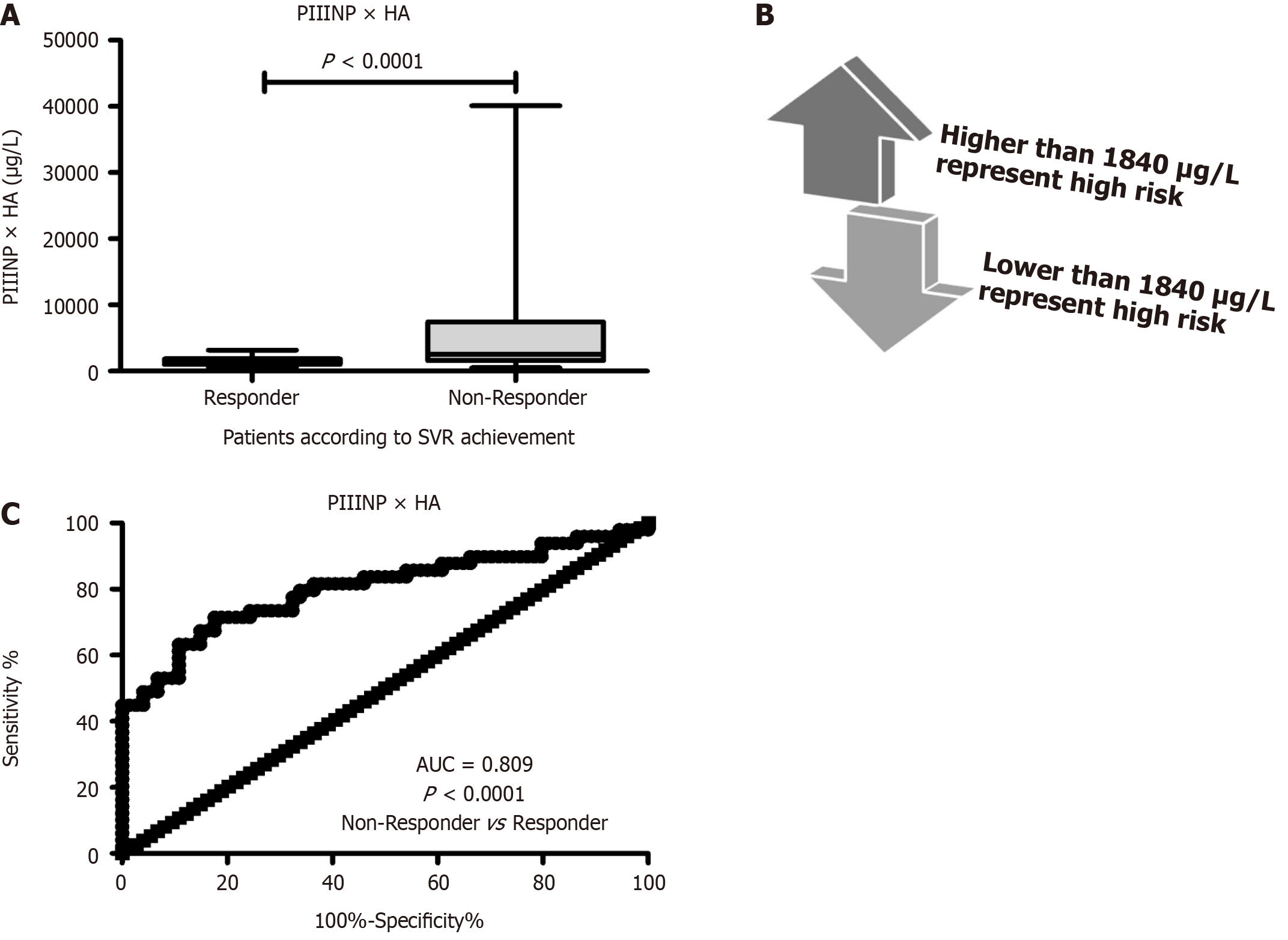
Stepwise logistic regression analysis revealed that multiplication of HA by PIIINP values is the beast model revealed a significant prediction of treatment failure (Figure 2A). Receiver operating characteristic analysis of HA × PIIINP for distinguishing non-responders from patients with SVR24 revealed that a cut-off point of ≤ 1840 μg/mL (Figure 2B) early and significantly (P < 0.0001) could predict SVR24 incidence in CHC patients receiving DAA treatment with AUC of 0.809 (Figure 2C). This multiplication revealed diagnostic performances (Table 2) that were superior to each protein alone. HA × PIIINP had a sensitivity of 74.7%, specificity 74%, PPV 68.3%, NPV 79.6% and accuracy of 74.3%. Moreover, compared to each marker alone, HA × PIIINP was more correlated significantly (P < 0.05) with fibrosis related parameters including elevated liver enzymes (r = 0.212), decreased albumin (r = -0.26), elevated APRI (r = 0.223) and elevated FIB-4 (r = 0.216) scores (Table 3).
| Categories | AUC (95%CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
| PIIINP ≥ 19.5 μg/L | 0.684 (0.59-0.78) | 69.3 | 50 | 51.0 | 68.5 | 58.3 |
| HA ≥ 82 μg/L | 0.766 (0.66-0.87) | 70.7 | 69 | 63.1 | 75.8 | 69.71 |
| PIIINP × HA > 1840 | 0.809 (0.72-0.90) | 74.7 | 74 | 68.3 | 79.6 | 74.3 |
| Variable | HA × PIIINP | HA | PIIINP | |||
| r | P value | r | P value | r | P value | |
| ALT (U/L) | 0.182 | 0.091 | 0.127 | 0.096 | 0.121 | 0.210 |
| AST (U/L) | 0.212 | 0.089a | 0.114 | 0.133 | 0.150 | 0.182 |
| ALP (U/L) | 0.191 | 0.059 | 0.112 | 0.214 | 0.071 | 0.261 |
| Albumin (g/dL) | -0.26 | 0.021a | -0.20 | 0.044a | -0.201 | 0.043a |
| Bilirubin (mg/dL) | 0.194 | 0.062 | 0.136 | 0.079 | 0.099 | 0.223 |
| Platelets (× 109/L) | -0.19 | 0.058 | -0.13 | 0.083 | -0.123 | 0.158 |
| FIB-4 | 0.216 | 0.022a | 0.186 | 0.041a | 0.185 | 0.041a |
| APRI | 0.223 | 0.019a | 0.188 | 0.041a | 0.181 | 0.041a |
Although DAAs introduction has improved CHC control and management[16], risk assessment tools to detect HCV cases at high treatment failure risk (failure to achieve SVR) could lead to interventions that improve SVR rates without required additional barriers to therapy access[17]. Using a group of Egyptian patients treated with DAAs for their CHC infection, we evaluated the association of two fibrogensis markers (HA and PIIINP) with achievement of SVR24 and their performances as a prediction model for treatment failure. This study included real-world Egyptian patients who received SOF/DCV and the response rate was 4225/4300 (98.25%). High SVR rates were achieved using DCV plus in difficult-to-treat and diverse populations[18]. Our findings were in accordance with other studies which concluded that SOF/DCV combination was associated with similarly high rate of SVR in HCV genotype 4 treatment[18-20].
Also, in our cohort of patients, treatment failure was associated (P < 0.05) with elevated liver enzymes (ALT, AST, and ALP) and bilirubin levels and decreased platelets count and serum albumin levels. Also, the increase in liver stiffness (fibrosis) was associated with treatment failure as non-responders had higher (P = 0.0001) values of APRI and FIB-4 scores than responders participants. Former studies found that the severity of hepatic dysfunction may affect the response rate to DAAs[21]. In similar large cohort of Egyptian patients, Omar et al[20] found that patients who achieved SVR were had lower liver enzymes activity, decreased bilirubin levels, lower FIB-4 score and higher albumin levels and platelets count. Other studies reported that the increase in liver fibrosis was associated with decreased SVR rates in HCV genotype 4 treated with SOF/DCV[22].
Although some studies demonstrated that HCV treatment with DAAs, in a significant number of patients, may be associated with reversal of hepatic fibrosis and inflammation[23], almost there was no study regarding the role of fibrogensis markers in treatment response to DAAs. This study revealed that baseline serum levels of HA (P < 0.0001) and PIIINP (P = 0.0003) were distinctly higher in non-responders than responders patients and both of them had a good ability for treatment success (SVR24) prediction (AUC of 0.766 for HA and 0.684 for PIIINP).
In virologic non-responder patients to IFN-α therapy, Leroy et al[11] reported that non-responder patients did not achieve histological scores improvement after therapy. They found that elevated HA and PIIINP levels were associated with treatment failure and IFN-α therapy is unable to reduce their levels and remained unchanged during follow-up[11]. Despite our study that focused on the prediction of treatment success, Leroy study focused on the changes and improvements in histological lesions before and after treatment and used HA and PIIINP as fibrogenesis markers to reveal the improvements in liver fibrosis. Also, the HCV treatment used in this study is based on DAA INF free regimen despite IFN-α therapy in Leroy et al’s study[11]. Moreover, Trocme et al[24], evaluated two fibrosis models, one included PIIINP and the other included HA, in CHC patients treated by IFN-α and ribavirin. Compared to non-responder patients, they found that values of each index significantly decreased in responders[24].
In this study, logistic regression analysis revealed that the most parsimonious predictive model for treatment success is HA × PIIINP with the highest predictive ability (AUC = 0.809). This model demonstrated diagnostic performances superior to each marker alone with good sensitivity (74.7%), specificity (74%), PPV (68.3%), NPV (79.6%) and accuracy (74.3%). Given treatment failure low incidence for DAAs, PPV was low as expected[25]. These performances were comparable to other predictive markers for DAAs treatment success in such Egyptian patients with HCV genotype 4. For example, the microRNA-122 showed AUC of 0.729, sensitivity of 60.34% and specificity of 66.67% in predicting the occurrence of SVR in such HCV Egyptian patients[16].
There is a great practical significance of the present HA × PIIINP predictive index. SOF/DCV non-responders in this study were subjected to VoseviTM.4 (SOF + velpatasvir + voxilaprevir) regimen which achieved a SVR12 in more than 97% of patients. Accordingly, HA × PIIINP predictive index helps in making effective treatment decisions. Across the general HCV-infected population, emerging such predictive models in the clinical practice could also provide a great cost-effectiveness and represent a cost-saving option for many costs including direct medical HCV care costs (health state complications adverse events, treatment monitoring and drug regimen cost).
These results suggested the remarkable role of fibrogensis markers HA and PIIINP in the prediction of HCV DAAs treatment response. Multiplying HA with PIIINP values increase the sensitivity to detect treatment success and thus may aim to improve cost-effectiveness, treatment duration and the disease control related to HCV infection. These results may encourage the future studies to evaluate the role of other fibrogensis markers in treatment response of DAAs and the control of HCV infection. This study is limited because it included patients from one center. Also, owing to limited resources, this study failed to control other potential confounding factors that may be related to treatment failure. Thus, other large multi-center studies may be needed to validate the use of HA and PIIINP in prediction of DAAs treatment response.
| 1. | Torre P, Coppola R, Masarone M, Persico M. Country-Wide HCV Elimination Strategies Need to Reach Older Patients in the General Population: The Italian Experience. Viruses. 2023;15:2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Yang J, Qi JL, Wang XX, Li XH, Jin R, Liu BY, Liu HX, Rao HY. The burden of hepatitis C virus in the world, China, India, and the United States from 1990 to 2019. Front Public Health. 2023;11:1041201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 3. | Zarębska-Michaluk D, Flisiak R, Janczewska E, Berak H, Mazur W, Janocha-Litwin J, Krygier R, Dobracka B, Jaroszewicz J, Parfieniuk-Kowerda A, Dobrowolska K, Rzymski P. Does a detectable HCV RNA at the end of DAA therapy herald treatment failure? Antiviral Res. 2023;220:105742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Baumert TF, Berg T, Lim JK, Nelson DR. Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges. Gastroenterology. 2019;156:431-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 5. | Ponnuvel S, Prakash A, Steve RJ, Doss GP, Goel A, Zachariah UG, Eapen CE, Rebekah G, Kannangai R, Fletcher GJ, Abraham P. Longitudinal assessment of HCV core antigen kinetics to monitor therapeutic response in the age of DAAs. PLoS One. 2023;18:e0282013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Charatcharoenwitthaya P, Wongpaitoon V, Komolmit P, Sukeepaisarnjaroen W, Tangkijvanich P, Piratvisuth T, Sanpajit T, Sutthivana C, Bunchorntavakul C, Sobhonslidsuk A, Chonprasertsuk S, Siripipattanamongkol C, Sethasine S, Tanwandee T; THASL Collaborating Group for the Study of the Use of Direct-acting Antivirals for Chronic Hepatitis C. Real-world effectiveness and safety of sofosbuvir and nonstructural protein 5A inhibitors for chronic hepatitis C genotype 1, 2, 3, 4, or 6: a multicentre cohort study. BMC Gastroenterol. 2020;20:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Park H, Lo-Ciganic WH, Huang J, Wu Y, Henry L, Peter J, Sulkowski M, Nelson DR. Machine learning algorithms for predicting direct-acting antiviral treatment failure in chronic hepatitis C: An HCV-TARGET analysis. Hepatology. 2022;76:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Rewisha E, Salman T, Alhaddad O, Raia GA, Naguib M, Rashad S, Abdelfattah A, Metwally K, Abdelsameea E. Hyaluronic acid as a potential marker for assessment of fibrosis regression after direct acting antiviral drugs in chronic hepatitis C patients. Clin Exp Hepatol. 2021;7:320-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Pettersson-Pablo P, Samyn D, Wasim J, Vink M. Reference interval for type III procollagen (PIIINP) using the Advia centaur PIIINP assay in adults and elderly. Scand J Clin Lab Invest. 2021;81:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Potier JFN, Durham AE, Modi R, Rosenberg W, Dash SA. Investigation of Serum Markers of Hepatic Fibrosis in Equids. J Equine Vet Sci. 2023;131:104937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Leroy V, De Traversay C, Barnoud R, Hartmann JD, Baud M, Ouzan D, Zarski JP. Changes in histological lesions and serum fibrogenesis markers in chronic hepatitis C patients non-responders to interferon alpha. J Hepatol. 2001;35:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | El-Akel W, El-Sayed MH, El Kassas M, El-Serafy M, Khairy M, Elsaeed K, Kabil K, Hassany M, Shawky A, Yosry A, Shaker MK, ElShazly Y, Waked I, Esmat G, Doss W. National treatment programme of hepatitis C in Egypt: Hepatitis C virus model of care. J Viral Hepat. 2017;24:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 863] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 14. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3800] [Article Influence: 190.0] [Reference Citation Analysis (0)] |
| 15. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3338] [Article Influence: 145.1] [Reference Citation Analysis (0)] |
| 16. | Elabd NS, Tayel SI, Elhamouly MS, Hassanein SA, Kamaleldeen SM, Ahmed FE, Rizk M, Gadallah AA, Ajlan SE, Sief AS. Evaluation of MicroRNA-122 as a Biomarker for Chronic Hepatitis C Infection and as a Predictor for Treatment Response to Direct-Acting Antivirals. Hepat Med. 2021;13:9-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Nabulsi NA, Martin MT, Sharp LK, Koren DE, Teply R, Zuckerman A, Lee TA. Predicting Treatment Failure for Initiators of Hepatitis C Virus Treatment in the era of Direct-Acting Antiviral Therapy. Front Pharmacol. 2020;11:551500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Shousha HI, Saad Y, Saleh DA, Dabes H, Alserafy M, ElShazly Y, Said M. Simple predictors of nonresponse to direct-acting antivirals in chronic hepatitis C patients. Eur J Gastroenterol Hepatol. 2020;32:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Pol S, Corouge M, Vallet-Pichard A. Daclatasvir-sofosbuvir combination therapy with or without ribavirin for hepatitis C virus infection: from the clinical trials to real life. Hepat Med. 2016;8:21-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Omar H, El Akel W, Elbaz T, El Kassas M, Elsaeed K, El Shazly H, Said M, Yousif M, Gomaa AA, Nasr A, AbdAllah M, Korany M, Ismail SA, Shaker MK, Doss W, Esmat G, Waked I, El Shazly Y. Generic daclatasvir plus sofosbuvir, with or without ribavirin, in treatment of chronic hepatitis C: real-world results from 18 378 patients in Egypt. Aliment Pharmacol Ther. 2018;47:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Verna EC, Morelli G, Terrault NA, Lok AS, Lim JK, Di Bisceglie AM, Zeuzem S, Landis CS, Kwo P, Hassan M, Manns MP, Vainorius M, Akushevich L, Nelson DR, Fried MW, Reddy KR. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J Hepatol. 2020;73:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | El-Khayat H, Fouad Y, Mohamed HI, El-Amin H, Kamal EM, Maher M, Risk A. Sofosbuvir plus daclatasvir with or without ribavirin in 551 patients with hepatitis C-related cirrhosis, genotype 4. Aliment Pharmacol Ther. 2018;47:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Cheng CH, Chu CY, Chen HL, Lin IT, Wu CH, Lee YK, Hu PJ, Bair MJ. Direct-acting antiviral therapy of chronic hepatitis C improves liver fibrosis, assessed by histological examination and laboratory markers. J Formos Med Assoc. 2021;120:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Trocme C, Leroy V, Sturm N, Hilleret MN, Bottari S, Morel F, Zarski JP. Longitudinal evaluation of a fibrosis index combining MMP-1 and PIIINP compared with MMP-9, TIMP-1 and hyaluronic acid in patients with chronic hepatitis C treated by interferon-alpha and ribavirin. J Viral Hepat. 2006;13:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Park H, Lo-Ciganic WH, Huang J, Wu Y, Henry L, Peter J, Sulkowski M, Nelson DR. Evaluation of machine learning algorithms for predicting direct-acting antiviral treatment failure among patients with chronic hepatitis C infection. Sci Rep. 2022;12:18094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/