Published online Jun 25, 2025. doi: 10.5501/wjv.v14.i2.106973
Revised: April 21, 2025
Accepted: June 7, 2025
Published online: June 25, 2025
Processing time: 103 Days and 15.5 Hours
The World Health Organization (WHO) recommends lumbar puncture (LP) procedures to assess the diagnosis of cryptococcal meningitis (CM) among patients with advanced human immunodeficiency virus (HIV) disease (AHD) with positive serum cryptococcal antigen (CrAg) and do not have evidence of CM.
To estimate pooled prevalence of uptake of LP, CM and mortality among patients with AHD.
PubMed, Cochrane Library and EMBASE were searched for articles published between January 2011 and December 2024. LP uptake was defined as percentage of people who underwent LP procedures among those with AHD (CD4 ≤ 200 cells/mm3 or WHO stage III/IV) and positive serum CrAg. Using random effects models, we computed the pooled estimate of LP uptake, CM and mortality and 95%CI. Stratified analyses were used to compare uptake of LP between studies that involved multiple vs single sites, and mortality analyses between patients with positive and negative serum CrAg were performed. Sensitivity analysis on LP uptake was done by excluding prospective cohort studies that reported 100% uptake.
A total of 32 studies with 46890 people with AHD screened for serum CrAg and 2730 (5.8%) had positive serum CrAg. Overall, pooled prevalence of LP uptake was 67.7% (95%CI: 54.0–81.5). The overall pooled prevalence of CM was 54.3% (95%CI: 39.7–69.0), and mortality was 6.2% (95% CI: 4.5–8.0). There is disparities in the pooled prevalence of LP uptake with studies involving multiple sites having lower prevalence compared to those that involved single sites (54.8% vs 84.7%, P = 0.004). By excluding prospective cohort studies that reported 100% uptake, the overall LP uptake was 54.5% (95%CI: 38.8–70.1). The pooled prevalence of CM was significantly lower among studies that involved multiple sites compared to those that involved single sites (6.8% vs 8.1%, P ≤ 0.001). Mortality was significantly twice as high among patients who had positive serum CrAg compared to those who had negative serum CrAg [risk ratio = 2.0 (95%CI: 1.6–2.5), P ≤ 0.001].
Nearly three to five in 10 people with AHD with positive serum CrAg did not have LP procedures done, indicating significant gaps in identifying patients with CM. Establishing a confirmed diagnosis of CM is critical to avoid exposing patients to subtherapeutic levels of antifungals preemptively. Capacity to perform LP and patient refusals are among the reasons for not performing the procedure. Capacity building in training health care providers to perform LP procedures and professional counselling to obtain patient consent are critical for appropriate treatment to reduce mortality associated with CM infection.
Core Tip: There is limited data on the compliance of the World Health Organization guideline on the management of patients with advanced human immunodeficiency virus disease (AHD). This systematic review and meta-analysis analyzed 46890 people with AHD screened for serum cryptococcal antigen (CrAg) from 32 studies conducted in Africa between 2011 and 2024. Of those screened, 2730 tested positive for serum CrAg. The uptake of lumbar puncture (LP) procedure among those with positive serum CrAg was between 55% and 68%. Mortality was higher among those with positive serum CrAg compared to those with negative serum CrAg. In Africa, among people with AHD who screened positive for CrAg, uptake of LP procedure is low, indicating gaps in the diagnosis of cryptococcal meningitis.
- Citation: Ally HM, Bakari HM, Mbishi JV, Ally ZM, Mbwana MS, Moshi L, Musoke R, Salim SM, Fussi HF, Mustafa AO, Bartlet J, Ramadhani HO. Uptake of lumbar puncture and mortality among patients with advanced human immunodeficiency virus disease who screened for serum cryptococcal-antigen in Africa. World J Virol 2025; 14(2): 106973
- URL: https://www.wjgnet.com/2220-3249/full/v14/i2/106973.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i2.106973
Cryptococcal meningitis (CM) is one of the most common causes of death among people with advanced human immunodeficiency virus (HIV) disease (AHD), particularly in low-income and mid-income countries[1]. Globally modelling analyses estimated an annual 152000 cases of CM leading to 112000 cryptococcal-related deaths in 2020[2]. The same modelling analyses reported that CM accounts for 19% of acquired immunodeficiency syndrome-related deaths globally.
In 2011, the World Health Organization (WHO) recommended screening for serum cryptococcal antigen (CrAg) in patients presenting with AHD (CD4 ≤ 100 cells/mm3 or WHO stage III/IV), and updated recommendations for CrAg screening were made for patients presenting with a higher CD4 count threshold ≤ 200 cells/mm3 in 2017[3]. The overall purpose of CrAg screening is to identify those with positive CrAg and provide preemptive antifungal medications for those without evidence of CM[4]. Detection of serum CrAg occurs before the onset of cryptococcal disease[5,6] and more than 25% of patients with positive serum CrAg develop CM[7]. Provision of preemptive antifungal medications to patients with positive serum CrAg is effective in preventing the development of CM[8,9]. For example, a systematic review that involved 31 studies showed that among those with CD4 count < 100 cells/mm3, if preemptive fluconazole was not administered, the incidence of CM was 21.4% compared to 5.7% when preemptive fluconazole was administered[10]. Another study also showed that compared to individuals who did not receive preemptive antifungal medications, those who received medications were associated with 40% reduction of developing CM[11].
As stated previously, the provision of preemptive antifungal medication requires exclusion of people who have evidence of CM. If lumbar puncture (LP) is not done, provision of preemptive antifungal medication to patients with undiagnosed subclinical CM exposes them to subtherapeutic levels of treatment and increases the risk of death. To diagnose people with evidence of CM, individuals who tested positive serum CrAg need to undergo a LP procedure to withdraw cerebrospinal fluid and test for CrAg in the fluid. LP is the gold standard procedure to establish the diagnosis of CM[12]. As with other medical procedures, LP requires resources, technical capacity and patient consent[13]. Failure to receive consent is reported as one of the most common barriers to performing LP procedures[14,15]. While fear of pain is the most common reason for LP refusal by patients, others have mistakenly associated LP procedure with death and paralysis[16].
While some literature showed higher mortality among patients with AHD who had positive serum CrAg compared to those with negative serum CrAg[17-19], others did not show statistically significant differences between the two groups[20,21]. These contradictory findings along with the need of clinical auditing to understand utility of WHO guidelines in the care of people with AHD prompted us to undertake a systematic review and meta-analysis.
In this systematic review and meta-analysis, we aimed to assess compliance with the WHO guidelines in identifying patients with evidence of CM. Specifically, this study addresses the following questions: (1) What is the prevalence of LP uptake among patients with AHD who screened positive for serum CrAg; (2) What is the prevalence of CM among patients with positive serum CrAg who underwent LP procedure; and (3) What is mortality among patients with positive serum CrAg compared to those with negative serum CrAg.
The protocol for this systematic review has been registered in the International Prospective Registry of Systematic Review with registration number CRD42024604056.
Ethical approval was not sought as this was a systematic review of published manuscripts.
We searched PubMed, Cochrane CENTRAL, EMBASE and clinicaltrials.gov for the articles published between January 2011 and December 2024. We chose 2011 as the lower limit because this is the time when WHO guidelines for the management of patients with AHD were initiated. Search terms were used to capture information on CrAg screening, LP procedure, CM and mortality among people who presented with AHD in Africa. We restricted our search to papers written in English. After uploading search results to Covidence Systematic Review Software (Melbourne, Australia), deduplication and screening was performed.
Studies were included based on the guidelines of systematic reviews and meta-analysis with prevalence approach (CoCoPop)[22]. The CoCoPoP acronym stands for condition/problem, context and study population. In this review, under: (1) Condition: We included studies that reported prevalence of LP uptake among patients with AHD with positive serum CrAg; (2) Context: We explored components of different studies that could explain variation in the reported prevalence of LP uptake such as study designs, number of study sites (single vs multiple sites), type of health care facilities (primary, secondary or tertiary), refusals, transferred out, and death prior to LP procedure; and (3) Population: We included people with AHD who screened for serum CrAg.
Observational studies that involved people with AHD who screened for serum CrAg in Africa, reported number and percentage of individuals who underwent LP procedure (among those with positive serum CrAg) and written in English were eligible for inclusion.
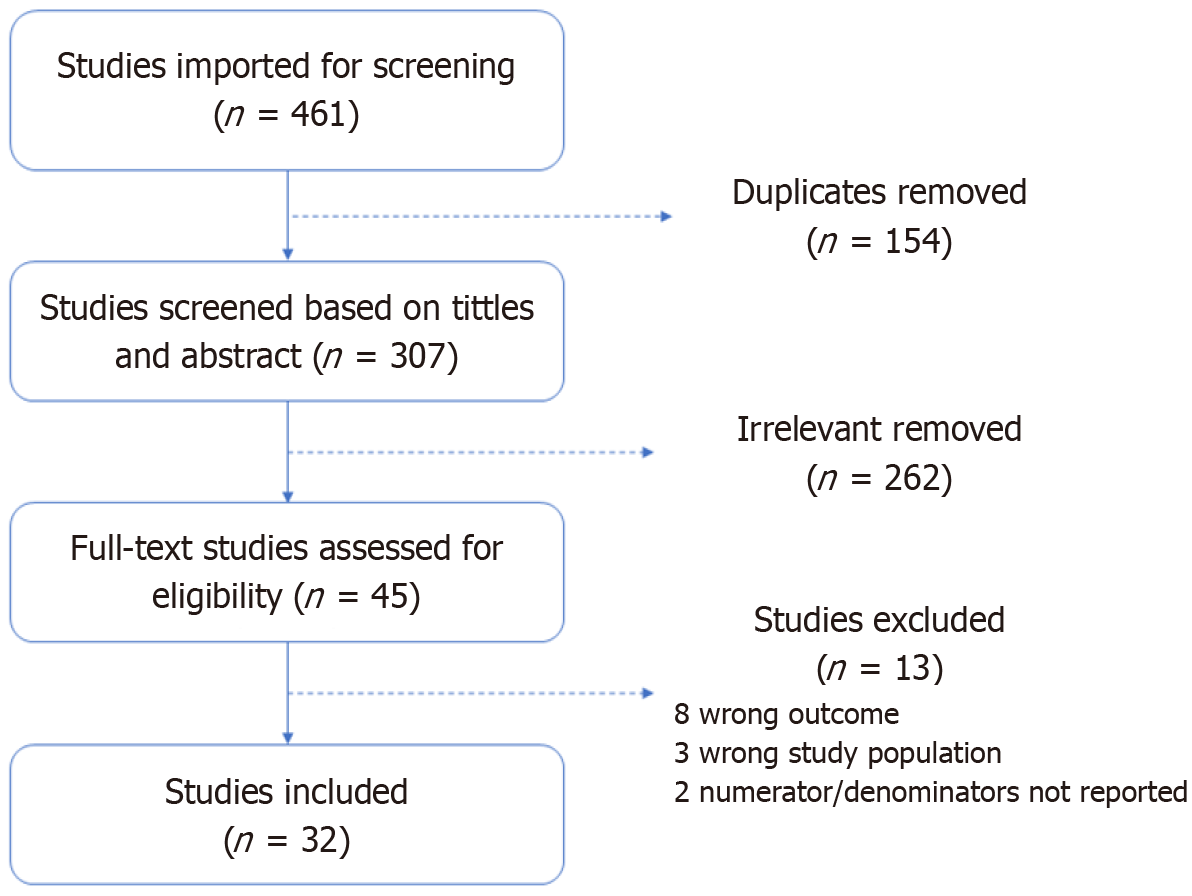
(1) Studies that reported the percentage of individuals who underwent LP procedure without actual numerators and denominators used to compute those percentages; and (2) Studies that were not written in English. Figure 1 describes the literature search process of all included studies as shown below. The reporting of this systematic review and meta-analysis was done according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[23].
All identified manuscripts were managed by Covidence software and following the duplication process, the final list was further reviewed by co-authors. Independently, two pairs of authors (Ally HM, Ally ZM, Musoke R, and Mustafa AO) carry out the study selection for inclusion in the assessment process. When disagreement for the manuscripts inclusion between two independent pairs of authors occurred, it was handled by the third pair of authors (Moshi L and Salim SM).
Using a pre-specified excel spread sheet template, two pairs of authors (Ally HM, Ally ZM, Musoke R, and Mustafa AO) independently abstracted the following data from the final list of manuscripts; authors, year of publication, the country in which the study was conducted, the year(s) in which data was collected, study design, study sites (single vs multi sites), number of people with AHD, number of people screened for serum CrAg, number of people screened positive for serum CrAg, number of people screened negative for serum CrAg, number of people who underwent LP procedures (among those screened positive for serum CM antigen), number of people with positive CM antigen in the cerebrospinal fluid (people with CM); number of people who died among those who screened positive for serum CrAg, and reasons for not performing LP procedure. We then compared data from two pairs of data abstractor, any discrepancies between the two pairs of reviewers was handled by consensus; and the third pair of reviewers (Mbwana MS and Moshi L) was consulted when necessary. Strategies in the Cochrane Handbook for Systematic Reviews of Interventions for data management were followed[24].
To assess the quality of studies, we used the Joanna Briggs Institute (JBI) tools for cross-sectional and cohort studies[25]. Independently, two pairs of reviewers (Ally HM, Ally ZM, Musoke R, and Mustafa AO) performed and rated the quality of the studies using the JBI tools. The tool encompassed nine questions with four responses: (1) Yes; (2) No; (3) Not clear; and (4) Not applicable. A score of 1 was assigned to a “Yes” response and 0 to a “No” response. The total score was summed up and categorized into three groups, with 0-3, 4-6 and 7-9 scores indicating low, medium and high quality.
Outcome variables: The main outcome of interest was the prevalence of LP uptake defined as percentage of individuals who underwent LP procedure among those with AHD and had positive serum CrAg. The secondary outcomes were: (1) The prevalence of CM; and (2) Mortality.
Exposure variables: This was a prevalence study with no main exposure variable; however, LP uptake and CM were compared by study sites (single vs multi-sites) and mortality was compared among individuals with positive and those with negative serum CrAg. We stratified the prevalence of LP uptake by whether studies involved single vs multiple sites because single site studies that involved high tier hospitals are more likely to report a high prevalence of LP uptake because these sites are better resourced. On the contrary, studies that involve multiple sites with different levels of care (low and medium tier) hospitals are more likely to report low levels of prevalence of LP uptake because these sites are more likely to be resource-constrained compared with high tier hospitals. As previously described, contradictory findings from prior studies on mortality among patients with positive serum CrAg compared to those with negative serum CrAg led to this stratification.
Using a random effects model, we computed pooled prevalence of LP procedure uptake, CM, and mortality. For the pooled uptake of LP, a sensitivity analysis was done by excluding prospective cohort studies that reported 100% uptake as opposed to retrospective studies that conducted clinical auditing by reviewing medical records. The Freeman-Tukey double arcsine transformation was used to stabilize variance of proportions prior to the computation of pooled estimates[26]. The stabilized variance addresses potential biases and ensure the robustness of our meta-analysis. Furthermore, this approach allows for the inclusion of all studies, including those with proportions at the boundaries, by stabilizing variance and facilitating the computation of confidence intervals that remain within admissible bounds. Subgroup analysis on the pooled estimates of LP procedure uptake, and prevalence of CM were performed to compare single vs multi-site studies and for mortality between individuals with positive serum CrAg vs negative serum CrAg. All comparisons of sub-group analysis were done using χ² tests. We evaluated heterogeneity across studies using the I2 statistic and Cochran’s Q test. The I2 statistics explain the variance attributable to study heterogeneity with scores of 75%, 50% and 25% indicating high, moderate and low heterogeneity, respectively[27]. The Egger regression asymmetry test was used to assess publication bias. During the assessments of publication bias and heterogeneity, when the P value was < 0.05, it implied their presence. We further conducted an influential analysis to explore sources of heterogeneity, using the leave-one-out method[28]. Studies with missing information, such as those that reported proportions of outcomes without actual numerators and/or denominators, were excluded from the analysis. All statistical tests were performed using STATA version 17 (Stata Corporation, College Station, Texas, United States).
Our search identified 461 articles. Of these, 154 articles were duplicates and deleted. The remaining 307 articles were eligible for title and abstract screening. Of the 45 articles eligible for full text review, 32 articles met inclusion criteria and were finally included in our analysis (Figure 1).
A total of thirty-two studies with 46890 people with AHD who screened for serum CrAg were analyzed. The review included studies conducted from 13 countries in sub–Saharan Africa (Table 1)[15,17-21,29-54]. The sample sizes of included studies ranged from 92 people to 19233 people with AHD. Twelve studies reported uptake of LP among studies that involved single sites[18,20,29-38], 19 among studies that reported multi-sites[15,17,19,21,39-53], and one study did not specify number of sites involved[54]. Thirty-one studies reported results of serum CrAg tests. Sixteen studies reported mortality and of these, eight reported mortalities among individuals with positive serum CrAg only and other eight studies reported mortality for both individuals with positive and negative serum CrAg. One study that did not report uptake was included in the analysis of mortality for individuals with positive versus negative serum CrAg.
| Ref. | Study setting and population | Number of sites | Country guidelines at the time of screening | Reasons for not performing LP | Design and data sources | Quality assessment |
| Boyd et al[39], 2022 | Adults ≥ 18 years with AHD based on CD4 < 200 cells/mm3 in Zimbabwe | Multiple sites | ART-naïve people with HIV were required to have CD4 count measurement and CrAg screening if CD4 count is ≤ 200 cells/mm3 | Undocumented | Cross-sectional study evaluated diagnostic accuracy of point of care finger prick whole blood compared with laboratory-based serum antigen testing | 7 |
| Blasich et al[40], 2021 | Adults ≥ 18 years with AHD based on CD4 < 100 cells/mm3 in South Africa. Identified during routine laboratory reflex screening | Multiple sites involving Helen Joseph and Tambo Memorial hospitals in South Africa | ART-naïve people with HIV were required to have CD4 count measurement and CrAg screening if CD4 count is ≤ 100 cells/mm3 | Undocumented | Prospective cohort study evaluated laboratory tests to quantify the amount of CrAg in plasma of patients with AHD and to gauge the risk of CM | 8 |
| Longley et al[15], 2016 | ART-naive patients with no prior history of cryptococcal disease, aged > 18 years, and with a CD4 cell count ≤ 100 cells/μL in South Africa | Two ART clinics in Cape Town, South Africa | ART-naïve people with HIV were required to have CD4 count measurement and CrAg screening if CD4 count is ≤ 100 cells/mm3 | Refusals | Prospective cohort with patients being followed for a period of one year to determine mortality among CrAg positive and negative individuals | 9 |
| Enock et al[41], 2022 | All people living with HIV who receive routine HIV care from facilities of different administrative level in five districts (2-Urban and 3-Rural) in Uganda | Fourteen facilities. The fourteen health facilities (six health center level three, three health center level four, three general referral hospitals, and two regional referral hospitals) | ART-naïve people with HIV were required to have CD4 count measurement and CrAg screening if CD4 count is ≤ 100 cells/mm3. Routine assessment with provider initiated | Attending rural facilities translating to training gaps and resources | Retrospective review of medical records from CD4 and CrAg registers standard Uganda Ministry of Health (MoH) tools that are used for documentation and generation of routine performance reports | 9 |
| Tiam et al[42], 2023 | Enrolled 15 years or older people with AHD (CD4 < 200 cells/mm3 or WHO stage III/IV) | Two largest hospitals ART clinics at the Motebang and Berea District. The hospital serves one-third of Lesotho’s population | Same-day serum CrAg screening test for all patients enrolling in care with CD4 count < 200 cells/mm3. Routine assessment with provider initiated | Undocumented | Prospective evaluation of routinely collected data from ART clinics. Follow up 6 months | 8 |
| Blankley et al[29], 2019 | Enrolled 19 years or older ART naïve people with AHD (CD4 < 200 cells/mm3 or WHO stage III/IV) at a semi-urban polyclinic in Epworth, Zimbabwe | Single site study at Epworth polyclinic (a nurse led with support from Me´decins Sans Frontières and Ministry of Health doctors | From 2015, recommended CrAg screening for those with CD4 < 100 cells/mm3. Routine assessment with provider initiated | Undocumented | Retrospective assessment of outcomes and management of patients with AHD | 9 |
| Heller et al[30], 2022 | In patient evaluation of AHD management practices at a tertiary hospital in Malawi | Single site at Kamuzu Central Hospital. A tertiary hospital located in Lilongwe | 2017: Recommended CrAg screening for those with CD4 < 100 cells/mm3. 2020: Recommended CrAg screening for those with CD4 < 200 cells/mm3. Routine assessment with provider initiated | Undocumented | Evaluation of outcome for patients with AHD using routinely collected data | 9 |
| Kanyama et al[31], 2022 | Enrolled 14 years or older in patients from a tertiary hospital. AHD diagnosis based on CD4 < 200 cells/mm3 | Single site at Kamuzu Central Hospital. A tertiary hospital located in Lilongwe | Between 1 August 2016 and 31 January 2017, CD4 cell count, urine lipoarabinomannan, urine X-pert and CrAg screening services for management of AHD were introduced. Routine assessment with provider initiated | Undocumented | Prospective evaluation of routinely collected data from medical wards among patients with AHD | 8 |
| Hurt et al[17], 2021 | Tested laboratory samples for patients with AHD based on CD4 count < 100 cells/mm3 | Data from 27 ART clinics and one central referral hospital in Botswana | Recommended CrAg screening for those with CD4 ≤ 100 cells/mm3. An evaluation of laboratory reflex CrAg screening | Undocumented | Evaluation of data from the Botswana. Harvard HIV reference laboratory | 8 |
| Deiss et al[32], 2021 | People with AHD CD4 < 200 cells/mm3 | Enrolled from Jose Macamo General Hospital, a tertiary hospital in Mozambique | Recommended CrAg screening for those with CD4 ≤ 200 cells/mm3 | Undocumented | Retrospective review of routinely collected clinical data | 9 |
| Braide et al[43], 2023 | People with AHD (CD4 < 200 cells/mm3 or WHO stage III/IV) | 28 healthcare facilities across 4 high-burden states in Nigeria | Recommended CrAg screening for those with AHD | Limited/lack of LP packs | Newly identified PLHIV were screened for AHD. Those with AHD were screened for Tuberculosis and CM | 8 |
| Bornstein et al[33], 2014 | HIV patients with CD4 < 100 cells/mm3 | Single site Tertiary Tikur Anbessa Hospital in Addis Ababa | CrAg screening for those with CD4 ≤ 100 cells/mm3 | Undocumented | Diagnostic evaluation between point of care finger stick and serum lateral flow assay | 8 |
| Faini et al[18], 2019 | Newly diagnosed PLHIV, ART-naïve adults ≥ 18 years-old, with CD4 < 150 cells/mm3 | Kilombero and Ulanga Antiretroviral Cohort involves patients attending Saint Francis Referral Hospital in Tanzania | CrAg screening for those with CD4 ≤ 100 cells/mm3. Routine laboratory-reflex CrAg screening | Undocumented | Prospective cohort to determine mortality among CrAg positive and negative individuals | 9 |
| Ndayishimiye et al[44], 2018 | PLHIV with CD4 < 100 cells/mm3 | 17 clinics and one hospital (Prince Mshiyeni Memorial Hospital) in South Africa | CrAg screening for those with CD4 ≤ 100 cells/mm3. Routine laboratory-reflex CrAg screening | Undocumented | Retrospective review of National Laboratory data and medical record charts | 7 |
| Temfack et al[34], 2018 | HIV-infected, ART naïve ambulatory adults (> 18 years) CD4 < 100 cells/mm3, no history of CM | Day Hospital of the Yaoundé Central Hospital in Cameroon. A tertiary hospital | CrAg screening for those with CD4 ≤ 100 cells/mm3 | Undocumented | Prospective cohort with 1 year of follow up to demine incidence of cryptococcal meningitis and mortality | 7 |
| Mamuye et al[20], 2016 | People living with HIV admitted at Tikur Anbessa | Tikur Anbessa (Black Lion) Hospital in Addis Ababa, a tertiary hospital | CrAg screening for those with CD4 ≤ 100 cells/mm3 | All CrAg positive patients underwent LP | Cross-sectional study to determine prevalve of CrAg | 8 |
| Pac et al[35], 2015 | Adults PLHIV with CD4 < 250 cells/mm3, but we reported those with CD < 200 cells/mm3 | Kiboga District Hospital HIV clinic, a rural government hospital | CrAg screening for those with CD4 ≤ 200 cells/mm3. Routine provider-initiated screening | Refusals | Prospective cohort to ascertain new cases of meningitis and mortality | 8 |
| Nalintya et al[45], 2018 | Adults living with HIV with CD4 < 9 cells/mm3 | 11 HIV clinics in Kampala | CrAg screening for those with CD4 ≤ 200 cells/mm3 | Refusals | Prospective cohort study of HIV-infected patients to determine mortality | 9 |
| Oyella et al[36], 2012 | Adults living with HIV with CD4 < 9 cells/mm3, no prior history of cryptococcal disease, not receiving fluconazole, both inpatients and outpatients | Mulago Hospital, a tertiary hospital in Uganda | CrAg screening for those with CD4 ≤ 9 cells/mm3 | Refusal, comatose | Cross-sectional study to determine prevalence of CrAg antigenemia | 9 |
| Ssebambulidde et al[46], 2019 | HIV-infected adults who presented with suspected meningitis and consented for LP | Mulago National Referral Hospital and Mbarara Regional Referral Hospital in Uganda | CrAg screening for those with CD4 ≤ 200 cells/mm3 | All CrAg positive patients underwent LP | Prospectively consented HIV-infected adults who presented with suspected meningitis to evaluate for the etiology of meningitis | 9 |
| Zono et al[47], 2024 | Asymptomatic outpatients with AHD (CD4 < 200 cells/mm3 | Multiple sites in Kinshasa, Democratic republic of Congo | CrAg screening for those with CD4 ≤ 200 cells/mm3 | Undocumented | Cross-sectional study to ascertain prevalence of CrAg and subtypes of Cryptococcal neoformans | 9 |
| Eigege et al[48], 2024 | PLHIV aged ≥ 10 years newly diagnosed and presenting with a CD4+ cell count < 200 cells/mm3 | 28 health care facilities in Nigeria | CrAg screening for those with CD4 < 200 cells/mm3 | Refusals (30%), lack of LP kits (27%), inability of patients to pay for LP (23%), pre-LP mortality (10%), lack of care worker competence (7%), and loss to follow-up | Programmatic evaluation of the implementation of AHD package of care | 8 |
| Smitson et al[21], 2024 | ART experienced, CD4 < 200 cells/mm3 | Two ART clinics in Addis Ababa | CrAg screening for those with CD4 ≤ 200 cells/mm3 | Undocumented | A retrospective study with 12-month of follow up to assess CrAg, mortality, loss to follow up | 9 |
| Eric et al[49], 2023 | People with AHD (CD4 < 200 cells/mm3 or WHO stage III/IV) | Mbale regional referral hospital and its associated clinics in Uganda | CrAg screening for those with CD4 ≤ 200 cells/mm3 or WHO stage III/IV) | All CrAg positive patients underwent LP | Cross-sectional study to determine prevalence of CrAg | 9 |
| Beyene Tufa et al[50], 2017 | Adults living with HIV, CD4 < 150 cells/mm3 | Adama and Asella hospitals in Ethiopia | CrAg screening for those with CD4 ≤ 200 cells/mm3 | All CrAg positive patients underwent LP | Case-control study comparing the 6-month survival outcomes among CrAg positive and negative patients | 8 |
| Wajanga et al[37], 2011 | Inpatients adults living with HIV, no history of CM, CD4 < 200 cells/mm3 | Bugando Medical Center, a tertiary hospital | CrAg screening for those with CD4 ≤ 200 cells/mm3 | All CrAg positive patients underwent LP | Prospective cohort to determine prevalence of CrAg | 9 |
| Magambo et al[51], 2018 | Outpatients adults living with HIV, no history of CM, CD4 < 200 cells/mm3 | Bugando Medical Center, a tertiary hospital and Sekoture Regional Hospital | CrAg screening for those with CD4 ≤ 200 cells/mm3 | All CrAg positive patients underwent LP | Cross-sectional study to determine prevalence of CrAg | 8 |
| Wake et al[52], 2018 | 16 years or older, PLHIV with CD4 ≤ 9 cells/mm3 | 17 primary care clinics and 5 hospitals in South Africa | CrAg screening for those with CD4 ≤ 200 cells/mm3. Laboratory reflex screening | Undocumented | Cross-sectional study to establish the prevalence of concurrent CM, and the relationship with blood CrAg titer | 8 |
| Wake et al[53], 2020 | Adults (18 years or older) PLHIV with CD4 ≤ 9 cells/mm3 | Helen Joseph and Tambo Memorial Hospitals in South Africa | CrAg screening for those with CD4 ≤ 200 cells/mm3. Laboratory reflex screening | Not applicable | Prospective cohort study to investigate causes of morbidity and mortality during 6 months of follow-up among CrAg-positive and CrAg-negative | 8 |
| Makadzange et al[19], 2021 | Adults (18 years or older) PLHIV with CD4 ≤ 9 cells/mm3, both ART naïve and ART experienced | 20 outpatient facilities in Harare, Zimbabwe | CrAg screening for those with CD4 ≤ 9 cells/mm3 | Refusals | Prospective cohort study to investigate mortality among CrAg-positive and CrAg-negative | 9 |
| Blanco-Arévalo et al[54], 2019 | ART-naïve or poorly ART-adherent PLHIV (CD4 counts < 200 cells/mm3 or WHO stage III/IV) | Manhiça district, Mozambique | No country policy but the study adopted WHO guideline to screen for CrAg among those with CD4 ≤ 9 cells/mm3 | Undocumented | Prospective cohort study to investigate CrAg and mortality | 8 |
| Lakoh et al[38], 2020 | Patients aged 18 years or older with a CD4. Less than 9 cells/mm3 both inpatients and outpatients | Connaught tertiary adult referral hospital in Freetown, Siera Leone | No country policy but the study adopted WHO guideline to screen for CrAg among those with CD4 ≤ 9 cells/mm3 | All CrAg positive patients underwent LP | Prospective cohort study to investigate CrAg and mortality | 8 |
Assessment of study quality showed that all studies were of high quality. For those with scores between 7 and 9, the common theme was smaller sample size or inappropriate sampling.
Figure 2A presents a meta-analysis of the prevalence of LP uptake across multiple studies[15,18-20,29-33,35-40,42,43,45-49,51,52,54]. The individual study estimates are displayed along with their corresponding 95%CI. The pooled prevalence of LP uptake was 67.7% (95%CI: 54.0%–81.5%). Sensitivity analysis was done by excluding prospectively collected clinical data for the studies that reported 100% uptake of LP. When these studies were excluded, the overall uptake of LP was 54.5% (95%CI: 38.8%–70.1%) (Supplementary Figure 1)[15,17-19,21,29,30,32-36,39-41,43-45,47-49,52].
Heterogeneity was observed in the meta-analysis (I2 = 99.6%), indicating considerable variability across studies and that the observed differences in prevalence estimates across studies are largely due to real differences rather than chance. Egger’s test was conducted to assess potential publication bias in the meta-analysis of LP uptake. The results indicated a Z value of 1.18 and a P value of 0.237, suggesting no significant evidence of publication bias. Additionally, the leave-one-out sensitivity analysis showed that all P values were less than 0.001, indicating that no single study disproportionately influenced the overall pooled prevalence estimate (Supplementary Figure 2)[15,17-21,29-52,54].
Figure 2B illustrates the meta-analysis of the prevalence of CM across multiple studies[15,18-20,29-33,35-40,42,43,45-49,51,52,54]. The forest plot displays individual study estimates along with their corresponding 95%CI. The overall pooled prevalence of CM was 54.3% (95%CI: 39.7%–69.0%).
Heterogeneity was observed in the meta-analysis (I2 = 97.7%), indicating variability across studies. Cochran’s Q test yielded a value of 1033.76 (df = 24, P < 0.001), confirming significant heterogeneity. Egger’s test was conducted to assess potential publication bias in the meta-analysis of CM prevalence. The results indicated a Z value of 0.19 (P = 0.8501), suggesting no significant evidence of publication bias. Additionally, the leave-one-out sensitivity analysis showed that all P values were less than 0.001, indicating that no single study disproportionately influenced the overall pooled estimate (Supplementary Figure 3)[15,18-20,29-33,35-40,42,43,45-49,51,52,54].
Figure 2C presents the meta-analysis findings on the prevalence of mortality across multiple studies[15,17-21,31,32,35,37,38,45,50,52-54]. The forest plot illustrates individual study estimates alongside their 95%CI. The pooled mortality prevalence was 6.2% (95%CI: 4.5%–8.0%), as indicated by the summary estimate at the bottom of the plot.
Heterogeneity was detected in the meta-analysis (I2 = 84.4%), reflecting variability across studies. Cochran’s Q test (Q = 96.14, df = 15, P < 0.001) confirmed significant between-study differences.
Egger’s test (Z = 4.36, P = 0.001) suggests potential publication bias, which may be influenced by small-study effects. However, the robustness of the findings, as indicated by the leave-one-out sensitivity analysis (all P values < 0.001), suggests that no single study disproportionately impacted the overall pooled estimate (Supplementary Figure 4)[15,17-21,31,32,35,37,38,45,50,52-54.
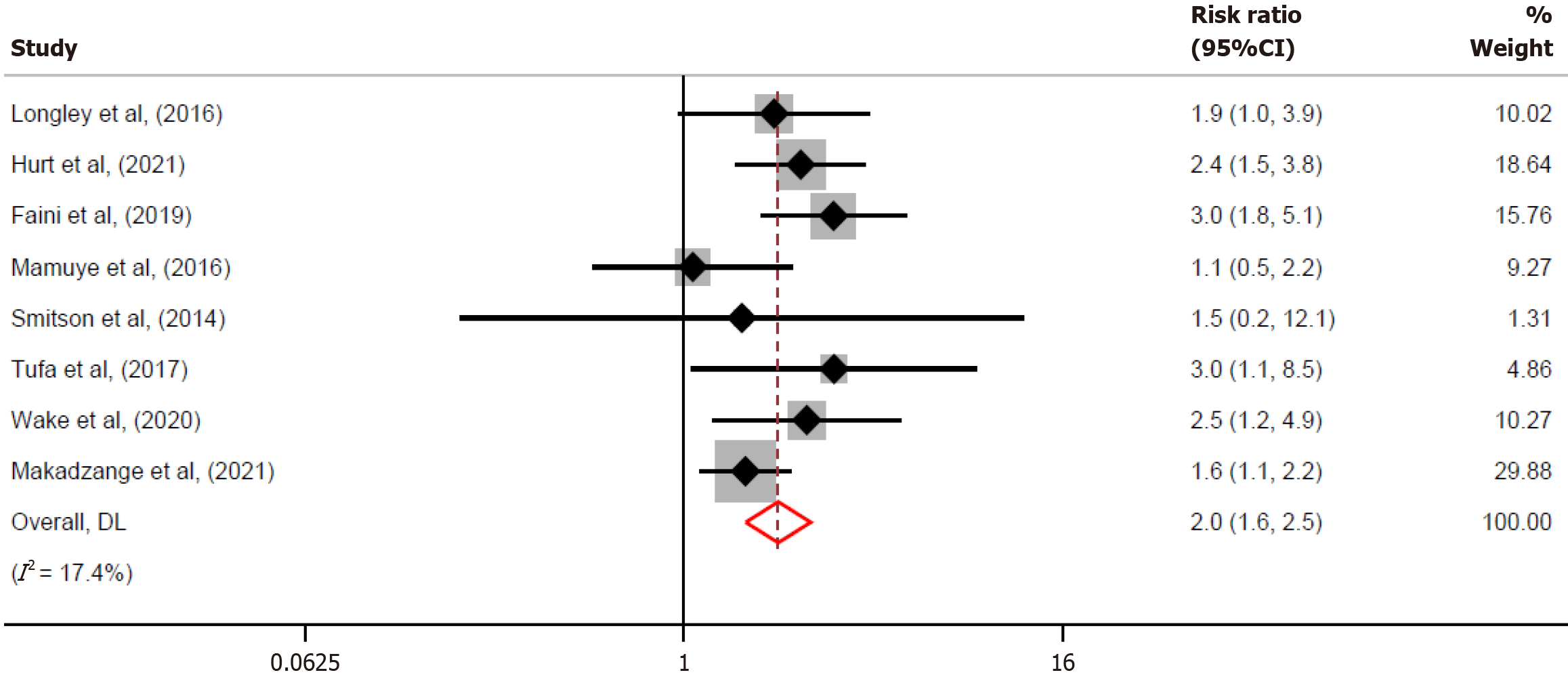
The meta-analysis indicates that mortality among people with positive CrAg was higher compared to those with negative CrAg, with an overall risk ratio of 2.0 (95%CI: 1.6–2.5) (Figure 3)[15,17-21,50,53]. The heterogeneity was low (I2 = 17.4%), indicating consistency across studies.
We evaluated uptake of LP procedure, prevalence of CM and mortality among patients presenting with AHD in Africa. The overall prevalence of LP uptake was between 54.5% and 67.7%. Among those with positive serum CrAg and underwent LP procedure, the prevalence of CM was 54.3%. The overall mortality was 22.5% among those with positive serum CrAg and 10.7% among those with negative serum CrAg.
Nearly three to five in 10 of the patients with positive serum CrAg did not have LP procedures done to establish a diagnosis of CM per WHO guidelines recommendations. As stated previously, 25% of patients with positive serum CrAg develop CM. Failure to perform LP procedures among patients with positive serum CrAg presents missed opportunity to identify those with CM. Because positive serum CrAg patients are given preemptive antifungals, providing preemptive antifungal medications to patients with subclinical CM predisposes them to subtherapeutic levels of drugs and increased risk of death. This is because treatment of CM requires more intensive antifungals than those provided preemptively. Moreover, exposure to the subtherapeutic levels of drugs may potentiate the development of drug resistance[55]. Overall, identification of patients with CM through cerebrospinal fluid examination is beneficial to avoid negative consequences associated with failure to establish the diagnosis through LP. Refusals, transfers out and deaths prior to LP procedure are some of the reasons for not performing the procedure[14,15,56]. Capacity (human resource, equipment and fear of bad outcomes) is another reason for not performing LP procedure[57]. Since CM is a common disease in patients with AHD and Africa being the most hit region with HIV[58], there is a need to invest in training healthcare workers to improve their skills to perform the procedure. Improving counselling skills to obtain patient consent and referrals to nearby facilities capable of performing LP procedures are critical to increase its uptake for accurate diagnosis and management of these patients. One clinical trial in Uganda showed that community engagement and social support showed a reduction of LP refusals from 31% in 2010 to 1% in 2017[59] ,indicating the role of these interventions to improve uptake of LP procedures.
We observed disparities in uptake of LP procedure between studies involved single sites in comparison to those involved multiple sites with those from multiple sites tending to have smaller prevalence of LP uptake. As expected, multi-site studies had mixed level healthcare facilities. Healthcare facilities at lower tiers are likely to be resource-constrained with impaired capacity to perform LP compared to higher tier facilities. Eight of the 12 studies from the current review that were from single sites were done from tertiary hospitals which are undoubtedly better resourced to undertake the task. These site-specific differences in LP uptake highlight the need for utilization of nationally representative data to evaluate compliance of WHO treatment guidelines. Data from single-site studies, specifically those from high tier hospitals, may overestimate LP prevalence uptake. In comparison, multi-site studies, which encompasses lower-level hospitals emphasize the problems faced by them, such as limited capacity of healthcare providers.
Mortality is high with 6.2% of patients who presented with AHD dying. Delayed seeking care impairs efforts to control the HIV epidemic. Stigma associated with HIV remains the main concern associated with delays in seeking care until patients develop AHD[60-62]. In this era of test and treat and increased access to ART, additional efforts to address stigma are required to achieve 95-95-95 targets and control the HIV epidemic.
Mortality was disproportionately high among those with positive serum CrAg compared to those with negative serum CrAg. A prior systematic review showed that uptake of preemptive antifungal medications among patients with positive serum CrAg and no evidence of CM was 85%, indicating gaps of preventive therapy among those in need[63]. Special attention should be paid to those with positive serum CrAg to increase uptake of preemptive antifungal medications and reduce mortality.
Several limitations were acknowledged in this study. This systematic review included data collected over nine years and because during this span, treatment guidelines varied and may explain disparities of LP uptake. Moreover, we included data from studies that involved single and multiple sites. Studies from single sites, particularly those from tertiary hospitals, tended to provide higher estimates of LP uptake compared to studies from multiple sites which encompasses healthcare facilities with mixed tiers. An ideal LP procedure uptake evaluation would be assessed using nationally representative data which comprises data from mixed level health care facilities. Moreover, by restricting search to publications written in English, we might have excluded potential publications written from other languages. Despite these limitations, the inclusion of 32 studies resulted in a large sample size and strengthened associations observed in this study. This clinical auditing review is important because it summarizes the effect size of the LP uptake giving opportunity to understand the extent of adherence with WHO recommendations in the care of people with AHD.
Our observations indicate adherence with WHO guidelines in the care of patients with AHD is less than expected with three to five in 10 patients with AHD and positive serum CrAg not undergoing LPs. To reduce the risk of predisposing patients with subclinical CM to subtherapeutic levels of antifungal medications, country HIV programs must prioritize periodic assessments of LP uptake and safeguard constant adherence to treatment guidelines. These periodic assessments are paramount to detect gaps and implement corrective measures. Improved training, equipment availability, and professional counselling to obtain patient consent are essential for achieving higher LP procedure uptake. Close monitoring for those with positive serum CrAg is needed to reduce mortality.
We acknowledged Emilie Ludeman for systematic search of the manuscripts and their compilation into the Covidence software.
| 1. | Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009;49:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Rajasingham R, Govender NP, Jordan A, Loyse A, Shroufi A, Denning DW, Meya DB, Chiller TM, Boulware DR. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis. 2022;22:1748-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 420] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 3. | Guidelines for The Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva: World Health Organization, 2018 . [PubMed] |
| 4. | Apisarnthanarak A, Mundy LM. The impact of primary prophylaxis for cryptococcosis on fluconazole resistance in Candida species. J Acquir Immune Defic Syndr. 2008;47:644-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Wake RM, Molloy SF, Jarvis JN, Harrison TS, Govender NP. Cryptococcal Antigenemia in Advanced Human Immunodeficiency Virus Disease: Pathophysiology, Epidemiology, and Clinical Implications. Clin Infect Dis. 2023;76:764-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Rajasingham R, Meya DB, Boulware DR. Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. J Acquir Immune Defic Syndr. 2012;59:e85-e91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Jarvis JN, Govender N, Chiller T, Park BJ, Longley N, Meintjes G, Bekker LG, Wood R, Lawn SD, Harrison TS. Cryptococcal antigen screening and preemptive therapy in patients initiating antiretroviral therapy in resource-limited settings: a proposed algorithm for clinical implementation. J Int Assoc Physicians AIDS Care (Chic). 2012;11:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Rajasingham R, Meya DB, Greene GS, Jordan A, Nakawuka M, Chiller TM, Boulware DR, Larson BA. Evaluation of a national cryptococcal antigen screening program for HIV-infected patients in Uganda: A cost-effectiveness modeling analysis. PLoS One. 2019;14:e0210105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | Temfack E, Bigna JJ, Luma HN, Spijker R, Meintjes G, Jarvis JN, Dromer F, Harrison T, Cohen JF, Lortholary O. Impact of Routine Cryptococcal Antigen Screening and Targeted Preemptive Fluconazole Therapy in Antiretroviral-naive Human Immunodeficiency Virus-infected Adults With CD4 Cell Counts <100/μL: A Systematic Review and Meta-analysis. Clin Infect Dis. 2019;68:688-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Li Y, Huang X, Chen H, Qin Y, Hou J, Li A, Wu H, Yan X, Chen Y. The prevalence of cryptococcal antigen (CrAg) and benefits of pre-emptive antifungal treatment among HIV-infected persons with CD4+ T-cell counts < 200 cells/μL: evidence based on a meta-analysis. BMC Infect Dis. 2020;20:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Jane LA, Wray AA. Lumbar Puncture. 2023 Jul 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 13. | Saylor D, Elafros M, Bearden D, Dallah I, Mathews M, Muchanga G, Mwale M, Mwenechanya M, Siddiqi OK, Winch PJ, Somwe SW, Birbeck GL. Patient, Provider, and Health Systems Factors Leading to Lumbar Puncture Nonperformance in Zambia: A Qualitative Investigation of the "Tap Gap". Am J Trop Med Hyg. 2023;108:1052-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Acharya S, Allam RR, Karanjkar VK, Rathod D, Mahajan R, Deshpande P, Palkar A, Todmal S, Koli S, Dhande S, Dale J, Yeldandi VV, Harshana A, Agarwal R, Upadhyaya S, Nyendak M. Implementation of point-of-care testing and prevalence of cryptococcal antigenaemia among patients with advanced HIV disease in Mumbai, India. BMJ Open. 2023;13:e070500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Longley N, Jarvis JN, Meintjes G, Boulle A, Cross A, Kelly N, Govender NP, Bekker LG, Wood R, Harrison TS. Cryptococcal Antigen Screening in Patients Initiating ART in South Africa: A Prospective Cohort Study. Clin Infect Dis. 2016;62:581-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | King M, Rwegerera G. An audit of consent practices and perceptions of lumbar puncture, Botswana inpatient setting experience. AFJEM. 2015;5:66-69. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Hurt WJ, Tenforde MW, Molefi M, Mitchell HK, Milton T, Azama MS, Goercke I, Mulenga F, Tlhako N, Tsholo K, Srivastava T, Leeme TB, Simoonga G, Muthoga C, Lechiile K, Mine M, Jarvis JN. Prevalence and Sequelae of Cryptococcal Antigenemia in Antiretroviral Therapy-Experienced Populations: An Evaluation of Reflex Cryptococcal Antigen Screening in Botswana. Clin Infect Dis. 2021;72:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Faini D, Kalinjuma AV, Katende A, Mbwaji G, Mnzava D, Nyuri A, Glass TR, Furrer H, Hatz C, Boulware DR, Letang E. Laboratory-Reflex Cryptococcal Antigen Screening Is Associated With a Survival Benefit in Tanzania. J Acquir Immune Defic Syndr. 2019;80:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Makadzange TA, Hlupeni A, Machekano R, Boyd K, Mtisi T, Nyamayaro P, Ross C, Vallabhaneni S, Balachandra S, Chonzi P, Ndhlovu CE. Survival following screening and preemptive antifungal therapy for subclinical cryptococcal disease in advanced HIV infection. AIDS. 2021;35:1929-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Mamuye AT, Bornstein E, Temesgen O, Blumberg HM, Kempker RR. Point-of-Care Testing for Cryptococcal Disease Among Hospitalized Human Immunodeficiency Virus-Infected Adults in Ethiopia. Am J Trop Med Hyg. 2016;95:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Smitson CCTA, Tsegaye M, Shiferaw A, Aseffa A, Blumberg H, Kempker R. No association of cryptococcal antigenemia with death or loss to follow up among hiv patients in Ethiopia. JIM. 2013;61. |
| 22. | Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 628] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 23. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 50772] [Article Influence: 10154.4] [Reference Citation Analysis (2)] |
| 24. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley and Sons, 2019. |
| 25. | Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Chapter 5: Systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. Australia: JBI, 2017. |
| 26. | Miller JJ. The Inverse of the Freeman–Tukey Double Arcsine Transformation. AM STAT. 1978;32:138-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48457] [Article Influence: 2106.8] [Reference Citation Analysis (4)] |
| 28. | Bakari HM, Alo O, Mbwana MS, Salim SM, Ludeman E, Lascko T, Ramadhani HO. Same-day ART initiation, loss to follow-up and viral load suppression among people living with HIV in low- and middle-income countries: systematic review and meta-analysis. Pan Afr Med J. 2023;46:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 29. | Blankley S, Gashu T, Ahmad B, Belaye AK, Ringtho L, Mesic A, Zizhou S, Casas EC. Lessons learned: Retrospective assessment of outcomes and management of patients with advanced HIV disease in a semi-urban polyclinic in Epworth, Zimbabwe. PLoS One. 2019;14:e0214739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Heller T, Damba D, Kumwenda T, Huwa J, Kamamia C, Nhlema A, Wallrauch C, Chawinga C, Kanyama C, Gondwe-Chunda L, Ngoma J, Matanje B, Tweya H. Implementing Advanced HIV Disease Care for Inpatients in a Referral Hospital in Malawi - Demand, Results and Cost Implications. Ann Glob Health. 2022;88:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Kanyama C, Chagomerana MB, Chawinga C, Ngoma J, Shumba I, Kumwenda W, Armando B, Kumwenda T, Kumwenda E, Hosseinipour MC. Implementation of tuberculosis and cryptococcal meningitis rapid diagnostic tests amongst patients with advanced HIV at Kamuzu Central Hospital, Malawi, 2016-2017. BMC Infect Dis. 2022;22:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Deiss R, Loreti CV, Gutierrez AG, Filipe E, Tatia M, Issufo S, Ciglenecki I, Loarec A, Vivaldo H, Barra C, Siufi C, Molfino L, Tamayo Antabak N. High burden of cryptococcal antigenemia and meningitis among patients presenting at an emergency department in Maputo, Mozambique. PLoS One. 2021;16:e0250195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Bornstein ETA, Temesgen O, Blumberg HM, Kempker R. Point of care screening for cryptococcal disease among hospitalized HIV infected adults in ethiopia. JIM. 2014;62:1. |
| 34. | Temfack E, Kouanfack C, Mossiang L, Loyse A, Fonkoua MC, Molloy SF, Koulla-Shiro S, Delaporte E, Dromer F, Harrison T, Lortholary O. Cryptococcal Antigen Screening in Asymptomatic HIV-Infected Antiretroviral Naïve Patients in Cameroon and Evaluation of the New Semi-Quantitative Biosynex CryptoPS Test. Front Microbiol. 2018;9:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Pac L, Horwitz MM, Namutebi AM, Auerbach BJ, Semeere A, Namulema T, Schwarz M, Bbosa R, Muruta A, Meya DB, Manabe YC. Implementation and operational research: Integrated pre-antiretroviral therapy screening and treatment for tuberculosis and cryptococcal antigenemia. J Acquir Immune Defic Syndr. 2015;68:e69-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Oyella J, Meya D, Bajunirwe F, Kamya MR. Prevalence and factors associated with cryptococcal antigenemia among severely immunosuppressed HIV-infected adults in Uganda: a cross-sectional study. J Int AIDS Soc. 2012;15:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Wajanga BM, Kalluvya S, Downs JA, Johnson WD, Fitzgerald DW, Peck RN. Universal screening of Tanzanian HIV-infected adult inpatients with the serum cryptococcal antigen to improve diagnosis and reduce mortality: an operational study. J Int AIDS Soc. 2011;14:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Lakoh S, Rickman H, Sesay M, Kenneh S, Burke R, Baldeh M, Jiba DF, Tejan YS, Boyle S, Koroma C, Deen GF, Beynon F. Prevalence and mortality of cryptococcal disease in adults with advanced HIV in an urban tertiary hospital in Sierra Leone: a prospective study. BMC Infect Dis. 2020;20:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Boyd K, Kouamou V, Hlupeni A, Tangwena Z, Ndhlovu CE, Makadzange AT; CryptoART Study Team. Diagnostic Accuracy of Point of Care Cryptococcal Antigen Lateral Flow Assay in Fingerprick Whole Blood and Urine Samples for the Detection of Asymptomatic Cryptococcal Disease in Patients with Advanced HIV Disease. Microbiol Spectr. 2022;10:e0107522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 40. | Blasich NP, Wake RM, Rukasha I, Prince Y, Govender NP. Association of semi-quantitative cryptococcal antigen results in plasma with subclinical cryptococcal meningitis and mortality among patients with advanced HIV disease. Med Mycol. 2021;59:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Enock K, Julius K, Griffith BC, Abila DB, Rutakingirwa MK, Kasibante J, Kandole KT, Kwizera R, Semeere A, Meya DB. Evaluation of the initial 12 months of a routine cryptococcal antigen screening program in reduction of HIV-associated cryptococcal meningitis in Uganda. BMC Health Serv Res. 2022;22:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 42. | Tiam A, Paulin H, Machekano R, Oboho I, Agyemang E, Mugyenyi FA, Maama-Maime L, Mengistu Y, Chatora T, Mungati M, Mokone M, Mots'oane T, Masheane A, Tukei V. Rapid antiretroviral therapy initiation in patients with advanced HIV disease: 6-month outcomes of an observational cohort evaluation in Lesotho. PLoS One. 2023;18:e0292660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 43. | Levy-Braide NOB, Abudiore O, Eigege W, Sowale O, Inyang A, Amamilo I, Rathakrishnan D, Moore A, Conroy J, Amole C, Lufadeju F, Wiwa O, Adesigbin C, Nwaokenneya P, Atu U, Patiko MI, Ikpeazu A, Oladele R, Agbaji O, Akanmu S. Lessons Learned from the introduction of advanced HIV disease package of care in Nigeria. In: International AIDS society. Australia: Melbourne, 2023. |
| 44. | Ndayishimiye E, Ross AJ. An audit of the screen-and-treat intervention to reduce cryptococcal meningitis in HIV-positive patients with low CD4 count. Afr J Prim Health Care Fam Med. 2018;10:e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Nalintya E, Meya DB, Lofgren S, Huppler Hullsiek K, Boulware DR, Rajasingham R. A Prospective Evaluation of a Multisite Cryptococcal Screening and Treatment Program in HIV Clinics in Uganda. J Acquir Immune Defic Syndr. 2018;78:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Ssebambulidde K, Bangdiwala AS, Kwizera R, Kandole TK, Tugume L, Kiggundu R, Mpoza E, Nuwagira E, Williams DA, Lofgren SM, Abassi M, Musubire AK, Cresswell FV, Rhein J, Muzoora C, Hullsiek KH, Boulware DR, Meya DB; Adjunctive Sertraline for Treatment of HIV-associated Cryptococcal Meningitis Team. Symptomatic Cryptococcal Antigenemia Presenting as Early Cryptococcal Meningitis With Negative Cerebral Spinal Fluid Analysis. Clin Infect Dis. 2019;68:2094-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Zono BB, Sacheli R, Kasumba DM, Situakibanza HN, Mavanga A, Anyshayi JM, Etondo M, Muwonga J, Moutschen M, Mvumbi GL, Hayette MP. Screening for cryptococcal antigenemia and meningeal cryptococcosis, genetic characterization of Cryptococcus neoformans in asymptomatic patients with advanced HIV disease in Kinshasa, Democratic Republic of Congo. Sci Rep. 2024;14:29959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Eigege W, Agbaji O, Otubu N, Abudiore O, Sowale O, Levy-Braide B, Inyang A, Rathakrishnan D, Amamilo I, Conroy J, Lufadeju F, Amole C, Wiwa O, Onotu D, Sanni K, Nwaokenneya P, Patiko M, Ikpeazu A, Oguche S, Oladele R, Akanmu S. Implementation of the advanced HIV disease package of care using a public health approach: lessons from Nigeria. BMC Public Health. 2024;24:3366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 49. | Eric E, Olupot-Olupot P, Bwayo D, Meya D, Katuramu R. Prevalence and Factors Associated With Cryptoccocal Antigenemia Among Patients With Advanced Human Immunodeficiency Virus in Eastern Uganda: A Facility-Based Cross-sectional Study. Open Forum Infect Dis. 2023;10:ofad351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 50. | Beyene Tufa T, Girma A, Rajasingham R, Boulware D. Survival in HIV-infected Asymptomatic Cryptococcal Antigenemia without CSF Positivity Treated with Fluconazole Did Not Differ from Cryptococcal Antigen (CrAg) Negative with CD4 <150. Open Forum Infect Di. 2017;4:S208-S208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 51. | Magambo KA, Kalluvya SE, Kapoor SW, Seni J, Chofle AA, Fitzgerald DW, Downs JA. Utility of urine and serum lateral flow assays to determine the prevalence and predictors of cryptococcal antigenemia in HIV-positive outpatients beginning antiretroviral therapy in Mwanza, Tanzania. J Int AIDS Soc. 2014;17:19040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Wake RM, Britz E, Sriruttan C, Rukasha I, Omar T, Spencer DC, Nel JS, Mashamaite S, Adelekan A, Chiller TM, Jarvis JN, Harrison TS, Govender NP. High Cryptococcal Antigen Titers in Blood Are Predictive of Subclinical Cryptococcal Meningitis Among Human Immunodeficiency Virus-Infected Patients. Clin Infect Dis. 2018;66:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 53. | Wake RM, Govender NP, Omar T, Nel C, Mazanderani AH, Karat AS, Ismail NA, Tiemessen CT, Jarvis JN, Harrison TS. Cryptococcal-related Mortality Despite Fluconazole Preemptive Treatment in a Cryptococcal Antigen Screen-and-Treat Program. Clin Infect Dis. 2020;70:1683-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 54. | Blanco-Arévalo ASJ, Murias A, Catorze N, Jordan A, Greene G, Santiago I, Nhampossa T, Letang E. Cryptococcal and tuberculosis coinfection: Case series identified through the implementation of an advanced HIV disease package of care linked to a TB active case finding strategy in rural Mozambique. HIV Med. 2019;20:2. |
| 55. | Di Paolo M, Hewitt L, Nwanko E, Ni M, Vidal-Diaz A, Fisher MC, Armstrong-James D, Shah A. A retrospective 'real-world' cohort study of azole therapeutic drug monitoring and evolution of antifungal resistance in cystic fibrosis. JAC Antimicrob Resist. 2021;3:dlab026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, Chijoka C, Masasi A, Kimaro G, Ngowi B, Kahwa A, Mwaba P, Harrison TS, Egwaga S, Jaffar S; REMSTART trial team. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. 2015;385:2173-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 57. | Thakur KT, Mateyo K, Hachaambwa L, Kayamba V, Mallewa M, Mallewa J, Nwazor EO, Lawal T, Mallum CB, Atadzhanov M, Boulware DR, Birbeck GL, Siddiqi OK. Lumbar puncture refusal in sub-Saharan Africa: A call for further understanding and intervention. Neurology. 2015;84:1988-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | UNAIDS. Global HIV statistics. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. |
| 59. | Kwizera R, Sadiq A, Ndyetukira JF, Nalintya E, Williams D, Rhein J, Boulware DR, Meya DB; COAT and ASTRO trial teams. Impact of community engagement and social support on the outcomes of HIV-related meningitis clinical trials in a resource-limited setting. Res Involv Engagem. 2020;6:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Belay GM, Endalamaw A, Ayele AD. Late presentation of HIV positive adults and its predictors to HIV/AIDS care in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Chone JS, Abecasis AB, Varandas L. Determinants of Late HIV Presentation at Ndlavela Health Center in Mozambique. Int J Environ Res Public Health. 2022;19:4568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 62. | Lofgren SM, Tsui S, Natala N, Nakasujja N, Sebuliba R, Ndyetukira JF, Arinda A, Akinyange V, Hullsiek KH, Nalintya E, Sadiq A, Pastick KA, Stadleman A, Meya D, Boulware DR. Differences in Reasons for Late Presentation to HIV Care in Uganda Among Men and Women. AIDS Behav. 2023;27:303-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Ally ZM, Mbishi JV, Mbwana MS, Bakari HM, Salim SM, Rodoshi ZN, Hundisa MI, Sileshi RM, Ayalew BD, Musoke R, Moshi L, Fakhoury YE, Ally HM, Ramadhani HO. Systematic review on the compliance of WHO guidelines in the management of patients with advanced HIV disease in Africa: The case of cryptococcal antigen screening. PLoS One. 2025;20:e0313453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/