Published online Dec 19, 2025. doi: 10.5498/wjp.v15.i12.112055
Revised: August 11, 2025
Accepted: September 4, 2025
Published online: December 19, 2025
Processing time: 134 Days and 5.7 Hours
Tic disorders (TDs) are a type of neurological and psychiatric disorder characterized by vocal or motor tics in the head, body, or limbs. Clinical studies have shown that Changmaxifeng granules (CG) can treat TDs. However, the pharmaceutical substances and mechanism of action of CG remain unclear.
To investigate the pharmaceutical substances and action mechanisms of CG against TDs, this study employs serum medicinal chemistry, network pharmacology, and molecular docking analysis.
Ultrahigh-performance liquid chromatography with quadrupole time-of-flight mass spectrometry was used to identify the blood-absorbed constituents of CG; Network pharmacology was then used to integrate these compounds with disease targets, followed by protein-protein interaction (PPI) networks analysis to pinpoint key proteins. Finally, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses combined with molecular docking elucidated the underlying mechanism of action.
Overall, 187 chemical components, including terpenoids, sugars, phenolic acids, and flavonoids, were identified in vitro. In addition, 75 components, namely 49 prototype components and 26 metabolites, were identified in vivo. The PPI results revealed 225 overlapping targets, with TNF, IL-6, FOS, VEGFA, and ESR1 being the major targets. GO and KEGG analyses were performed to identify key signaling pathways and biological processes. Paeonol, evofolin B, aspalathin, and paeoniflorin were identified as potential pharmacodynamic substances based on the results of the “compound-target” network. The maximum binding energy between the core target and the active ingredient was less than -4.7 kcal/mol, indicating that the pharmacophore exhibited a strong affinity toward the core ingre
This study elucidated the in vitro and in vivo chemical components of CG and outlined their potential targets and action mechanisms. This study provides a basis for further research into the action mechanism and clinical application of CG.
Core Tip: Clinical studies have shown that Changmaxifeng granules (CG) can treat tic disorders. However, the pharmaceutical substances and mechanism of action of CG remain unclear. In this study, we combined serum pharmacochemistry with network pharmacology to identify the chemical constituents of CG that enter the bloodstream and to predict their potential molecular targets, thereby laying the groundwork for elucidating the underlying mechanisms of action.
- Citation: Xie LD, Wu JP, Liu SS, Zong Z, Hu Y, Ling N, Han B, Li WL, Yao HY. Investigating the pharmaceutical substances and action mechanisms of Changmaxifeng granules against tic disorders. World J Psychiatry 2025; 15(12): 112055
- URL: https://www.wjgnet.com/2220-3206/full/v15/i12/112055.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i12.112055
Tic disorder (TD) is a mental developmental disorder that most commonly affects children between the ages of 5 and 10 years and is characterized by involuntary blinking, strange face-making, head twisting, eyebrow squeezing, and lower limb twitching[1]. Symptoms are aggravated by mental stress, diminished through concentration, and absent while sleeping. The duration of the condition usually ranges from a few months to a year, and the condition severely affects children’s learning, daily life, and physical and mental development[2]. The clinical manifestations of this disorder are diverse and often associated with various comorbidities, such as attention deficit hyperactivity disorder, obsessive compulsive disorder, anxiety, and depression[3]. The pathogenesis of TD is complex and currently remains unclear[4]. However, there has been a gradual increase in the incidence of TD, globally[5]. Most medications used to treat TD are psychotropic drugs, which can relieve symptoms but have been clinically shown to be ineffective in the long term. For example, the most common drugs used for TD treatment include dopamine receptor blockers, which can lead to adverse effects, such as cognitive impairment[6]. Modern studies have shown that traditional Chinese medicine (TCM) can help regulate neurotransmitter release and metabolism through dopaminergic synapses, neuroligand-receptor interactions, and other pathways, exerting a multicomponent, multitarget, and multipathway anti-TD effect with better clinical outcomes, fewer side effects, and a low relapse rate[7-9].
Jiren Pharmaceutical Co., Ltd. (Heilongjiang Province, China) developed Changmaxifeng Tables (CT; State Drug License Z20140013) based on a famous clinical prescription. CT comprises five types of Chinese medicines: Paeoniae Radix Alba [Baishao (BS)], Gastrodiae Rhizoma [Tianma (TM)], Polygalae Radix [Yuanzhi (YZ)], Acori Tatarinowii Rhizoma [Shichangpu (SCP)], and Margaritifera Concha [Zhenzhumu (ZZM)]. Modern pharmacological studies have found that Chinese medicines such as BS, TM, and YZ possess varying degrees of neuroprotective effects[10-13]. Gai[14] reported that CT exhibits higher clinical efficacy and safety than Thiabetic, and it was recommended as a first-line drug.
After experimental evaluation, it was found that the tablets were changed to granules could improve drug compliance; however, the pharmacodynamic composition and action mechanisms of Changmaxifeng granules (CG) remain unclear. This has become an obstacle to the marketing of CG as a new drug. Therefore, according to the concept of “effective, unique, transmission and traceability, measurable and prescription matching” in Chinese medicine quality markers, investigating the blood components of Chinese medicines is important to understand their pharmacodynamic composition and action mechanism in vivo. Thus, this study analyzed the composition of CG and its components that are absorbed into the blood via and ultrahigh-performance liquid chromatography with quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS/MS) technology. Based on the blood components alongside network pharmacology and molecular docking, we initially discovered the pharmacodynamic composition and action mechanism of CG against TD. Therefore, this approach lays the foundation for understanding the pharmacodynamic composition and action mechanism of CG and development of new CG dosage forms. Meanwhile, it provides strong support for the completion of new drug application.
LE2O4E/O2 electronic analytical balances (Mettler-Toledo International Inc); Acquity high performance liquid chromatography, Xevo G2 Q TOF mass spectrometer (Waters Corporation, United States), chromatographic column (Waters ACQUITY UPLC BEH C18 2.1 mm × 100 mm, 1.7 μm), UC-250E ultrasonic cleaner (Shanghai Jingqi Instrument Co., Ltd., Shanghai, China), high-speed freezing centrifuge (Shanghai Lixin Scientific Instrument Co. Shanghai, China).
CG was provided by Jiren Pharmaceutical Co., Ltd. (Heilongjiang Province, China), 1 g/bag; methanol, acetonitrile, and formic acid were of mass spectral grade (Thermo Fisher Scientific Ltd., Batch No. 20210323, 202100517, 20190312). Paeoniflorin, paeonilactone, asparagine, 3,6'-dierucoylsucrose, and 1,2,3,4,6-O-galloylglucose were obtained from Chengdu Push Biotechnology Co., LTD. (Batch No. PS000825, PS021057, PS012103, PS011608, and PS013974, respectively). Gallic acid was procured from Shanghai Yuanye Biotechnology Co., Ltd. (Batch No. AN1127SA14).
Twelve 4-week-old specific pathogen-free-grade Sprague-Dawley rats (six male and six female) were provided by Changchun Yisi Experimental Animals Technology Limited Co., Ltd. (Certificate No. 01021731571831465). The animals were housed in separate cages (n = 6) in a 12-hour alternating light and dark environment at a temperature of 18-21 °C and a relative humidity of 50% ± 5%. The experimental animals were fed standard chow and allowed to eat and drink freely throughout the experimental period. All animals were handled in accordance with the procedures described in the Guidelines for the Management and Use of Laboratory Animals (People’s Republic of China), which were reviewed by the Animal Ethics Committee of Harbin University of Commerce and found to be in accordance with the standards (Ethical Approval No. HSDYXY-2024055).
The animal protocol was designed to minimize pain or discomfort to the animals. Intragastric gavage administration was carried out with conscious animals, using straight gavage needles appropriate for the animal size. All animals were anesthetized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for plasma collection.
Establishment of a chemical composition database of CG: By searching domestic and foreign literature (CNKI, PubMed, and Web of Science), TCM literature databases, and organizing the chemical composition of five types of TCMs in CG, a comprehensive database was established.
Chromatographic and mass spectrometric conditions: Chromatographic conditions: The time point with the most transitional components was selected to perform the UPLC-Q-TOF-MS analysis; an ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm) was used. The binary gradient elution system comprised 0.1% formic acid aqueous solution and acetonitrile. The chromatogram was monitored at 275 nm and obtained using the following linear gradient elution program: B started at 2% and increased linearly from 2% to 10% (0-2 minute), 10%-16% (2-4 minute), 16%-31% (4-8 minute), 31%-46% (8-12 minute), 46%-68% (12-17 minute), 68%-80% (17-20 minute), and finally 80%-2% (20-23 minute). The injection volume of each sample was 2.0 μL. The column temperature was maintained at 30 °C, and the mobile phase was pumped at a flow rate of 0.3 mL/min.
MS conditions: MS was conducted on an Agilent-1100 HPLC/6520 Q-TOF-MS system (Waters Corp., United States) equipped with an electrospray ionization source in the negative ion mode. The collision energy was 20-45 mV, and the volume flow rate of the desolvation gas was 800 L/h. The temperatures of the desolvation gas and ion source were 400 °C and 100 °C, respectively. The spray voltage was 3.0 kV. The mass data were recorded within a scan range of 50-2000 Da.
Preparation of test and control solutions: Approximately 4 g of CG was added to a stoppered conical flask containing 25 mL of 50% methanol. The mixture was subjected to ultrasonic treatment for 10 minutes, cooled, shaken well, and centrifuged at 4000 r/min for 10 minutes. The supernatant was then passed through a 0.22-μm microporous membrane for filtration, and the filtrate was obtained.
Paeoniflorin, paeonilactone, asparagine, 1,2,3,4,6-O-galloylglucose, gallic acid, and 3,6'-dierucoylsucrose were added in appropriate amounts to a volumetric flask containing 10 mL of 50% methanol. The mixture was passed through a 0.22-μm microporous filtration membrane, and the filtrate was obtained.
Animal grouping and drug administration: The rats were randomly divided into blank and drug administration groups (n = 6), half of which were male and female. In the drug administration group, rats were gavaged with an aqueous solution of CG at a dose of 0.95 g/kg/day (The clinical pediatric doses of CG are 3 g/day for children aged 3-6 years and 6 g/day for children aged 7-11 years. Using the FDA body-surface-area conversion factor, the rat equivalent dose is calculated as: Human dose × 6.3/40 kg approximately 0.95 g/kg/day), by oral gavage once daily for 5 consecutive days. while the blank group was gavaged with an equal amount of saline. The drugs were administered once daily for 5 days. Blood was collected at 0.5-hour, 1-hour, 1.5-hour, and 2-hour after the last administration of the drugs and centrifuged in sodium heparin tubes for further preservation.
Plasma sample collection and pretreatment: Plasma sample collection: Rats were used as experimental subjects, and the aqueous solution of CG was gavaged. Blood was collected from the fundus venosus at 0.5-hour, 1-hour, 1.5-hour, and 2-hour, and all Eppendorf tubes and capillaries were infiltrated with heparin before blood collection. The collected blood was centrifuged at 3000 r/minute for 15 minutes, and the supernatant was removed with a pipette gun to obtain the plasma sample.
Plasma sample processing: A four-fold amount of methanol was added to 3 mL of plasma to precipitate proteins, and then vortexed and mixed for 2 minutes. After standing for 5 minutes, the plasma was centrifuged for 10 minutes at 13000 r/minute. The supernatant was aspirated and blow dried using a nitrogen blower. Two times the amount of methanol was added, and the process was repeated. The blow-dried solid was reconstituted with 0.5 mL of methanol, vortexed for 30 seconds, and centrifuged for 10 minutes at 13000 r/minute. The supernatant was aspirated and passed through a 0.22-μm microporous filter membrane to obtain a serum sample, which was retained for measurement.
Collection and screening of blood-entry components and TD targets: By inputting the names of components absorbed into blood from the TCMSP database (https://old.TCMsp-e.com/TCMsp.php), we retrieved information on the components of CG that were absorbed into blood and exported the corresponding effective targets of the screened active ingredients. Converting these targets into human genetic proteins using the UniProt database (https://www.uniprot.org/) using “TD” as a keyword, we searched the GeneCards (https://www.genecards.org/), OMIM (https://omim.org), CTD (http://ctdbase.org/), and DisGeNET (https://www.disgenet.org/) databases to obtain tar
Active compounds and disease targets were retained only if they met the following thresholds: Oral bioavailability > 30% and drug-likeness > 0.18 in TCMSP; GeneCards (v5.15) and DisGeNET (v7.0): All entries with a relevance/association score > 0 were retained without further cut-off to avoid missing any potential targets.
Common target gene-protein interaction network construction and Hub gene screening: The intersecting target genes were imported into the STRING database (https://cn.string-db.org/), and the minimum required interaction score was set to the highest confidence (0.900). Protein species was selected as “Homo sapiens”, and the off-node was deleted. The interconnections between genes were predicted.
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses: Cross-target genes were imported into the Metascape website, the species was set to human, the screening threshold for enrichment analysis was P < 0.01, and Gene Ontology (GO) biological process, cellular localization, and molecular function analyses and Kyoto Encyclopedia of Genes and Genomes (KEGG)signaling pathway enrichment analyses were performed for the cross-targets, respectively. The results were integrated to draw GO enrichment analysis histograms and KEGG enrichment analysis bubble diagrams.
CG blood component-target-pathway-disease pull network analysis and construction: CG ingestion components were incorporated into Cytoscape 3.7.2 software to construct a network of “blood components-potential targets-associated pathways-anti-TD effects” and the analysis was visualized.
To balance biological relevance with computational efficiency, we prioritized compounds for docking based on (1) Degree centrality in the compound-target network (degree value > 100); (2) Plasma abundance; and (3) Literature-documented activity against core targets. The three-dimensional structures of the core proteins were downloaded from the PDB database (https://www.rcsb.org/), and the protein molecules were imported into the software Discovery Studio 2019 to remove water molecules, add hydrogen atoms, and set the atom types. The structures of the compounds were down
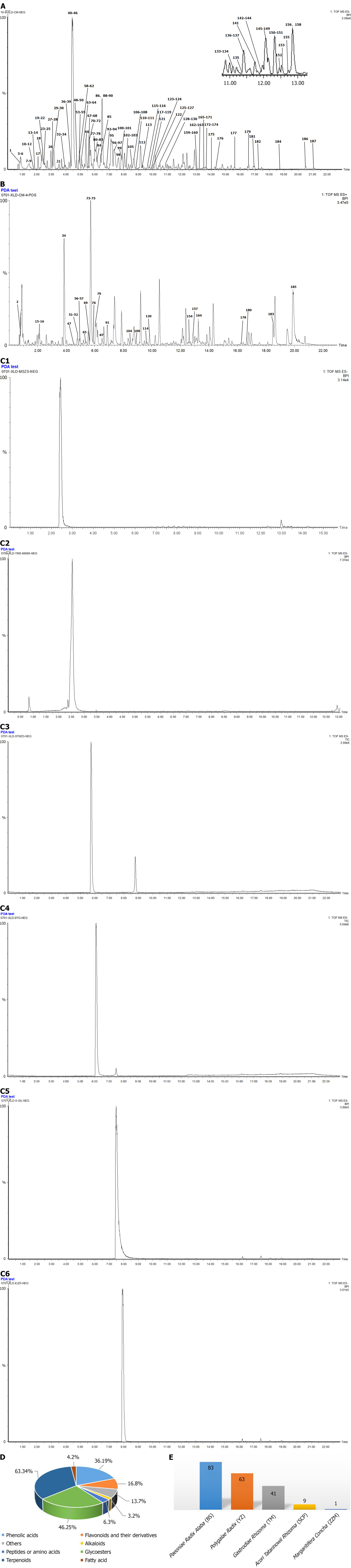
The chemical components of CG were analyzed using UPLC-Q-TOF-MS/MS, and the MS information was compared with the control, combined with the self-constructed library and UNIFI software. A total of 187 compounds were identified in the positive and negative ion modes, including phenolic acids, terpenes, flavonoids, and saccharides. Among them, 83 compounds were characterized in BS, 44 compounds in TM, 63 compounds in YZ, 9 compounds in SCP, and 1 compound in ZZM. The information and sources of each constituent and control quality profile are shown in Table 1 and Figure 1.
| No. | tR (minute) | Ion mode | Deviation (ppm) | Fragment; ions | Theoretical value | Molecular formula | Ingredient name | Source |
| 1 | 0.81 | [M-H]- 215.0348 | 1.86 | 149.0428 | 216.0423 | C12H8O4 | 1-furan-2-yl-2-(4-hydroxy-phenyl)ethane-1,2-dione | TM |
| 2 | 0.82 | [M+H]+ 175.1197 | 1.14 | 116.0725; 158.0872 | 174.1117 | C6H14N4O2 | Arginine | BS |
| 3 | 0.84 | [M-H]- 165.039 | -5.45 | 131.0469 | 166.0477 | C5H10O6 | Arabic acid | TM |
| 4 | 0.85 | [M-H]- 195.0492 | -6.66 | 165.0399; 179.0583; 129.0202 | 195.0583 | C6H12O7 | Gluconic acid | BS/TM |
| 5 | 1.03 | [M-H]- 133.0139 | 1.5 | 71.0096; 115.0034 | 134.0215 | C4H6O5 | D-(+)-malic acid | TM |
| 6 | 1.05 | [M-H]- 115.0034 | 2.61 | 71.0133 | 116.011 | C4H4O4 | Fumaric acid | TM |
| 7 | 1.37 | [M-H]- 128.035 | 1.56 | 96.9606 | 129.0426 | C5H7NO3 | L-pyroglutamic acid | TM |
| 8 | 1.49 | [M-H]- 191.0206 | 7.33 | 111.0066 | 192.027 | C6H8O7 | Citric acid | BS/TM |
| 9 | 1.49 | [M-H]- 173.0096 | 5.78 | 111.0066; 129.0202 | 174.0164 | C6H6O6 | Trans-aconitic acid or its isomer | BS |
| 10 | 1.71 | [M-H]- 188.0566 | 3.72 | 128.035; 144.0627; 173.0038 | 189.0637 | C7H11NO5 | N-acetylglutamic acid | TM |
| 11 | 1.72 | [M-H]- 343.0668 | 0.87 | 191.0206 | 344.0743 | C14H16O10 | 5-galloylquinic acid | BS |
| 12 | 1.8 | [M-H]- 243.0642 | -6.17 | 107.0534 | 244.0736 | C14H12O4 | Gastrodibenzin B/C | TM |
| 13 | 1.9 | [M-H]- 375.1257 | -9.06 | 341.1122; 300.9911 | 376.1369 | C16H24O10 | 8-debenzoylpaeoniflorin | BS |
| 14 | 1.95 | [M-H]- 493.1178 | -3.04 | 169.0121; 313.0574 | 494.1272 | C19H26O15 | 6'-O-galloylsucrose or its isomer | BS |
| 15 | 2.13 | [M+H]+ 268.1019 | -10.01 | 119.0341; 136.0614; 268.1090 | 267.0968 | C10H13N5O4 | Adenosine | BS |
| 16 | 2.13 | [M+H]+ 136.0614 | -6.61 | 91.0423; 119.0389 | 135.0545 | C5H5N5 | AdenIne | TM |
| 17 | 2.15 | [M-H]- 331.0651 | -4.23 | 316.1480; 271.4559 | 332.0743 | C13H16O10 | 6-O-galloylglucose or its isomer | BS |
| 18 | 2.28 | [M-H]- 493.1178 | -3.04 | 169.0121; 313.0574 | 494.1272 | C19H26O15 | 6'-O-galloylsucrose or its isomer | BS |
| 19 | 2.37 | [M-H]- 282.0842 | 1.42 | 133.0139; 150.0412 | 283.0917 | C10H13N5O5 | Guanosine | BS/TM |
| 201 | 2.46 | [M-H]- 169.0121 | -9.47 | 125.0238; 107.0082 | 170.0215 | C7H6O5 | Gallic acid | BS/TM |
| 21 | 2.46 | [M-H]- 125.0238 | -0.8 | 81.0333 | 126.0317 | C6H6O3 | Maltol | BS |
| 22 | 2.48 | [M-H]- 125.0238 | -0.8 | 107.7866; 95.0097; 77.0962 | 126.0317 | C6H6O3 | Pyrogallol | BS |
| 23 | 2.62 | [M-H]- 331.1049 | 6.04 | 313.0575 | 332.1107 | C14H20O9 | Mudanoside A | BS |
| 241 | 2.62 | [M-H]- 285.0993 | 6.66 | 268.0276 | 286.1053 | C13H18O7 | Gastrodin | TM |
| 25 | 2.79 | [M-H]- 359.1365 | 6.4 | 329.0024; 271.0459 | 360.142 | C16H24O9 | 1-O-β-D-glucopyranosyl-paeoniflorin | BS |
| 26 | 3.03 | [M-H]- 493.1178 | -3.04 | 169.0121; 313.0574 | 494.1272 | C19H26O15 | 6'-O-galloylsucrose or its isomer | BS |
| 27 | 3.14 | [M-H]- 164.0698 | -8.53 | 103.0476; 131.0419 | 165.079 | C9H11NO2 | L-phenylalanine | TM/ZZM |
| 28 | 3.15 | [M-H]- 331.0651 | -4.23 | 313.0497; 271.0459 | 332.0743 | C13H16O10 | 6-O-galloylglucose or its isomer | BS |
| 29 | 3.24 | [M-H]- 493.1178 | -3.04 | 169.0121; 313.0574 | 494.1272 | C19H26O15 | 6'-O-galloylsucrose or its isomer | BS |
| 30 | 3.27 | [M-H]- 313.0574 | 4.47 | 113.0234; 137.0263 | 314.0638 | C13H14O9 | 2-carboxyphenyl-α-D-glucopyranuronic acid | TM |
| 31 | 3.44 | [M-H]- 447.1514 | 2.46 | 89.0203; 341.0718; 389.0217 | 448.1581 | C19H28O12 | Gastrodin A | TM |
| 32 | 3.85 | [M-H]- 527.1414 | 2.47 | 479.0424 | 528.1479 | C23H28O14 | Galloyl desbenzoylpaeoniflorin | BS |
| 33 | 3.98 | [M-H]- 589.1218 | -1.53 | 167.0366; 259.0247 | 590.1305 | C24H30O15S | Paeoniflorin E sulfite | BS |
| 34 | 4 | [M+H]+ 385.1481 | -4.67 | 323.103 | 384.142 | C18H24O9 | Tenuifoliside D | YZ |
| 35 | 4.01 | [M-H]- 361.1504 | 1.38 | 315.0695 | 362.1577 | C16H26O9 | 6-O-copyranosyl-lactinlide | BS |
| 36 | 4.24 | [M-H]- 343.1396 | 0.87 | 310.0282 | 343.1393 | C16H24O8 | Mudanpioside F/mudanpioside G | BS |
| 37 | 4.24 | [M-H]- 459.1151 | 2.61 | 401.1037 | 460.1217 | C19H24O13 | Parishin E/parishin G | TM |
| 38 | 4.24 | [M-H]- 173.0096 | 5.78 | 125.0287; 137.0071 | 174.0164 | C6H6O6 | Trans-aconitic acid or its isomer | TM |
| 39 | 4.33 | [M-H]- 633.0732 | 7.58 | 555.1772 | 634.0806 | C27H22O18 | Strictinin | BS |
| 40 | 4.37 | [M-H]- 461.129 | -1.08 | 93.0323; 137.0225 | 462.1373 | C19H26O13 | Sibiricose A3 | YZ |
| 41 | 4.41 | [M-H]- 705.1734 | 4.68 | 543.1223; 259.0247; 121.0279 | 706.1779 | C29H38O18S | Isomaltopaeoniflorinsulfite or its isomer | BS |
| 42 | 4.48 | [M-H]- 451.1218 | -4.88 | 169.0065; 245.0836; 289.0727 | 452.1319 | C21H24O11 | Catechin glucoside | BS |
| 43 | 4.5 | [M-H]- 203.0833 | 5.91 | 116.0501; 159.0521 | 204.0899 | C11H12N2O2 | Tryptophan | SCP |
| 44 | 4.54 | [M-H]- 459.1151 | 2.61 | 400.9901 | 460.1217 | C19H24O13 | Parishin E/parishin G | TM |
| 45 | 4.54 | [M-H]- 421.0773 | 0.47 | 259.0247 | 422.0849 | C19H18O11 | Isomangiferin | TM |
| 46 | 4.55 | [M-H]- 543.1121 | -9.39 | 121.0279; 259.0247 | 544.1251 | C23H28O13S | Paeoniflorin sulfite | BS |
| 47 | 4.56 | [M+H]+ 579.1458 | -7.77 | 409.1026 | 578.1424 | C30H26O12 | Procyanidin B1/B2 | BS |
| 48 | 4.69 | [M-H]- 483.0744 | -6.42 | 125.0189; 151.0002; 169.0121 | 484.0853 | C20H20O14 | Paeoniflorin sulfite | BS |
| 49 | 4.76 | [M-H]- 577.1319 | -4.68 | 289.0727 | 578.1424 | C30H26O12 | Procyanidin B1/B2 | BS |
| 50 | 4.79 | [M-H]- 495.1466 | -7.47 | 465.1419; 137.0225 | 496.1581 | C23H28O12 | Oxypaeoniflorin or its isomer | BS |
| 51 | 4.81 | [M+H]+ 414.1312 | -5.55 | 162.0233; 308.0806 | 413.1257 | C17H23N3O7S | Sulfur-(4-hydroxybenzyl)-glutathione | TM |
| 52 | 4.81 | [M+H]+ 576.1881 | 3.12 | 320.0902 | 575.1785 | C23H33N3O12S | S-gastrodin-glutathione | TM |
| 53 | 4.82 | [M-H]- 175.0605 | -0.57 | 161.0244 | 176.0685 | C7H12O5 | 2-Isopropylmalic acid | TM |
| 54 | 4.92 | [M-H]- 635.0884 | 4.41 | 169.0121; 313.0574 | 636.0963 | C27H24O18 | 1,3,6-Tri-O-galloyl-β-D-glucose | BS |
| 55 | 4.95 | [M-H]- 517.1526 | -5.99 | 160.0174; 175.0432; 193.0479 | 518.1636 | C22H30O14 | Sibiricose A5 | YZ |
| 56 | 4.97 | [M+H]+ 139.0400 | 3.6 | 77.0374 | 138.0317 | C7H6O3 | Salicylic acid | TM/YZ/SCP |
| 57 | 4.98 | [M+H]+ 291.0884 | 5.15 | 247.0267; 263.1024 | 290.079 | C15H14O6 | Catechin | BS |
| 58 | 4.99 | [M-H]- 245.0836 | 8.98 | 190.026 | 246.0892 | C14H14O4 | Peonol | BS |
| 59 | 5 | [M-H]- 289.0727 | 5.19 | 123.0444; 205.0483 | 290.079 | C15H14O6 | Epicatechin | BS |
| 60 | 5 | [M-H]- 183.0303 | 5.46 | 78.0115; 124.0151; 168.0370 | 184.0372 | C8H8O5 | Methyl gallate | BS |
| 61 | 5 | [M-H]- 547.1702 | 7.13 | 205.0483 | 548.1741 | C23H32O15 | Sibiricose A1 | YZ |
| 62 | 5.19 | [M-H]- 205.0359 | 5.36 | 111.0112 | 206.0427 | C7H10O7 | 3-Hydroxy-3-(methoxycarbonyl) pentan | TM |
| 63 | 5.36 | [M-H]- 785.0837 | 0 | 125.0279 | 786.0916 | C34H26O22 | Tellimagrandin I | BS |
| 64 | 5.36 | [M-H]- 889.2623 | 1.01 | 85.0282; 111.0066; 780.1116 | 890.2692 | C38H50O24 | Parishin V | TM |
| 65 | 5.39 | [M+H]+ 374.1462 | -0.53 | 136.0614 | 373.1386 | C17H19N5O5 | N-(4-hydroxypheny) adenosine | TM |
| 66 | 5.43 | [M-H]- 727.2141 | 7.56 | 129.0202 | 728.2164 | C32H40O19 | Parishin B | TM |
| 67 | 5.57 | [M-H]- 757.2173 | -2.38 | 71.0133; 453.0717 | 758.2269 | C33H42O20 | Parishin M | TM |
| 68 | 5.63 | [M-H]- 687.2103 | -4.8 | 635.0635; 165.0502; 121.0279 | 688.2215 | C30H40O18 | 6'-O-β-D-glucopyranosylalbiflorin | BS |
| 69 | 5.66 | [M+H]+ 342.1699 | -1.75 | 237.0880; 297.0598 | 341.1627 | C20H23NO4 | N-Methylhernagine | BS/TM |
| 701 | 5.71 | [M-H]- 479.1568 | 2.09 | 525.1579 | 480.1632 | C23H28O11 | Albiflorin | BS |
| 71 | 5.71 | [M-H]- 525.158 | -5.33 | 165.0558; 167.0309; 327.1120; 363.1698 | 526.1686 | C24H30O13 | Mudanpioside E | BS |
| 72 | 5.72 | [M-H]- 727.2141 | 7.56 | 111.0066; 161.0465 | 728.2164 | C32H40O19 | Parishin C | TM |
| 73 | 5.72 | [M+H]+ 319.1151 | -9.71 | 301.1047 | 318.1103 | C17H18O6 | Evofolin B | SCP |
| 74 | 5.73 | [M+H]+ 197.0822 | 4.06 | 151.078; 179.0684 | 196.0736 | C10H12O4 | Paeonilactone B | BS |
| 75 | 5.73 | [M+H]+ 133.0648 | -3.76 | 77.0374; 105.0681; 115.0545 | 132.0575 | C9H8O | Cinnamaldehyde | BS/SCP |
| 76 | 5.98 | [M+H]+ 539.1373 | -5.19 | 503.1316 | 538.1323 | C24H26O14 | Sibiricaxanthone A | YZ |
| 77 | 6.00 | [M-H]- 285.0404 | 1.75 | 257.0396; 268.0276 | 286.0477 | C15H10O6 | Kaempferol | BS/TM |
| 78 | 6.00 | [M-H]- 537.1243 | -0.19 | 315.0464; 387.0604 | 538.1323 | C24H26O14 | Sibiricaxanthone B | YZ |
| 79 | 6.08 | [M+H]+ 463.1623 | 4.1 | 151.0780; 179.0684 | 462.1526 | C23H26O10 | Lactiflorin | BS |
| 801 | 6.09 | [M-H]- 479.1568 | 2.09 | 77.0387; 121.0329; 165.0607; 327.1063; 449.1483; 959.3233 | 480.1632 | C23H28O11 | Paeoniflorin | BS |
| 81 | 6.09 | [M-H]- 121.0279 | -9.09 | 77.0387 | 122.0368 | C7H6O2 | 4-hydroxybenzaldehyde | TM |
| 82 | 6.1 | [M-H]- 165.0558 | 3.64 | 121.0279; 77.0387 | 166.063 | C9H10O3 | Paeonol | BS |
| 83 | 6.16 | [M-H]- 431.1373 | 7.19 | 327.1041 | 432.142 | C22H24O9 | Heptemthoxyflavone | BS |
| 84 | 6.23 | [M-H]- 567.1356 | 1.06 | 345.0594; 315.0541; 272.0318 | 568.1428 | C25H28O15 | Polygalaxanthone VIII | YZ |
| 85 | 6.42 | [M-H]- 495.1466 | -7.47 | 427.1026; 137.0225 | 496.1581 | C23H28O12 | Oxypaeoniflorin or its isomer | BS |
| 86 | 6.48 | [M-H]- 787.104 | 5.84 | 169.0121; 456.0666; 617.0786 | 788.1072 | C34H28O22 | 1,3,4,6-tetragalloylglucose | BS |
| 87 | 6.55 | [M+H]+ 199.0596 | -5.02 | 163.0358; 153.0205 | 198.0528 | C9H10O5 | Ethyl gallate | BS |
| 88 | 6.64 | [M-H]- 995.3086 | 5.43 | 423.0960; 728.2154 | 996.3111 | C45H56O25 | Parishin A | TM |
| 89 | 6.69 | [M-H]- 433.1162 | 6.23 | 111.0020; 271.0603; 397.1084 | 434.1213 | C21H22O10 | Dihydroxyflavone-glucoside | BS |
| 90 | 6.73 | [M-H]- 300.9986 | 0.66 | 271.0603; 245.9800 | 302.0063 | C14H6O8 | Ellagic acid | BS |
| 91 | 6.89 | [M+H]+ 271.0762 | -0.37 | 147.0451; 153.0151; 273.0834 | 272.0685 | C15H12O5 | Naringenin | BS |
| 92 | 6.9 | [M-H]- 667.1871 | -0.45 | 205.0483; 461.1384 | 668.1952 | C30H36O17 | Tenuifoliside B | YZ |
| 93 | 6.92 | [M-H]- 433.1162 | 6.23 | 119.0471; 151.0002; 271.0603 | 434.1213 | C21H22O10 | Isosalipurposide | BS |
| 94 | 6.94 | [M-H]- 223.0618 | 5.38 | 93.0365; 121.0279 | 224.0685 | C11H12O5 | Sinapinic acid | TM |
| 95 | 7.18 | [M-H]- 631.1628 | -5.55 | 465.1419; 313.1419; 271.0459; 169.0121 | 632.1741 | C30H32O15 | Galloylpaeoniflorin or its isomer | BS |
| 96 | 7.25 | [M-H]- 631.1628 | -5.55 | 465.1419; 313.1419; 271.0459; 169.0121 | 632.1741 | C30H32O15 | Galloylpaeoniflorin or its isomer | BS |
| 971 | 7.44 | [M-H]- 939.1152 | 5.11 | 769.0880; 617.0786; 447.0591; 169.0121 | 940.1182 | C41H32O26 | 1,2,3,4,6-pentagalloylglucose | BS |
| 98 | 7.71 | [M-H]- 631.1628 | -5.55 | 465.1419; 313.1419; 271.0459; 169.0121 | 632.1741 | C30H32O15 | Galloylpaeoniflorin or its isomer | BS |
| 99 | 7.81 | [M-H]- 611.1589 | -3.76 | 465.1042; 287.0197 | 612.169 | C27H32O16 | Polygalaxanthone VII | YZ |
| 1001 | 7.82 | [M-H]- 753.2277 | -4.65 | 205.0518; 223.0675; 367.1056; 529.1638; 547.1773 | 754.232 | C34H42O19 | 3,6′-disinapoyl sucrose | YZ |
| 101 | 7.97 | [M-H]- 631.1628 | -5.55 | 465.1419; 313.1419; 271.0459; 169.0121 | 632.1741 | C30H32O15 | Galloylpaeoniflorin or its isomer | BS |
| 102 | 8.13 | [M-H]- 631.1628 | -5.55 | 465.1419; 313.1419; 271.0459; 169.0121 | 632.1741 | C30H32O15 | Galloylpaeoniflorin or its isomer | BS |
| 103 | 8.2 | [M-H]- 723.2156 | 2.77 | 631.1628 | 724.2215 | C33H40O18 | Arillanin A | YZ |
| 104 | 8.5 | [M+H]+ 481.1678 | -6.65 | 105.0323; 436.1096 | 480.1632 | C23H28O11 | Mudanpioside I | BS |
| 105 | 8.52 | [M-H]- 503.1763 | -0.4 | 209.0763; 485.1499 | 504.1843 | C22H32O13 | Polygalatenosides E | YZ |
| 106 | 8.64 | [M-H]- 651.1879 | -7.06 | 137.0225; 281.0656; 443.1159 | 652.2003 | C30H36O16 | Tenuifoliside-652 | YZ |
| 107 | 8.7 | [M-H]- 1453.4358 | -6.81 | 145.0315; 1039.3169; 1119.3817; 1161.3540 | 1454.4535 | C65H82O37 | Tenuifoliose Q | YZ |
| 108 | 8.74 | [M-H]- 509.1694 | 6.87 | 479.1472; 169.0121; 121.0279 | 510.1737 | C24H30O12 | Mudanpioside D | BS |
| 109 | 8.78 | [M+H]+ 481.1678 | -6.65 | 77.0374 | 480.1632 | C23H28O11 | Albiflorin R1 | BS |
| 110 | 8.79 | [M-H]- 647.1489 | 8.34 | 121.0279; 213.0223; 259.0247; 525.0979 | 648.1513 | C30H32O14S | Benzoylpaeoniflorin sulfonate | BS |
| 111 | 8.81 | [M-H]- 283.0826 | 2.83 | 255.8088 | 284.0894 | C13H16O7 | Benzyl glucoside | BS |
| 112 | 9.18 | [M-H]-783.1761 | -1.53 | 169.0121; 465.1325; 631.1519 | 784.1851 | C37H36O19 | 3’-6’-D-O-galloylpaeoniflorin | BS |
| 113 | 9.25 | [M-H]- 681.2019 | -1.76 | 179.0349; 281.0656; 443.1159 | 682.2109 | C31H38O17 | Tenuifoliside A | YZ |
| 114 | 9.61 | [M+H]+ 271.0609 | 1.11 | 95.0152; 207.0684 | 270.0528 | C15H10O5 | Galangin | SCP |
| 115 | 9.65 | [M-H]- 269.0438 | -4.46 | 251.0329 | 270.0528 | C15H10O5 | Baicalein | BS |
| 116 | 9.66 | [M-H]- 599.1808 | 7.18 | 137.0276 | 600.1843 | C30H32O13 | Benzoyloxypaeoniflorin | BS |
| 117 | 9.71 | [M-H]- 1525.4702 | 2.23 | 1379.4265 | 1526.4746 | C68H86O39 | Tenuifoliose F | YZ |
| 118 | 9.74 | [M-H]- 1495.4609 | 3.14 | 1161.3540; 1203.3713; 1349.4047 | 1496.4641 | C67H84O38 | Tenuifoliose L | YZ |
| 119 | 9.82 | [M-H]- 1253.3766 | -0.48 | 753.2396; 809.2495; 955.3134; 1077.3359 | 1254.385 | C56H70O32 | Tenuifoliose T | YZ |
| 120 | 9.85 | [M+H]+ 314.1379 | -4.14 | 93.0645 | 313.1314 | C18H19NO4 | N-trans-Feruloyltyramine | TM |
| 121 | 9.88 | [M-H]- 312.1232 | -1.28 | 281.2414 | 313.1314 | C18H19NO4 | Feruloyl tyramine | SCP |
| 122 | 10.01 | [M-H]- 447.2253 | 5.14 | 59.0121; 89.0244 | 448.2308 | C21H36O10 | Geraniol-primeveroside | BS |
| 123 | 10.12 | [M-H]- 767.2444 | 5.87 | 223.0672; 237.0764; 529.1638 | 768.2477 | C35H44O19 | Tenuifoliside C | YZ |
| 124 | 10.17 | [M-H]- 1223.3766 | 8.17 | 145.0315; 307.0751; 955.2988; 1077.3217; 1101.3361 | 1224.3745 | C55H68O31 | Tenuifoliose S | YZ |
| 125 | 10.43 | [M-H]- 1295.3929 | 3.94 | 997.3134; 1119.3524 | 1296.3956 | C58H72O33 | Tenuifoliose C/tenuifoliose E | YZ |
| 126 | 10.57 | [M-H]- 237.0761 | -0.84 | 206.0752; 222.0520 | 238.0841 | C12H14O5 | 4-hydroxy-3,5-dimethoxylcinnamate | YZ |
| 127 | 10.67 | [M-H]- 1397.621 | -1.07 | - | 1398.6303 | C64H102O33 | Arillatanoside C | YZ |
| 128 | 10.79 | [M-H]- 445.2051 | -5.17 | 311.0902 | 446.2152 | C21H34O10 | Β-pinen-10-yl-β-vicianoside | BS |
| 129 | 10.83 | [M-H]- 1307.3984 | 8.11 | 307.0827; 653.1922; 1161.3689 | 1308.3956 | C59H72O33 | Tenuifoliose J/tenuifoliose I | YZ |
| 130 | 10.88 | [M-H]- 1265.5903 | 7.9 | 425.3125; 455.3217; 499.1701; 585.1967; 1235.5663 | 1266.5881 | C59H94O29 | Desacylsenegasaponin B | YZ |
| 131 | 10.96 | [M-H]- 1541.6667 | -8.63 | 425.3125; 1317.3502 | 1542.6879 | C74H110O34 | Onjisaponin H | YZ |
| 132 | 10.98 | [M-H]- 1411.6322 | -4.25 | - | 1412.646 | C65H104O33 | Desacylsenegasaponin III | YZ |
| 133 | 11.2 | [M-H]- 1235.5818 | 9.79 | 337.1186; 455.3217; 555.1955 | 1236.5775 | C58H92O28 | Arillatanoside A | YZ |
| 134 | 11.29 | [M-H]- 1381.6334 | 4.2 | 701.1796; 1157.5767; 1351.6196 | 1382.6354 | C64H102O32 | Polygalasaponin XIX | YZ |
| 135 | 11.36 | [M-H]- 711.2166 | 4.22 | 694.1769 | 712.2215 | C32H40O18 | Telephiose C | SCP |
| 136 | 11.47 | [M-H]- 1249.5913 | 4.8 | 425.3125; 455.3217; 1025.5084; 1219.5607 | 1250.5932 | C59H94O28 | Onjisaponin Tf | YZ |
| 137 | 11.5 | [M-H]- 1525.6748 | 3.21 | 1157.5618; 1351.6196; 1463.7104 | 1526.6777 | C70H110O36 | Onjisaponin Te | YZ |
| 138 | 11.53 | [M-H]- 1379.6213 | 6.81 | 425.3125; 455.3217; 1235.5817 | 1380.6198 | C64H100O32 | Onjisaponin TG | YZ |
| 139 | 11.54 | [M-H]- 1103.5229 | -4.08 | 455.3146 | 1104.5353 | C53H84O24 | Polygalasaponin XXVIII | YZ |
| 140 | 11.55 | [M-H]- 1307.3984 | 8.11 | 307.0827; 653.1922; 1161.3689 | 1308.3956 | C59H72O33 | Tenuifoliose J/tenuifoliose I | YZ |
| 141 | 11.71 | [M-H]- 741.2241 | -0.13 | 179.0583; 684.2599 | 742.232 | C33H42O19 | Parishin K | TM |
| 142 | 11.9 | [M-H]- 1367.4008 | -5.92 | 1027.3135; 1191.3721 | 1368.4167 | C61H76O35 | Tenuifoliose O | YZ |
| 143 | 11.91 | [M-H]- 1265.3884 | 8.85 | 631.1901; 753.2276; 1143.3408; 1223.3461 | 1266.385 | C57H70O32 | Tenuifoliose K | YZ |
| 144 | 11.97 | [M-H]- 1349.4048 | 4.82 | 1039.3309; 1161.3689; 1203.3713 | 1350.4061 | C61H74O34 | Tenuifoliose H | YZ |
| 145 | 12.18 | [M-H]- 583.1800 | -2.74 | 553.1725; 121.0279 | 584.1894 | C30H32O12 | Benzoylpaeoniflorin | BS |
| 146 | 12.18 | [M-H]- 629.1896 | 4.13 | 121.0279 | 630.1949 | C31H34O14 | Mudanpioside B/mudanpioside J | BS |
| 147 | 12.19 | [M-H]- 1379.4103 | 1.01 | 347.0917; 1161.3540 | 1380.4167 | C62H76O35 | Tenuifoliose A | YZ |
| 148 | 12.22 | [M-H]- 1409.418 | -1.06 | 825.3434; 1069.3090; 1111.3448; 1173.3512; 1191.3871 | 1410.4273 | C63H78O36 | Tenuifoliose N | YZ |
| 149 | 12.22 | [M-H]- 1325.3993 | 0.75 | 661.2083; 1149.3740; 1203.3866 | 1326.4061 | C59H74O34 | Senegose B/C | YZ |
| 150 | 12.38 | [M+H]+ 585.1997 | 4.27 | 77.0375; 105.0323 | 584.1894 | C30H32O12 | Benzoylalbiflorin | BS |
| 151 | 12.4 | [M-H]- 629.1896 | 4.13 | 121.0279 | 630.1949 | C31H34O14 | Mudanpioside B/mudanpioside J | BS |
| 152 | 12.46 | [M-H]- 1307.3984 | 8.11 | 307.0827; 653.1922; 1161.3689 | 1308.3956 | C59H72O33 | Tenuifoliose J/tenuifoliose I | YZ |
| 153 | 12.51 | [M-H]- 327.2147 | -7.33 | 171.1021 | 328.225 | C18H32O5 | (12Z, 15Z) -9,10,11-trihydroxy-12,15-octadecadienoic acid | TM |
| 154 | 12.62 | [M+H]+ 303.0843 | -8.58 | 245.0417; 261.0185 | 302.079 | C16H14O6 | Onjixanthone I | YZ |
| 155 | 12.76 | [M-H]- 677.355 | 1.92 | 629.0362 | 678.3615 | C36H54O12 | Sibiricasaponin A | YZ |
| 156 | 12.83 | [M-H]- 679.3682 | -1.77 | 425.3086; 455.3146 | 680.3772 | C36H56O12 | Tenuifolin | YZ |
| 157 | 12.89 | [M+H]+ 295.2275 | 0.68 | 221.0807 | 294.2195 | C18H30O3 | 13(S)-HOTrE | BS |
| 158 | 12.9 | [M-H]- 329.2322 | -1.82 | 171.1021; 211.1272; 229.1422 | 330.2406 | C18H34O5 | 9,12,13-TriHOME | BS |
| 159 | 13 | [M-H]- 1703.7188 | -8.22 | 157.1910; 1479.7279 | 1704.7407 | C80H120O39 | Onjisaponin A | YZ |
| 160 | 13.09 | [M-H]- 1631.7034 | -5.09 | 425.3125; 455.3123; 567.1970; 1601.6403 | 1632.7195 | C77H116O37 | Onjisaponin O | YZ |
| 161 | 13.18 | [M-H]- 1571.6836 | -4.45 | 567.1865; 1347.6085; 1541.6838 | 1572.6984 | C75H112O35 | Onjisaponin B/onjisaponin D | YZ |
| 162 | 13.23 | [M-H]- 1617.6907 | -3.34 | 425.3035; 455.3123; 1393.8030 | 1618.7039 | C76H114O37 | Polygalasaponin XLIV | YZ |
| 163 | 13.26 | [M-H]- 1673.7109 | -6.81 | 425.3035; 1617.6730 | 1674.7301 | C79H118O38 | Z-polygalasaponin XXXII | YZ |
| 164 | 13.26 | [M+H]+ 347.1114 | -4.9 | 289.0669; 331.1214 | 346.1053 | C18H18O7 | 1,2,3,6,7-pentamethoxyxanthone | YZ |
| 165 | 13.33 | [M-H]- 1469.6482 | 3.13 | 1439.6489 | 1470.6515 | C67H106O35 | Polygalasaponin XXXXII | YZ |
| 166 | 13.34 | [M-H]- 1469.6482 | -7.28 | 405.1388 | 1470.6667 | C71H106O32 | Onjisaponin Z | YZ |
| 167 | 13.42 | [M-H]- 1485.6475 | -4.24 | 425.3125; 1455.6677 | 1486.6616 | C71H106O33 | Onjisaponin E | YZ |
| 168 | 13.45 | [M-H]- 1409.6310 | -4.82 | 425.3125; 455.3217; 1185.6506; 1379.6375 | 1410.6456 | C69H102O30 | Onjisaponin Y | YZ |
| 169 | 13.52 | [M-H]- 1587.6880 | 1.57 | 155.3035; 583.1805; 1455.6344 | 1588.6933 | C75H112O36 | Onjisaponin F | YZ |
| 170 | 13.52 | [M-H]- 1455.6344 | -6.05 | 237.0832; 425.3125; 455.3123; 1425.36638 | 1456.6511 | C70H104O32 | Onjisaponin G/onjisaponin J | YZ |
| 171 | 13.58 | [M-H]- 1733.7567 | 7.67 | 455.3217; 669.2285 | 1734.7512 | C81H122O40 | Onjisaponin S | YZ |
| 172 | 13.62 | [M-H]- 1599.6833 | -1.38 | 1455.6344; 1537.7080 | 1600.6933 | C76H112O36 | Onjisaponin Gg/onjisaponin K | YZ |
| 173 | 13.66 | [M-H]- 1425.6309 | -1.26 | 425.3035; 455.3123; 1395.6298; 1426.6205 | 1426.6405 | C69H102O31 | Senegasaponin B | YZ |
| 174 | 13.72 | [M-H]- 1323.6033 | 1.74 | 425.3035; 455.3217; 1293.5858 | 1324.6088 | C65H96O28 | Onjisaponin TH | YZ |
| 175 | 13.99 | [M-H]- 1263.5869 | 5.54 | 425.3125; 455.3123; 1233.6174 | 1264.5877 | C63H92O26 | Onjisaponin MF | YZ |
| 176 | 14.31 | [M-H]- 317.0649 | -3.78 | 259.0338 | 318.074 | C16H14O7 | 6,8-dihydroxy-1,2,4-trimethyoxyxanthone | YZ |
| 177 | 15.66 | [M-H]- 455.3146 | -3.29 | 441.2885; 427.2561 | 456.324 | C29H44O4 | 30-demethylated hederagenin | BS |
| 178 | 16.3 | [M+H]+ 373.2014 | -0.27 | 331.0904 | 372.1937 | C22H28O5 | Pyrethrin II | BS |
| 179 | 16.57 | [M-H]- 471.3503 | 6.15 | 393.3143 | 472.3553 | C30H48O4 | Hederagenin or its isomer | BS |
| 180 | 16.65 | [M+H]+ 520.3367 | -6.92 | 86.0983; 184.0721 | 519.3325 | C26H50NO7P | 2-dioleoyl-sn-glycero-3-phosphocholine | TM |
| 181 | 16.99 | [M-H]- 471.3503 | 6.15 | 393.3143 | 472.3553 | C30H48O4 | 23-hydroxybetulinic acid | BS |
| 182 | 17.29 | [M-H]- 233.1523 | -8.15 | 218.8748; 197.9078 | 234.162 | C15H22O2 | Acoronene | SCP |
| 183 | 18.5 | [M+H]+ 301.1426 | 8.63 | 127.0094; 132.9692 | 300.1321 | C13H20N2O6 | 5-butyluridine | SCP |
| 184 | 18.69 | [M-H]- 293.1782 | 9.89 | 245.8978 | 294.1831 | C17H26O4 | 6-gingerol | TM |
| 185 | 20.34 | [M+H]+ 297.2404 | -8.75 | 253.1753; 261.0256 | 296.2351 | C18H32O3 | 13-HODE | TM |
| 186 | 20.57 | [M-H]- 455.3519 | -1.32 | 441.7197; 355.7012 | 456.3603 | C30H48O3 | Oleanolic acid or its isomer | BS |
| 187 | 21.12 | [M-H]- 455.3519 | -1.32 | 441.7197; 355.7012 | 456.3603 | C30H48O3 | Oleanolic acid or its isomer | BS |
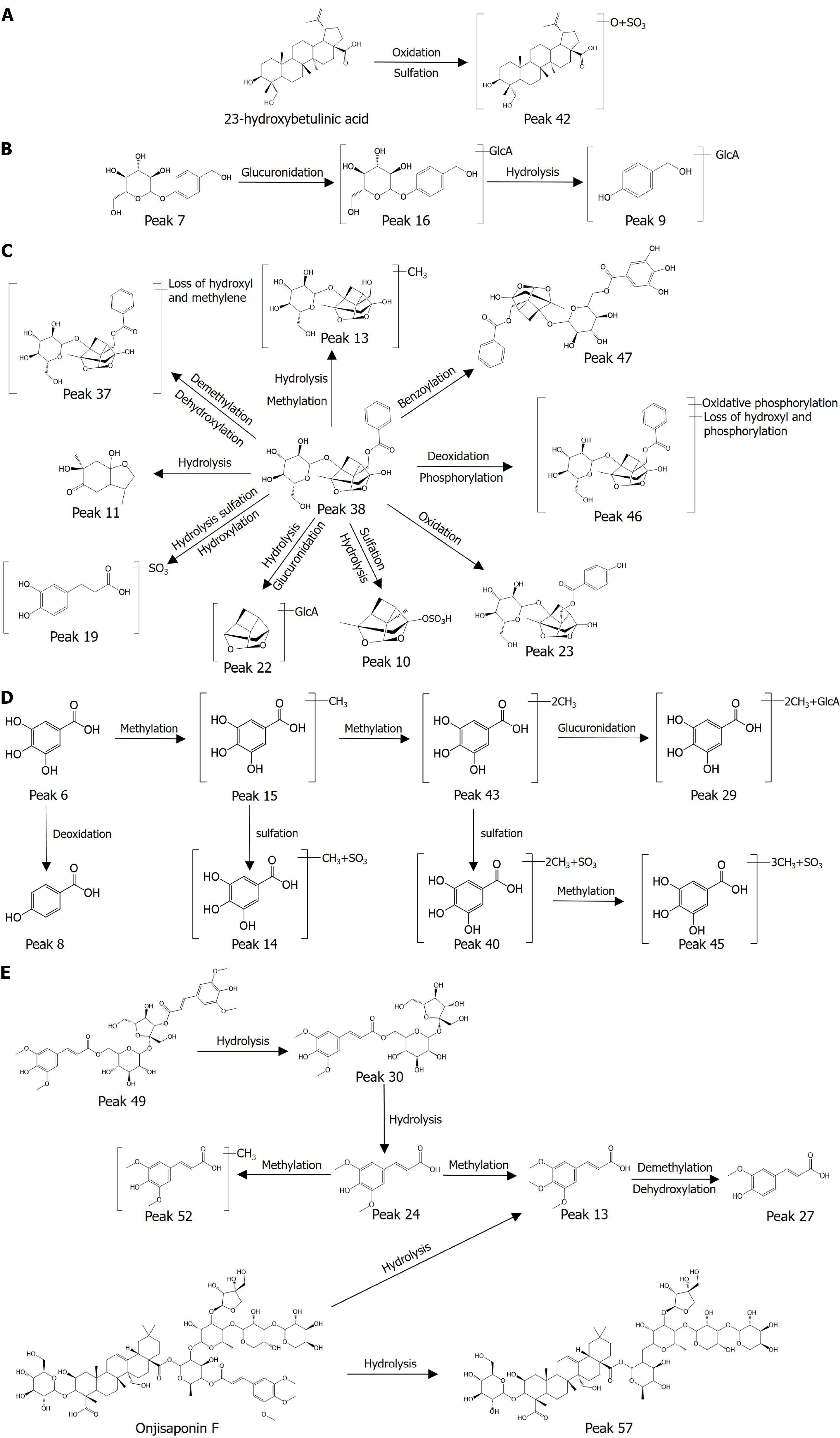
Identification of terpenoids: In total, 63 terpenoids have been characterized in CG, mainly from BS and YZ. Most terpenes combine with sugars to form glycosides. The characterized monoterpenes include paeoniflorin, albiflorin, benzoylpaeoniflorin, etc. The characterized triterpenes include onjisaponin Y, arillatanoside A, desacylsenegasaponin III, etc. The molecular ion peak during cleavage usually begins with a glycosidic bond break, forming a genin. Subsequently, the genin continues to lose some substituents, including -OH and -CH3.
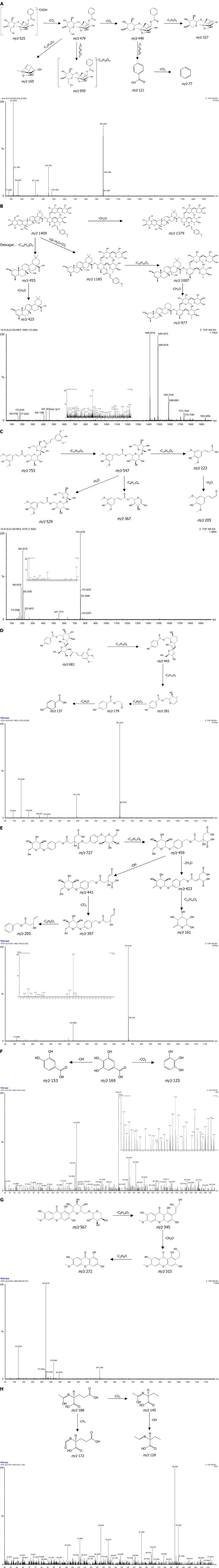
The monoterpenes represented by paeoniflorin mostly contain a pinane skeleton. Fragment ion peaks at m/z 165 were observed. The quasi-molecular ion peaks of [M-H]- are usually lower, and they mostly exist as [M+CH3COOH]-. Taking peak 80 (tR = 6.09) as an example, the primary mass spectrogram in negative ion mode exists [M+CH3COOH]- and peaks at m/z 525, which has a lower [M-H]- abundance at m/z 479. The secondary mass spectrometry showed the presence of
The triterpenoids represented by onjisaponin Y are mostly combined with sugar to form saponins. These saponins belong to the oleanolic acid type of pentacyclic triterpenes, and the C3 position is mostly replaced by glucose. The C28 position is also linked to rhamnose, galactose, and other monosaccharides. Therefore, the cleavage pattern of their mass spectra mostly showed glycosidic bond breaking, and one or more H2O and CO2 molecules might have been lost during the cleavage process. According to the cleavage characteristics of parent nucleus of onjisaponin Y, its characteristic fragment ions were m/z 455 and m/z 425. Taking peak 168 (tR = 13.45) as an example, the peak with m/z 1409.6310 was detected as an [M-H]- peak in the negative ion mode. In the secondary MS, there were peaks at m/z 1379, which were presumed to be [M-H-CH2O]-, peaks at m/z 1185 were presumed to be [M-H-Glc-H2O-CO2]-, and peaks at m/z 1007 were presumed to be [M-H-Glc-H2O-CO2-C10H10O3]-, followed by CH2OH removal to obtain the peaks at m/z 977. The peaks at m/z 455 and m/z 425 were attributed to breakage of sugar bonds, dehydration, decarboxylation, and CH2OH cleavage of parent nucleus of onjisaponin Y. The cleavage pattern and secondary mass spectra are shown in Figure 2B.
Identification of carbohydrate components: Overall, 41 carbohydrate components were characterized in CG, and these components were mainly from TM and YZ. Sugar esters, which use sucrose as the parent nucleus and connect different monosaccharides with glycosidic bonds at different positions, are mainly present in YZ. Sugar ester components are mainly categorized into monosaccharide and oligosaccharide esters. Oligosaccharide esters are linked to different amounts of glucose and contain different acyl groups, mainly acyl, benzoyl, cinnamoyl, and feruloyl. Glycose esters are present in many plants, but oligosaccharides containing three or more glucose molecules in the parent nucleus are mainly found in YZ. Oligosaccharides are considered compounds unique to YZ, and Fargesia oligosaccharides may have some specific pharmacological activities[15].
Oligosaccharides were detected at peak 100 (tR = 7.82) as an [M-H]- peak with an ion peak at m/z 753 in the negative ion mode. m/z 547 was present in the secondary mass spectrogram, presumed to be [M-H-C11H10O4]-; m/z529 was presumed to be [M-H-C11H12O5]-, m/z 367 presumed to be [M-H-C11H12O5-C6H10O5]-, and m/z 205 was presumed to be [M-H-C12H14O5-C6H10O5-C4H6O3]-. After comprehensive analysis and comparison with the control, peak 100 was confirmed to be 3,6'-dierucoyl sucrose. The cleavage pattern and secondary mass spectra are shown in Figure 2C.
Sugar esters are exemplified by peak 113 (tR = 9.25), where an ion peak at m/z 681 was detected in the negative ion mode as the [M-H]- peak. In the secondary mass spectrum, m/z 443 was presumed to be [M-H-C12H14O5]-, m/z 281 was presumed to be [M-H-C12H14O5-C6H10O5]-, and m/z 179 was presumed to be [M-H-C11H12O5-C6H10O5-C4H6O3]-. The hydrolysis of one molecule of CO continued to drop off to form the peak of m/z 137. After comprehensive analysis, peak 113 was presumed to be tenuifoliside A. The cleavage pattern and secondary mass spectra are shown in Figure 2D.
Glycosides are mainly present in TM, and these components, represented by palisarin, undergo ester bond breaking during the cleavage process. Taking peak 66 (tR = 5.43) as an example, the ion peak at m/z 727 was detected as [M-H]- peak in the negative ion mode. In the secondary mass spectrum, m/z 459 was presumed to be [M-H-C13H17O6]-, m/z 423 was presumed to be [M-H-C13H17O6-2H2O]-, m/z 161 was presumed to be [M-H-C13H17O6-2H2O-C13H10O6]-, m/z 441 was presumed to be [M-H-C13H17O6-OH]-, m/z 397 was presumed to be [M-H-C13H17O6-OH-CO2]-, and m/z 205 was presumed to be [M-H-C13H17O6-OH-CO2-C6H8O7]-. After the comprehensive analysis, peak 66 was presumed to be palisarin B. The cleavage pattern and second-level mass spectra are shown in Figure 2E.
Identification of phenolic acid: A total of 36 phenolic acid components were characterized in CG, and these phenolic acid components were mainly found in BS and TM. The molecular weights of these analogs are relatively small, and they tend to -OH, -COOH, and other groups during the cleavage process, and the phenyl ring fragment ions of m/z 77 are generally characteristic fragments.
As an example, peak 21 (tR = 2.46), an ion peak at m/z 169 was detected at the negative ion mode as the [M-H]- peak. The presence of m/z 125 in the secondary mass spectrum was presumed to be [M-H-CO2]-, and m/z153 was presumed to be [M-H-OH]-. After comprehensive analysis and comparison with the control, peak 21 was confirmed to be gallic acid. The cleavage pattern and secondary mass spectra are shown in Figure 2F.
Identification of flavonoid components: After UPLC-Q-TOF-MS/MS detection, the structural type of flavonoid com
For example, peak 84 (tR = 5.43) was detected as an [M-H]- peak at m/z 567 at the negative ion mode. In the secondary mass spectrum, m/z 345 was present, which was presumed to be [M-H-C8H14O7]-, m/z 315 was presumed to be [M-H-C8H14O7-CH2O]-, and m/z 272 was presumed to be [M-H-C8H14O7-2H2O-C2H4O]-. After comprehensive analysis, peak 84 was presumed to be polygalaxanthone VIII. The cleavage pattern and secondary mass spectra are shown in Figure 2G.
Identification of other components: In addition to the above components, alkaloids, peptides, and other components, such as amino acids and fatty acids, were detected in CG. Taking peak 10 (tR = 1.71) as an example, the ion peak at m/z 188 was detected as [M-H]- peak at the negative ion mode. The presence of m/z 145 in the secondary mass spectrum was presumed to be [M-H-CO2]-, m/z 128 was presumed to be [M-H-CO2-OH]-; m/z 172 was presumed to be [M-H-OH]-; and peak 10 was presumed to be N-acetyl-L-glutamic acid after comprehensive analysis. The cleavage pattern and secondary mass spectra are shown in Figure 2H.
UPLC-Q-TOF-MS/MS analysis of blood component
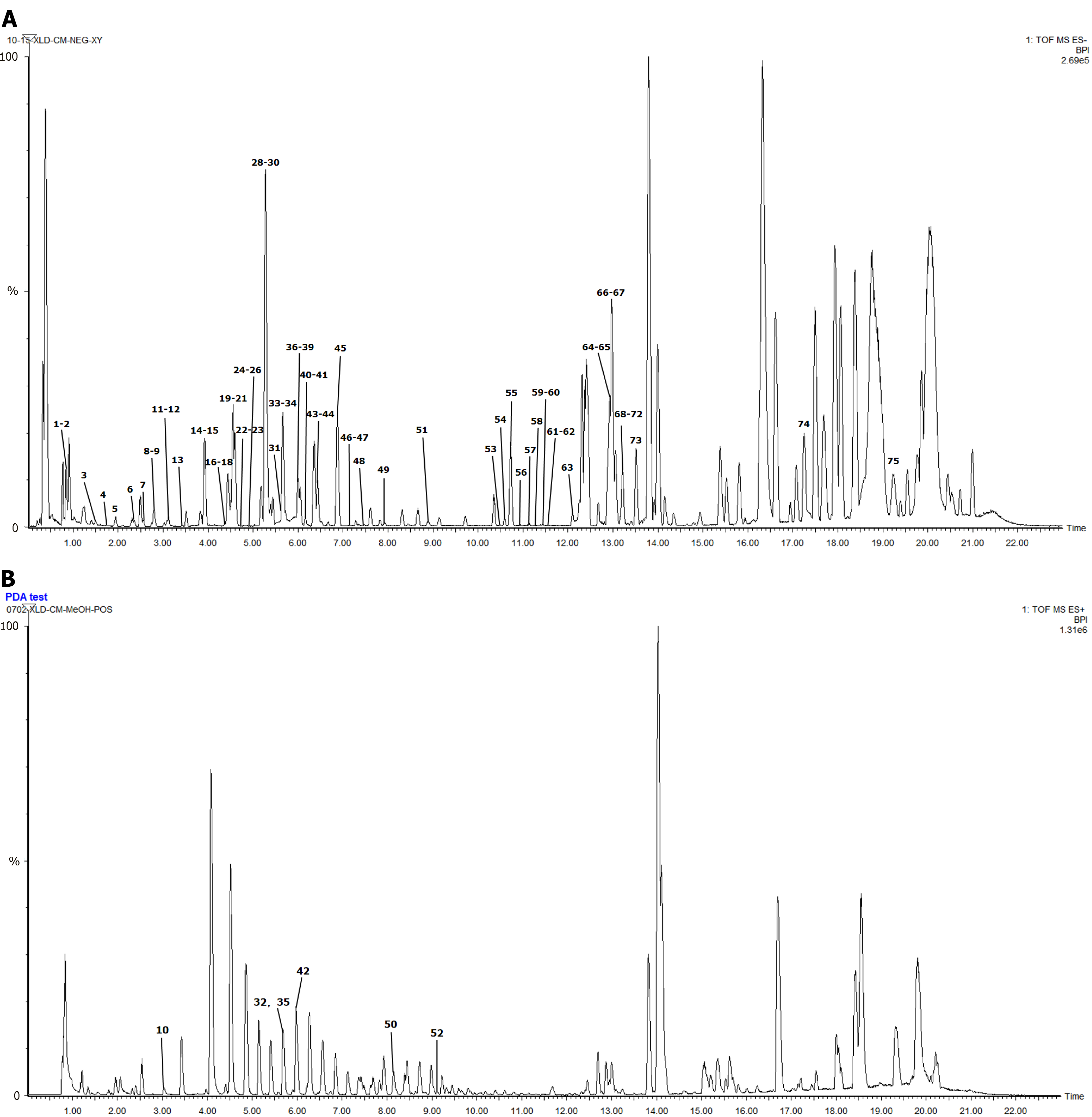
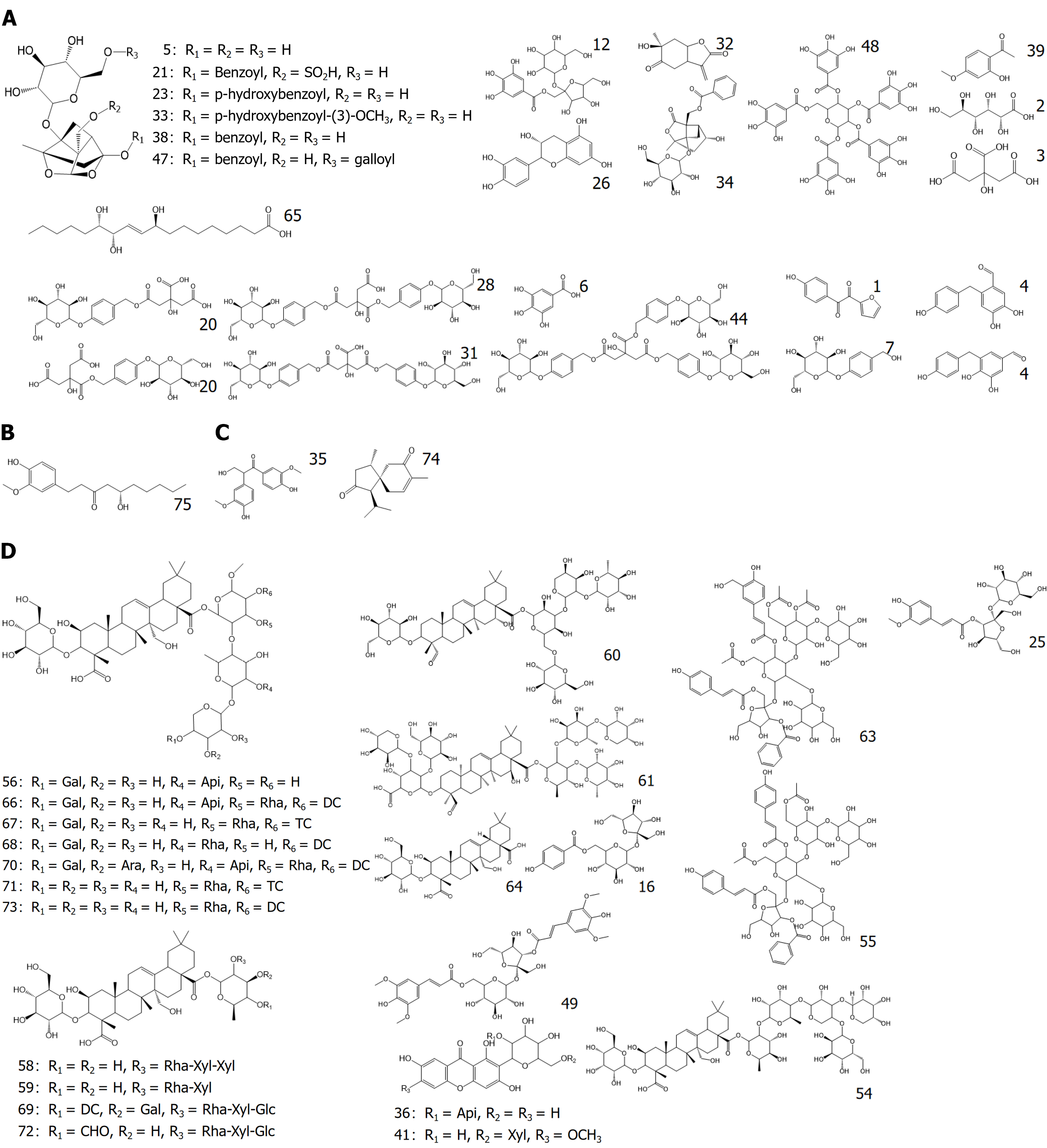
We compared the plasma total ion current chromatograms of rats before and after CG administration, combined with the self-constructed libraries of in vivo and in vitro components, UNIFI software, and control quality spectra information. A total of 75 chemical components were characterized, namely 49 prototypical components, of which 16 were from BS, 11 from TM, 22 from YZ, and 2 from SCP. Twenty-six metabolites, including paeoniflorin, aspalathosin, gallic acid, 3,6'-dierucoyl sucrose, Siberian farnesose A5, onjisaponin F, and 23-hydroxybetulinic acid, were metabolized, and metabolic reactions such as hydrolysis, methylation, glucuronidation, and sulfate esterification mainly occurred. Specific compound information is presented in Table 2 and Figure 3, the structural formulas of the blood-entry prototype components are presented in Figure 4.
| No. | tR/minute | Ion mode | Deviation (ppm) | Fragment ions | Theoretical value | Molecular formula | Ingredient name | Note |
| 1 | 0.83 | [M-H]- 215.0329 | -6.98 | 149.0457 | 216.0423 | C12H8O4 | 1-Furan-2-yl-2-(4-hydroxy-phenyl)ethane-1,2-dione | P |
| 2 | 0.85 | [M-H]- 195.0516 | 5.64 | 165.0439; 179.0590 | 195.0583 | C6H12O7 | Gluconic acid | P |
| 3 | 1.46 | [M-H]- 191.0206 | 7.33 | 111.0066 | 192.027 | C6H8O7 | Citric acid | P |
| 4 | 1.75 | [M-H]-243.0651 | -2.47 | 107.9124 | 244.0736 | C14H12O4 | Gastrodibenzin B/C | P |
| 5 | 1.9 | [M-H]- 375.1257 | -9.06 | 341.1122; 300.9911 | 376.1369 | C16H24O10 | 8-Debenzoylpaeoniflorin | P |
| 6 | 2.4 | [M-H]- 169.0121 | 9.47 | 125.0244; 107.1245 | 170.0215 | C7H6O5 | Gallic acid | P |
| 7 | 2.59 | [M-H]- 285.0973 | -0.35 | 268.0276 | 286.1053 | C13H18O7 | Gastrodin | P |
| 8 | 2.81 | [M-H]- 137.0243 | 2.92 | 109.0268 | 138.0317 | C7H6O3 | The product of gallic acid takes off two oxygen atoms or its isomer | M |
| 9 | 2.81 | [M-H]- 299.0766 | -0.33 | 175.0088; 283.0656; 285.0088 | 300.0845 | C13H16O8 | Gastrodin hydrolysis of pyranose, glucuronidation | M |
| 10 | 3.01 | [M+H]+ 265.0752 | 2.26 | 218.0879 | 264.0668 | C10H16O6S | C10H14O3 sulfate | M |
| 11 | 3.12 | [M-H]- 199.0981 | 5.52 | 137.0908; 162.8295; 181.0398 | 200.1049 | C10H16O4 | Paeonimetabolin II | M |
| 12 | 3.18 | [M-H]- 493.1193 | 0 | 169.0118; 313.0659 | 494.1272 | C19H26O15 | 1'-O-Galloylsucrose | P |
| 13 | 3.43 | [M-H]- 389.1425 | -5.91 | 375.0822 | 390.1526 | C17H26O10 | The product of paeoniflorin loses C7H4O and methylation | M |
| 14 | 3.92 | [M-H]- 262.9856 | -2.28 | 125.0146; 168.0027; 183.0244 | 263.994 | C8H8O8S | Methyl gallate sulfate | M |
| 15 | 3.97 | [M-H]- 183.0300 | 3.82 | 125.0287; 167.0704 | 184.0372 | C8H8O5 | Methyl gallate | M |
| 16 | 4.34 | [M-H]- 461.1273 | -4.77 | 175.0088; 285.0088 | 462.1373 | C19H26O13 | Gastrodin glucuronidation | M |
| 17 | 4.34 | [M-H]- 461.1273 | -4.77 | 93.0382; 137.0294 | 462.1373 | C19H26O13 | Sibiricose A3 | P |
| 18 | 4.39 | [M-H]- 705.1751 | 7.09 | 259.0203; 121.0329 | 706.1779 | C29H38O18S | Sulfitation of isomaltosyl paeoniflorin | M |
| 19 | 4.51 | [M-H]- 261.0062 | -2.68 | 171.0163; 215.0649 | 262.0147 | C9H10O7S | 3,4-dihydroxyphenylpropionic acid sulfate | M |
| 20 | 4.54 | [M-H]- 459.1132 | -1.52 | 400.9565 | 460.1217 | C19H24O13 | Parishin E/Parishin G | P |
| 21 | 4.56 | [M-H]- 543.1189 | 3.13 | 121.0281; 259.0273 | 544.1251 | C23H28O13S | Paeoniflorin sulfite | P/M |
| 22 | 4.74 | [M-H]- 345.1527 | -6.37 | 169.1254; 175.0088 | 346.1628 | C16H26O8 | C10H18O2-glucuronidation | M |
| 23 | 4.8 | [M-H]- 495.1483 | -4.04 | 137.0345 | 496.1581 | C23H28O12 | Oxypaeoniflora | P/M |
| 24 | 4.87 | [M-H]- 223.0607 | 0.45 | 164.0299; 176.0824; 208.0458 | 224.0685 | C11H12O5 | Sinapinic acid | M |
| 25 | 4.91 | [M-H]- 517.1567 | 1.93 | 160.0218; 175.0377; 193.0743 | 518.1636 | C22H30O14 | Sibiricose A5 | P |
| 26 | 4.95 | [M-H]- 289.071 | -0.69 | 123.0399; 205.0580 | 290.079 | C15H14O6 | L-Epicatechin | P |
| 27 | 5.16 | [M-H]- 193.0500 | -0.52 | 133.0153; 151.0411 | 194.0579 | C10H10O4 | Ferulic acid | M |
| 28 | 5.39 | [M-H]- 727.2063 | -3.16 | 111.0058 | 728.2164 | C32H40O19 | Parishin B | P |
| 29 | 5.44 | [M-H]- 373.0803 | 8.58 | 197.0426; 359.0924 | 374.0849 | C15H18O11 | Dimethylgallic acid glucuronidation | M |
| 30 | 5.44 | [M-H]- 547.1671 | 1.46 | 175.0319; 190.0279; 205.0330; 223.0607 | 548.1741 | C23H32O15 | Sibiricose A1 | P/M |
| 31 | 5.67 | [M-H]- 727.2062 | -3.3 | 129.0162 | 728.2164 | C32H40O19 | Parishin C | P |
| 32 | 5.68 | [M+H]+ 197.0800 | -7.1 | 151 | 196.0736 | C10H12O4 | Paeonilactone B | P |
| 33 | 5.69 | [M-H]- 525.1628 | 3.81 | 165.0551; 363.1675 | 526.1686 | C24H30O13 | Mudanpioside E | P |
| 34 | 5.7 | [M-H]- 479.1568 | 2.09 | 525.1579 | 480.1632 | C23H28O11 | Albiflorin | P |
| 35 | 5.72 | [M+H]+ 319.1173 | -2.82 | 301.1047 | 318.1103 | C17H18O6 | Evofolin B | P |
| 36 | 5.99 | [M-H]- 537.1202 | -7.82 | 315.0473 387.0947 | 538.1323 | C24H26O14 | Albiflorin B | P |
| 37 | 6.03 | [M-H]- 449.1454 | 1.34 | 77.0388; 197.0390 | 450.1526 | C22H26O10 | Paeoniflorin dehydroxymethylene | M |
| 38 | 6.05 | [M-H]- 479.1569 | 2.3 | 121.0281; 77.0388 | 480.1632 | C23H28O11 | Paeoniflorin | P |
| 39 | 6.09 | [M-H]- 165.0551 | -0.61 | 121.0329 | 166.063 | C9H10O3 | Paeonol | P |
| 40 | 6.16 | [M-H]- 276.9997 | -7.58 | 197.0451 | 278.0096 | C9H10O8S | Sulfation of dimethylgallic acid | M |
| 41 | 6.17 | [M-H]- 567.1345 | -0.88 | 315.055 | 568.1428 | C25H28O15 | Polygalaxanthone VIII | P |
| 42 | 6.18 | [M+H]+ 569.3103 | -7.9 | 525.3032 | 568.307 | C30H48O8S | Oxidized-23-hydroxybetulinic acid sulfate | M |
| 43 | 6.5 | [M-H]- 197.0451 | 0.51 | 151.0840; 167.0474 | 198.0528 | C9H10O5 | Dimethylgallic acid | M |
| 44 | 6.56 | [M-H]- 995.3008 | -2.41 | 423.0817; 728.2076 | 996.3111 | C45H56O25 | Parishin A | P |
| 45 | 6.88 | [M-H]- 291.0198 | 7.9 | 197.0329; 211.0615 | 292.0253 | C10H12O8S | Trimethyl gallate sulfate | M |
| 46 | 7.15 | [M-H]- 631.1572 | -1.43 | 121.0281; 589.0381 | 632.1659 | C30H33O13P | Benzoylpaeoniflorin lost oxygen and oxidative phosphorylation | M |
| 47 | 7.18 | [M-H]- 631.1682 | 3.01 | 465.0559; 313.0582; 271.0066; 169.0118 | 632.1741 | C30H32O15 | Galloylpaeoniflorin | P |
| 48 | 7.43 | [M-H]- 939.1154 | 5.32 | 769.0880; 617.0786; 447.0591; 169.0121 | 940.1182 | C41H32O26 | Pentagalloylglucose | P |
| 49 | 7.91 | [M-H]- 753.2276 | 4.51 | 205.0518; 223.0541; 529.1585; 547.1569 | 754.232 | C34H42O19 | 3,6'-Disinapoyl sucrose | P |
| 50 | 8.09 | [M+H]+ 333.0979 | 1.5 | 303.0536 | 332.0896 | C17H16O7 | Oxypaeoniflorin loses C6H10O6 andketonisation | M |
| 51 | 8.9 | [M-H]- 137.0243 | 2.92 | 109.0268 | 138.0317 | C7H6O3 | The product of gallic acid takes off two oxygen atoms or its is isomer | M |
| 52 | 9.12 | [M+H]+ 241.1073 | -1.24 | 227.1124 | 240.0998 | C12H16O5 | Sinapic acid methylation | M |
| 53 | 10.52 | [M-H]- 237.0764 | 0.42 | 103.0904; 163.0302; 193.0258; 222.0247 | 238.0841 | C12H14O5 | 3,4,5-Trimethoxycinnamic acid | M |
| 54 | 10.66 | [M-H]- 1397.6211 | -1 | - | 1398.6303 | C64H102O33 | Arillatanoside C | P |
| 55 | 10.84 | [M-H]- 1307.3827 | -3.9 | 307.1561; 1161.3540 | 1308.3956 | C59H72O33 | Tenuifoliose J | P |
| 56 | 10.9 | [M-H]- 1265.5747 | -4.42 | 425.3125; 455.3123; 499.1799; 585.2072; 1235.5510 | 1266.5881 | C59H94O29 | desacylsenegasaponin B | P |
| 57 | 11.11 | [M-H]- 1367.6119 | 0.88 | 425.3035; 687.2446; 1143.5181; 1137.5907 | 1368.6198 | C63H100O32 | Onjisaponin F-C12H12O4 | M |
| 58 | 11.23 | [M-H]- 1235.5663 | -2.75 | 337.2389; 455.3123; 555.1955 | 1236.5775 | C58H92O28 | Arillatanoside A | P |
| 59 | 11.44 | [M-H]- 1103.5256 | -1.63 | 455.3123 | 1104.5353 | C53H84O24 | Polygalasaponin XXVIII | P |
| 60 | 11.5 | [M-H]- 1249.5913 | 4.8 | 425.3125; 455.3123; 1025.5084 | 1250.5932 | C59H94O28 | Onjisaponin Tf | P |
| 61 | 11.54 | [M-H]- 1525.6748 | 3.21 | 1157.4280; 1351.5072; 1463.5935 | 1526.6777 | C70H110O36 | Onjisaponin Te | P |
| 62 | 11.56 | [M-H]- 1307.3827 | -3.9 | 307.1561; 1161.3540 | 1308.3956 | C59H72O33 | Tenuifoliose I | P |
| 63 | 12.16 | [M-H]- 1379.4103 | 1.01 | 1161.3391 | 1380.4167 | C62H76O35 | Tenuifoliose A | P |
| 64 | 12.8 | [M-H]- 679.3673 | -3.09 | 425.3035; 455.3123 | 680.3772 | C36H56O12 | Tenuifolin | P |
| 65 | 12.9 | [M-H]- 329.2345 | 5.16 | 171.0906; 211.0615; 229.2078 | 330.2406 | C18H34O5 | 9,12,13-TriHOME | P |
| 66 | 13 | [M-H]- 1703.7188 | -8.22 | 157.0906; 1479.6775 | 1704.7407 | C80H120O39 | Onjisaponin A | P |
| 67 | 13.1 | [M-H]- 1631.7034 | -5.09 | 425.3125; 455.3217; 567.2073; 1601.6753 | 1632.7195 | C77H116O37 | Onjisaponin O | P |
| 68 | 13.21 | [M-H]- 1571.6836 | -4.45 | 567.1970; 1347.6245; 1541.6838 | 1572.6984 | C75H112O35 | Onjisaponin B | P |
| 69 | 13.25 | [M-H]- 1617.6938 | -1.42 | 425.3035; 455.3123; 1393.6237 | 1618.7039 | C76H114O37 | Polygalasaponin XLIV | P |
| 70 | 13.28 | [M-H]- 1673.7109 | -6.81 | 425.3125; 1617.6731 | 1674.7301 | C79H118O38 | Z-polygalasaponin XXXII | P |
| 71 | 13.33 | [M-H]- 1469.6482 | -7.28 | 405.1388 | 1470.6667 | C71H106O32 | Onjisaponin Z | P |
| 72 | 13.33 | [M-H]- 1469.6482 | 3.13 | 1439.566 | 1470.6515 | C67H106O35 | Polygalasaponin XXXXII | P |
| 73 | 13.48 | [M-H]- 1409.6310 | -4.82 | 425.3125; 455.3217; 1379.7347 | 1410.6456 | C69H102O30 | Onjisaponin Y | P |
| 74 | 17.29 | [M-H]- 233.1522 | -8.58 | 218.8733; 197.8061 | 234.162 | C15H22O2 | Acoronene | P |
| 75 | 19.22 | [M-H]- 293.1770 | 5.8 | 245.899 | 294.1831 | C17H26O4 | 6-Gingerol | P |
Metabolism is divided into one-phase and two-phase metabolism. One-phase metabolism involves the introduction of functional groups, and lipid-soluble compounds are hydrolyzed, oxidized, or subjected to other reactions to generate large polar substances. The two-phase metabolism is dominated by binding reactions in which polar groups are combined with endogenous substances, resulting in an increase in polarity and easy elimination[16].
The metabolic process analysis using gallic acid as an example. Peak at m/z 169, which was found at tR = 2.40, analyzed, and confirmed to be gallic acid. Peak 16 (tR = 3.97), m/z 183, which differed from gallic acid by 14 (CH2), existed with the same fragment m/z 125 as gallic acid and m/z 167 was presumed to be [M-H-O]-, which was analyzed and confirmed to be methyl gallic acid. Similarly, peak 43 (tR = 3.97, m/z 197) was identified as dimethyl gallic acid. Peak 16 (tR = 3.92, m/z 263), which had a molecular formula of C8H8O8S, also showed the presence of the same fragments as gallic acid, m/z 125, and m/z 168, as [M-H-SO3-CH3]-, which was presumed to be gallic acid sulfate glycoside. Similarly, peak 41 (tR = 6.16, m/z 277) was presumed to be dimethyl gallic acid sulfate glycoside. Peak 32 (tR = 5.44, m/z 373), which differed from gallic acid by 204, was the weight of two CH3 and a glucuronide group, showing the presence of m/z 197 for [M-H-GlcA-2CH3]- and m/z 359 for [M-H-CH3]-, and this peak is presumed to be a dimethyl gallic acid glucuronide. Peak 45 (tR = 6.88, m/z 291), differing from peak 41 by 14. m/z 197, a dimethyl gallic acid sulfate glycoside ion peak, was present. This peak is presumed to be trimethyl gallic acid glucuronide. Peak 50 (tR = 8.90, m/z 137), which differs from gallic acid by 32; m/z 109 for [M-H-CO]- was present. It is hypothesized that this peak represents the product of gallic acid degradation of 2 oxygen atoms. The above results show that gallic acid mainly undergoes methylation, sulfation, glucuronidation, and deoxygenation. The metabolic pathways of the components are illustrated in Figure 5.
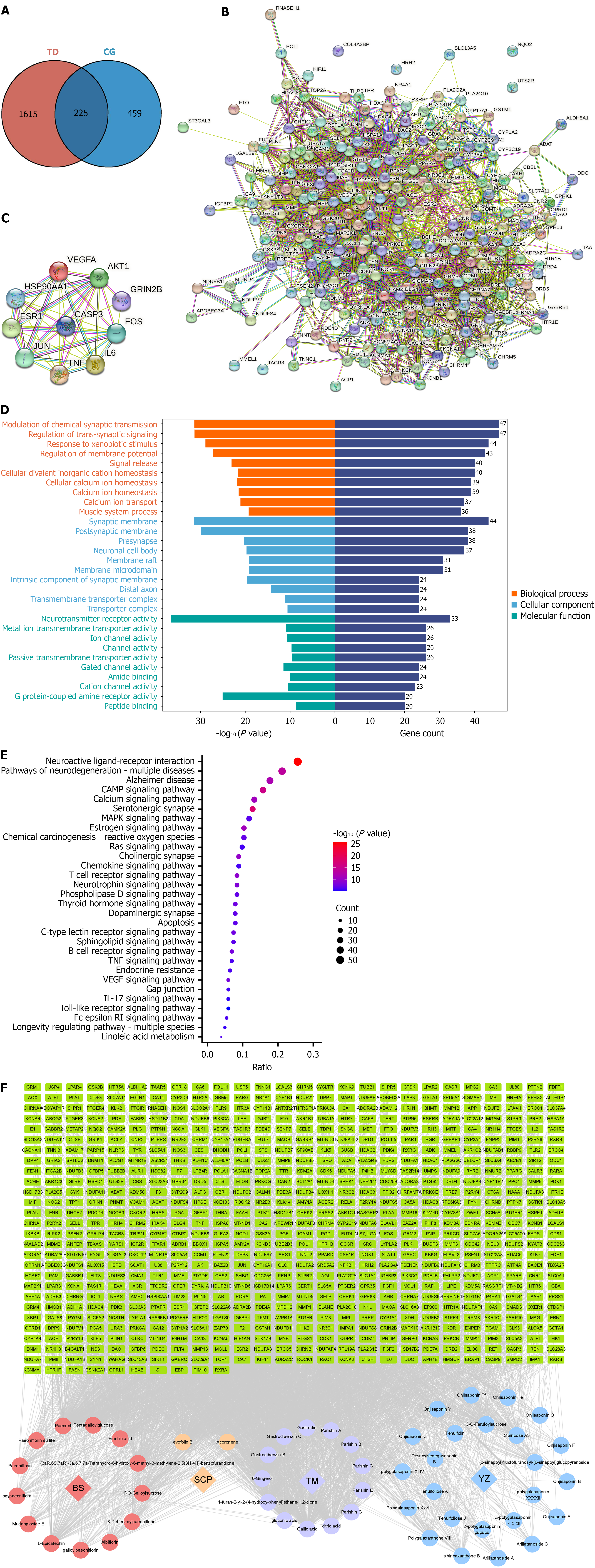
Target screening of CG for treating TD: UPLC-Q-TOF-MS/MS was used to characterize 49 prototype compounds and 26 metabolites. Metabolite backtracking yielded 7 prototype components, and comparison and de-emphasis yielded 50 incoming prototype components. Entering these 50 components into the TCMSP database yielded 684 targets. Screening yielded 1840 TD targets. After the intersection, 225 targets were obtained. The Venn diagram of the intersecting targets is shown in Figure 6A.
Protein interaction network construction and Hub gene screening results: The common targets obtained were imported into the STRING database to construct a protein interaction network diagram (Figure 6B). The genes with the top 10 degree values were screened, which were AKT1, TNF, IL6, CASP3, FOS, VEGFA, JUN, HSP90AA1, and ESR1(Figure 6C).
GO functional enrichment and KEGG pathway enrichment analyses: A total of 5420 GO enrichment results were obtained by importing the crossed genes into the database, of which 4395 were biological processes, mainly including modulation of chemical synaptic transmission, response to xenobiotic stimulus, regulation of membrane potential, and cellular ion homeostasis. A total of 383 cellular compositions were enrichment, mainly including the synaptic membrane, neuronal cell body, membrane rafts, and membrane microdomain. Six hundred and forty-two were molecular functions, mainly involving neurotransmitter receptor activity, metal ion transmembrane transporter activity, and ion channel activity. A total of 273 signaling pathways were enriched by KEGG analysis, including neuroactive ligand-receptor interaction, pathways of neurodegeneration-multiple diseases, and cyclic AMP signaling pathway. The GO and KEGG enrichment results were imported into the network drawing platform and visualized. The results are shown in Figure 6D and E and Tables 3 and 4. The results indicated that CG exerted anti-TD effects through multiple targets and pathways.
| GO term | Subgroup | -log10 (P value) | Count | -log10 (q value) |
| Modulation of chemical synaptic transmission | Biological process | 31.3 | 47 | 28.23 |
| Regulation of trans-synaptic signaling | Biological process | 31.3 | 47 | 28.23 |
| Response to xenobiotic stimulus | Biological process | 28.8 | 44 | 26.4 |
| Regulation of membrane potential | Biological process | 27.1 | 43 | 24.8 |
| Signal release | Biological process | 23.0 | 40 | 21.0 |
| Cellular divalent inorganic cation homeostasis | Biological process | 21.6 | 40 | 19.7 |
| Cellular calcium ion homeostasis | Biological process | 21.9 | 39 | 19.9 |
| Calcium ion homeostasis | Biological process | 21.5 | 39 | 19.6 |
| Calcium ion transport | Biological process | 21.0 | 37 | 19.2 |
| Muscle system process | Biological process | 19.2 | 36 | 17.5 |
| Synaptic membrane | Cellular component | 31.4 | 44 | 29.0 |
| Postsynaptic membrane | Cellular component | 29.8 | 38 | 27.8 |
| Presynapse | Cellular component | 20.3 | 38 | 18.7 |
| Neuronal cell body | Cellular component | 19.7 | 37 | 18.2 |
| Membrane raft | Cellular component | 19.2 | 31 | 17.8 |
| Membrane microdomain | Cellular component | 19.2 | 31 | 17.8 |
| Intrinsic component of synaptic membrane | Cellular component | 19.6 | 24 | 18.1 |
| Distal axon | Cellular component | 14.2 | 24 | 13.0 |
| Transmembrane transporter complex | Cellular component | 11.1 | 24 | 10.0 |
| Transporter complex | Cellular component | 10.6 | 24 | 9.6 |
| Neurotransmitter receptor activity | Molecular function | 36.5 | 33 | 34.0 |
| Metal ion transmembrane transporter activity | Molecular function | 10.9 | 26 | 9.3 |
| Ion channel activity | Molecular function | 10.6 | 26 | 9.1 |
| Channel activity | Molecular function | 9.7 | 26 | 8.3 |
| Passive transmembrane transporter activity | Molecular function | 9.6 | 26 | 8.3 |
| Gated channel activity | Molecular function | 11.5 | 24 | 9.8 |
| Amide binding | Molecular function | 10.0 | 24 | 8.5 |
| Cation channel activity | Molecular function | 10.5 | 23 | 9.0 |
| G protein-coupled amine receptor activity | Molecular function | 25.0 | 20 | 22.8 |
| Peptide binding | Molecular function | 8.7 | 20 | 7.4 |
| Description | Gene ratio | Count | -log10 (P value) | -log10 (q value) |
| Neuroactive ligand-receptor interaction | 52/203 | 52 | 25.8 | 23.78 |
| Pathways of neurodegeneration - multiple diseases | 43/203 | 43 | 13.7 | 12.38 |
| Alzheimer disease | 36/203 | 36 | 11.9 | 10.68 |
| cAMP signaling pathway | 32/203 | 32 | 15.7 | 14.18 |
| Calcium signaling pathway | 27/203 | 27 | 10.8 | 9.7 |
| Serotonergic synapse | 26/203 | 26 | 17.9 | 16.2 |
| MAPK signaling pathway | 24/203 | 24 | 6.6 | 6.1 |
| Estrogen signaling pathway | 21/203 | 21 | 10.9 | 9.8 |
| Chemical carcinogenesis - reactive oxygen species | 21/203 | 21 | 7.1 | 6.5 |
| Ras signaling pathway | 20/203 | 20 | 6 | 5.6 |
| Cholinergic synapse | 18/203 | 18 | 9.8 | 8.8 |
| Chemokine signaling pathway | 18/203 | 18 | 6.1 | 5.7 |
| T cell receptor signaling pathway | 17/203 | 17 | 9.47 | 8.5 |
| Neurotrophin signaling pathway | 17/203 | 17 | 8.5 | 7.7 |
| Phospholipase D signaling pathway | 17/203 | 17 | 7 | 6.5 |
| Thyroid hormone signaling pathway | 16/203 | 16 | 7.5 | 6.9 |
| Dopaminergic synapse | 16/203 | 16 | 7 | 6.4 |
| Apoptosis | 16/203 | 16 | 6.8 | 6.3 |
| C-type lectin receptor signaling pathway | 15/203 | 15 | 7.6 | 6.9 |
| Sphingolipid signaling pathway | 15/203 | 15 | 6.7 | 6.2 |
| B cell receptor signaling pathway | 14/203 | 14 | 8 | 7.3 |
| TNF signaling pathway | 14/203 | 14 | 6.3 | 5.8 |
| Endocrine resistance | 13/203 | 13 | 6.2 | 5.8 |
| VEGF signaling pathway | 12/203 | 12 | 7.9 | 7.2 |
| Gap junction | 12/203 | 12 | 5.9 | 5.5 |
| IL-17 signaling pathway | 12/203 | 12 | 5.6 | 5.3 |
| Toll-like receptor signaling pathway | 12/203 | 12 | 5.1 | 4.9 |
| Fc epsilon RI signaling pathway | 11/203 | 11 | 6.2 | 5.8 |
| Longevity regulating pathway - multiple species | 10/203 | 10 | 5.7 | 5.34 |
Construction results of the component-target-pathway-disease topological network of CG: The 49 blood-entry prototype components and corresponding targets were imported into Cytospace 3.10.0 software and visualized. The results are shown in Figure 6F, with red representing BS and its key components, yellow representing SCP and its key components, blue representing YZ and its key components, purple representing TM and its key components, and green representing the key targets of each component’s action. The results showed that the blood-entry components and the key targets acted closely with each other, indicating that CG could exert anti-TD effects through multicomponents and multitargets. According to the degree value, paeonol, evofolin B, gastrodin, paeoniflorin, and other components may be potential pharmacodynamic substances, and FOS, AKT1, ESR1, and TNF may be potential targets.
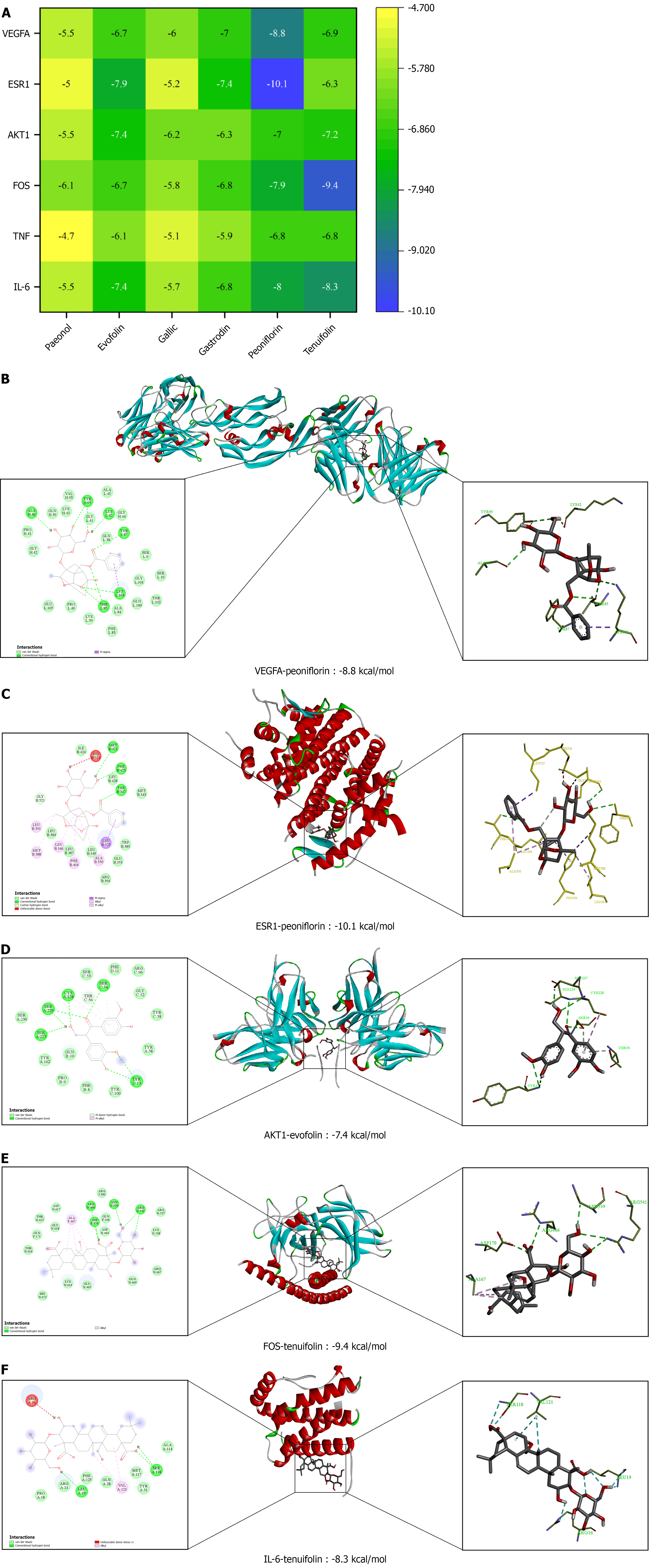
According to the degree value (degree value > 100) of the “component-target” network, Plasma abundance and the active compounds reported in the literature, paeonol, evofolin B, gallic acid, gastrodin, peoniflorin, and tenuifolin were selected as key components; IL-6, TNF, FOS, AKT1, ESR1, and VEGFA were selected as the core proteins; and molecular docking was performed on the key components and core proteins to verify the binding between the key components and the core targets. The smaller binding energies indicated easy binding of the components to the targets. The results showed that the binding energies of most of the key components and the core targets were less than -5 kcal/mol, indicating that the key components were tightly bound to the core targets with high affinity and might have strong pharmacodynamic activities. The binding process is dominated by hydrogen bonding. In addition to that, there are van der Waals forces, hydrophobic effects, and other forces. For example, during the binding process between tenuifolin and IL-6, SER-118 and LEU-19 form hydrogen bonds with tenuifolin, ARG-24 binds to tenuifolin via van der Waals forces, and VAL-121 and tenuifolin bind via hydrophobic interaction. The molecular docking results are shown in Figure 7A, and a part of the docking process is shown in Figure 7B-F. The positive control of the core protein and its inhibitor are shown in Table 5.
| Receptor-ligand | VEGFA-Ginsenoside Rg3 | ESR1-Tamoxifen | AKT1-MK2206 | FOS-224 | TNF-SPD304 | IL-6-LMT-28 |
| Binding energies (kcal/mol) | -9.3 | -10.3 | -10.0 | -8.6 | -9.8 | -8.8 |
Herein, UPLC-Q-TOF-MS/MS was used to analyze the chemical composition of CG, mainly terpenoids, glycosides, flavonoids, and phenolic acid components. The plasma samples were examined for pretreatment methods and analyzed for composition, and the results of five methods of plasma treatment with methanol, acetonitrile, ethyl acetate, water-saturated n-butanol, and SPE solid-phase extraction were compared. The results showed that the methanol-treated plasma samples were well separated and had many components suitable for compositional analyses; and the results of the characterization of the compositions showed that a total of 49 prototype components were found, which were mainly terpenoids, saccharides, and phenolic acids. After screening the blood-entry components with TD targets and analyzing them via network pharmacology, a total of 225 intersecting targets were obtained. Through GO, KEGG, “component-target” network construction, and molecular biology analyses, alongside relevant literature reports, the active components and related targets of CG were initially identified. Molecular docking revealed that the binding affinities of Tenuifolin-FOS, Tenuifolin-IL-6, Paeoniflorin-ESR1, and Paeoniflorin-VEGFA are comparable to those of the positive control, indicating similar binding potency. Subsequent studies will focus on cell-based pharmacodynamic evaluation and target verification of Tenuifolin and Paeoniflorin, along with quantitative analysis and pharmacokinetic profiling, to elucidate their in vivo behavior.
A total of 26 metabolites were identified using UPLC-Q-TOF-MS/MS, primarily resulting from glucuronidation, methylation, sulfation, and hydrolysis. Oxidation and hydrolysis are indicative of phase-I metabolism, whereas glucuronidation and sulfation are characteristic of phase-II metabolism. Upon entry into the body, phase-I enzymes located in the liver and intestine are activated, facilitating hydrolysis and other reactions that modify the molecular structure, enhance polarity and water solubility, and ultimately alter the compound's biological activity. Onjisaponin F was not detected in plasma as the intact parent compound; rather, its hydrolysis product, ferulic acid, was identified. Ferulic acid has been documented to inhibit inflammatory pathways by reducing the production of pro-inflammatory mediators such as TNF-α, thereby mitigating inflammation[17]. This observation is consistent with our network-pharmacology findings. Furthermore, research has demonstrated that ferulic acid enhances the Akt/CRMP2 signaling pathway, promoting neuronal regeneration and synaptic remodeling, and exerting neuroprotective effects[18]. These observations suggest that hydrolytic reactions occurring in vivo have the potential to produce small-molecule metabolites with notable biological activity. During phase-II metabolism, xenobiotics undergo conjugation with glucuronic acid, activated sulfate, or other polar groups through the action of phase-II enzymes, resulting in the formation of more water-soluble me
TD is a class of psychiatric disorders with arrhythmic tics as the main manifestation, and the drugs used to treat TD are mainly psychotropic, including dopamine receptor blockers, antiepileptic drugs, and α-adrenergic receptor agonists. However, these drugs had a high rate of relapse and obvious adverse effects[22,23]. Modern pharmacological studies have found that TD is associated with dysfunctional neuroimmune interactions, and it is possible that inflammatory neuronal components are involved in the pathogenesis of TD[24,25]. Microglia are immune cells present in neural tissues and are usually involved in inflammation, nerve injury and other diseases[26]. Microglia activity has been reported to be high in patients with TD[27]. Microglia may differentiate into proinflammatory microglia that secrete proinflammatory factors, such as IL-2, TNF-α, and other inflammatory factors or into anti-inflammatory microglia that secrete anti-inflammatory factors and upregulate neuroprotective factors when stimulated by the disease[28,29]. Kutuk et al[30] found that serum levels of IL-1β, TNF-α, IL-6, and IL-4 are significantly elevated in children with TD. Most cells in the nervous system can produce IL-6, which modulates dopaminergic and cholinergic neurons, thereby regulating neuronal excitability and sleep. TNF-α can modulate the activation of microglia and astrocytes and influence the blood-brain barrier. We therefore hypothesize that these inflammatory factors cross the immature blood-brain barrier, infiltrate the striatum, activate microglia, and lead to hyperactivity of the direct pathway and suppression of the indirect pathway, ultimately producing motor tics such as eye-blinking and shoulder-shrugging. The PI3K/AKT signaling pathway is also a common inflammatory pathway with various biological effects[31]. TNF serves as an upstream signal to activate PI3K/AKT, while PI3K/AKT in turn negatively regulates TNF-mediated inflammation. In our protein-protein interaction network, TNF/AKT1 ranked among the top 10 hub genes; therefore, we postulate that the PI3K/AKT pathway can influence the therapeutic efficacy of CG in TD[32]. Some studies have found that the use of PI3K inhibitors can alleviate TD symptoms and downregulate the levels of inflammatory factors, suggesting that the PI3K/AKT signaling pathway mediates the pathological process of TD. Tao et al[33] reported that the levels of serum inflammatory factors, such as IL-6 and TNF-α, in patients with TD were significantly higher than those in the control group. Therefore, the occurrence of TD is closely related to the inflammatory signaling pathway. In addition, other studies have found that the core targets of TD include JUN and FOS[34], which is consistent with the results of this study.
Currently, Chinese medicines, such as Tianmagoutengyin, CG, Ning Dong Granule, and Jing An Oral Liquid, are first-line medicines for the treatment of TD. Most of these Chinese medicines are based on BS, TM, and YZ, which are characterized by good therapeutic effects, fewer adverse effects, and simultaneous action on multiple targets. After UPLC-Q-TOF-MS/MS and network pharmacology screening, paeoniflorin could enter the bloodstream and is a potential medicinal substance. Modern pharmacological studies have found that paeoniflorin is widely found in plants of the family Paeoniaceae, and it has neuronal cell-protecting, anti-inflammatory, and other activities[35,36]. Zhao et al[37], found that paeoniflorin exerted neuroprotective effects by attenuating brain damage through the PI3K/AKT signaling pathway by means of transmission electron microscopy, immunofluorescence, liquid chromatography-mass spectrometry, and molecular docking. In addition, gastrodin and tenuifolin may be potential pharmacodynamic substances. Some studies have shown that gastrodin and tenuifolin can exert neuroprotective effects by acting on targets such as TNF-α and IL-6[38,39]. The molecular docking results also showed that paeoniflorin, gastrodin, and tenuifolin have strong binding power to the core targets.
This study is the first to integrate serum pharmacochemistry, network pharmacology, and molecular docking to characterize the in vitro and in vivo chemical constituents of CG in pediatric TDs. The fragmentation patterns and metabolic pathways of the relevant compounds were elucidated, providing a solid foundation for identifying the active substances of CG. The combined approach offers a comprehensive view of CG’s mechanism of action. Emphasizing CG’s potential as a safer alternative to psychotropic drugs addresses an unmet need in the treatment of TD. Moreover, it provides robust evidence for new-drug registration. To date, the marketing-authorization dossier for CG has been accepted by the National Medical Products Administration (acceptance No. CXZS2500020) and granted priority-review status.
In summary, CG effectively improves TD through its multicomponent and multitarget synergistic effects. The core mechanism may involve the inhibition of inflammatory factors to alleviate inflammation. These findings provide a theoretical basis for the treatment of TD with CG and lay the foundation for the subsequent interactions between components and targets. In future studies, we plan to employ a lipopolysaccharide-induced microglial inflammation model. Western blot, ELISA and RT-qPCR will be used to quantify TNF-α, IL-6, and so on protein levels, providing direct experimental validation of the predicted anti-inflammatory pathway.
Herein, serum pharmacology was analyzed using UPLC-Q-TOF-MS/MS, which provided a basis for the further investigation of the material basis and action mechanism of CG against TD. Overall, 187 components were characterized in CG, and 75 components were characterized in plasma, of which 49 were prototypes and 26 were metabolites. CG might exert its therapeutic effect on TD through the action of paeoniflorin, gastrodin, gallic acid, and tenuifolin on key targets such as TNF, IL-6, FOS, and VEGFA. So far, CG have been included in the priority approval of TCM new drugs, in China. However, this study has some limitations. It relies on network pharmacology to predict the key targets. To ensure the accuracy of the key targets, timely experimental verification is required. Therefore, to solve this problem, our group will perform in vivo and in vitro experiments for validation in subsequent studies to thoroughly investigate the mechanism by which CG exerts anti-TD effects.
| 1. | Woods DW, Himle MB, Stiede JT, Pitts BX. Behavioral Interventions for Children and Adults with Tic Disorder. Annu Rev Clin Psychol. 2023;19:233-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Miller-Fleming TW, Allos A, Gantz E, Yu D, Isaacs DA, Mathews CA, Scharf JM, Davis LK. Developing a phenotype risk score for tic disorders in a large, clinical biobank. Transl Psychiatry. 2024;14:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Ueda K, Black KJ. A Comprehensive Review of Tic Disorders in Children. J Clin Med. 2021;10:2479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 4. | Müller-Vahl KR, Szejko N, Verdellen C, Roessner V, Hoekstra PJ, Hartmann A, Cath DC. European clinical guidelines for Tourette syndrome and other tic disorders: summary statement. Eur Child Adolesc Psychiatry. 2022;31:377-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Li F, Cui Y, Li Y, Guo L, Ke X, Liu J, Luo X, Zheng Y, Leckman JF. Prevalence of mental disorders in school children and adolescents in China: diagnostic data from detailed clinical assessments of 17,524 individuals. J Child Psychol Psychiatry. 2022;63:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 6. | Roessner V, Eichele H, Stern JS, Skov L, Rizzo R, Debes NM, Nagy P, Cavanna AE, Termine C, Ganos C, Münchau A, Szejko N, Cath D, Müller-Vahl KR, Verdellen C, Hartmann A, Rothenberger A, Hoekstra PJ, Plessen KJ. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part III: pharmacological treatment. Eur Child Adolesc Psychiatry. 2022;31:425-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 7. | Liu YK, Cui H, Yang J, Wang JH, Wu LQ, Ji XH, An B, Liu XM, Wan WL, Wang H, Zhou GJ, Zhang W, Qiu LY, Guo K, Zhang XX. [Clinical advantages of traditional Chinese medicine in treatment of pediatric diseases]. Zhongguo Shiyan Fangjixue Zazhi. 2024;30:224-231. [DOI] [Full Text] |
| 8. | Zhao Q, Hu Y, Yan Y, Song X, Yu J, Wang W, Zhou S, Su X, Bloch MH, Leckman JF, Chen Y, Sun H. The effects of Shaoma Zhijing granules and its main components on Tourette syndrome. Phytomedicine. 2024;129:155686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Feng P, Chen Y, Sun K, Wei X, Ding Y, Shang J, Shi Z, Xu X, Guo J, Tian Y. Volatile oil from Acori graminei Rhizoma affected the synaptic plasticity of rats with tic disorders by modulating dopaminergic and glutamatergic systems. J Ethnopharmacol. 2024;335:118676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Zhao L, Li AY. Gastrodin - A potential drug used for the treatment of Tourette Syndrome. J Pharmacol Sci. 2021;145:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Wang L, Jin G, Yu H, Li Q, Yang H. Protective effect of Tenuifolin against Alzheimer's disease. Neurosci Lett. 2019;705:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Luo XQ, Li A, Yang X, Xiao X, Hu R, Wang TW, Dou XY, Yang DJ, Dong Z. Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2. Chin Med. 2018;13:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Liu H, Song Z, Liao DG, Zhang TY, Liu F, Zhuang K, Luo K, Yang L, He J, Lei JP. Anticonvulsant and Sedative Effects of Eudesmin isolated from Acorus tatarinowii on mice and rats. Phytother Res. 2015;29:996-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Gai LL. [Clinical efficacy of Changma Xifeng tablet combined with clonidine transdermal patch in the treatment of children with tic disorder]. Zhongguo Xiandai Yaowu Yingyong. 2023;17:153-156. [DOI] [Full Text] |
| 15. | Chen Q, Jia T, Wu X, Chen X, Wang J, Ba Y. Polygalae Radix Oligosaccharide Esters May Relieve Depressive-like Behavior in Rats with Chronic Unpredictable Mild Stress via Modulation of Gut Microbiota. Int J Mol Sci. 2023;24:13877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 16. | Peng YS, Liu B, Wang RF, Zhao QT, Xu W, Yang XW. Hepatic metabolism: a key component of herbal drugs research. J Asian Nat Prod Res. 2015;17:89-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Wang Z, Wan H, Tong X, He Y, Yang J, Zhang L, Shao C, Ding Z, Wan H, Li C. An integrative strategy for discovery of functional compound combination from Traditional Chinese Medicine: Danhong Injection as a model. Biomed Pharmacother. 2021;138:111451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Li W, Liu X, Qiao H. Downregulation of hippocampal SIRT6 activates AKT/CRMP2 signaling and ameliorates chronic stress-induced depression-like behavior in mice. Acta Pharmacol Sin. 2020;41:1557-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Bock KW. Roles of human UDP-glucuronosyltransferases in clearance and homeostasis of endogenous substrates, and functional implications. Biochem Pharmacol. 2015;96:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Portenoy RK, Thaler HT, Inturrisi CE, Friedlander-Klar H, Foley KM. The metabolite morphine-6-glucuronide contributes to the analgesia produced by morphine infusion in patients with pain and normal renal function. Clin Pharmacol Ther. 1992;51:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Wu L, Wang W, Zhang X, Zhao X, Yu G. Anti-HBV activity and mechanism of marine-derived polyguluronate sulfate (PGS) in vitro. Carbohydr Polym. 2016;143:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Can A, Vermilion J, Mink JW, Morrison P. Pharmacological Treatment of Tourette Disorder in Children. J Child Adolesc Psychopharmacol. 2024;34:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Gilbert DL, Dubow JS, Cunniff TM, Wanaski SP, Atkinson SD, Mahableshwarkar AR. Ecopipam for Tourette Syndrome: A Randomized Trial. Pediatrics. 2023;151:e2022059574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Liao C, Vuokila V, Catoire H, Akçimen F, Ross JP, Bourassa CV, Dion PA, Meijer IA, Rouleau GA. Transcriptome-wide association study reveals increased neuronal FLT3 expression is associated with Tourette's syndrome. Commun Biol. 2022;5:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 25. | Tsetsos F, Yu D, Sul JH, Huang AY, Illmann C, Osiecki L, Darrow SM, Hirschtritt ME, Greenberg E, Muller-Vahl KR, Stuhrmann M, Dion Y, Rouleau GA, Aschauer H, Stamenkovic M, Schlögelhofer M, Sandor P, Barr CL, Grados MA, Singer HS, Nöthen MM, Hebebrand J, Hinney A, King RA, Fernandez TV, Barta C, Tarnok Z, Nagy P, Depienne C, Worbe Y, Hartmann A, Budman CL, Rizzo R, Lyon GJ, McMahon WM, Batterson JR, Cath DC, Malaty IA, Okun MS, Berlin C, Woods DW, Lee PC, Jankovic J, Robertson MM, Gilbert DL, Brown LW, Coffey BJ, Dietrich A, Hoekstra PJ, Kuperman S, Zinner SH, Wagner M, Knowles JA, Jeremy Willsey A, Tischfield JA, Heiman GA, Cox NJ, Freimer NB, Neale BM, Davis LK, Coppola G, Mathews CA, Scharf JM, Paschou P; Tourette Association of America International Consortium for Genetics, Barr CL, Batterson JR, Berlin C, Budman CL, Cath DC, Coppola G, Cox NJ, Darrow S, Davis LK, Dion Y, Freimer NB, Grados MA, Greenberg E, Hirschtritt ME, Huang AY, Illmann C, King RA, Kurlan R, Leckman JF, Lyon GJ, Malaty IA, Mathews CA, McMahon WM, Neale BM, Okun MS, Osiecki L, Robertson MM, Rouleau GA, Sandor P, Scharf JM, Singer HS, Smit JH, Sul JH, Yu D; Gilles de la Tourette GWAS Replication Initiative, Aschauer HAH, Barta C, Budman CL, Cath DC, Depienne C, Hartmann A, Hebebrand J, Konstantinidis A, Mathews CA, Müller-Vahl K, Nagy P, Nöthen MM, Paschou P, Rizzo R, Rouleau GA, Sandor P, Scharf JM, Schlögelhofer M, Stamenkovic M, Stuhrmann M, Tsetsos F, Tarnok Z, Wolanczyk T, Worbe Y; Tourette International Collaborative Genetics Study, Brown L, Cheon KA, Coffey BJ, Dietrich A, Fernandez TV, Garcia-Delgar B, Gilbert D, Grice DE, Hagstrøm J, Hedderly T, Heiman GA, Heyman I, Hoekstra PJ, Huyser C, Kim YK, Kim YS, King RA, Koh YJ, Kook S, Kuperman S, Leventhal BL, Madruga-Garrido M, Mir P, Morer A, Münchau A, Plessen KJ, Roessner V, Shin EY, Song DH, Song J, Tischfield JA, Willsey AJ, Zinner S; Psychiatric Genomics Consortium Tourette Syndrome Working Group, Aschauer H, Barr CL, Barta C, Batterson JR, Berlin C, Brown L, Budman CL, Cath DC, Coffey BJ, Coppola G, Cox NJ, Darrow S, Davis LK, Depienne C, Dietrich A, Dion Y, Fernandez T, Freimer NB, Gilbert D, Grados MA, Greenberg E, Hartmann A, Hebebrand J, Heiman G, Hirschtritt ME, Hoekstra P, Huang AY, Illmann C, Jankovic J, King RA, Kuperman S, Lee PC, Lyon GJ, Malaty IA, Mathews CA, McMahon WM, Müller-Vahl K, Nagy P, Neale BM, Nöthen MM, Okun MS, Osiecki L, Paschou P, Rizzo R, Robertson MM, Rouleau GA, Sandor P, Scharf JM, Schlögelhofer M, Singer HS, Stamenkovic M, Stuhrmann M, Sul JH, Tarnok Z, Tischfield J, Tsetsos F, Willsey AJ, Woods D, Worbe Y, Yu D, Zinner S. Synaptic processes and immune-related pathways implicated in Tourette syndrome. Transl Psychiatry. 2021;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Gao C, Jiang J, Tan Y, Chen S. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct Target Ther. 2023;8:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 799] [Article Influence: 266.3] [Reference Citation Analysis (0)] |
| 27. | Frick L, Pittenger C. Microglial Dysregulation in OCD, Tourette Syndrome, and PANDAS. J Immunol Res. 2016;2016:8606057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Zhao H, Lv Y, Xu J, Song X, Wang Q, Zhai X, Ma X, Qiu J, Cui L, Sun Y. The activation of microglia by the complement system in neurodegenerative diseases. Ageing Res Rev. 2025;104:102636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Zhao MJ, Zhao Q, Yang CL, Zhou HY, Sun XJ, Guo XY, Huang SJY. [Modified Xiehuangsan Regulates Microglial Polarization andTLR4/MyD88/NF-κB Pathway to Treat Tic Disorders in Rats]. Zhongguo Shiyan Fangjixue Zazhi. 2025;31:10-18. [DOI] [Full Text] |
| 30. | Kutuk MO, Tufan AE, Kilicaslan F, Gokcen C, Aksu GG, Yektas C, Kandemir H, Celik F, Mutluer T, Buber A, Karadag M, Coban N, Coskun S, Hangul Z, Altintas E, Acikbas U, Giray A, Aka Y, Basturk B, Kutuk O. Cytokine expression profiles in children and adolescents with tic disorders. Sci Rep. 2024;14:15101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Yeung YT, Aziz F, Guerrero-Castilla A, Arguelles S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr Pharm Des. 2018;24:1449-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 387] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 32. | Hongyan L, Chunyan W, Yue'e Y. LY294002, a PI3K inhibitor, attenuates Tourette syndrome in rats. Metab Brain Dis. 2017;32:1619-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Tao Y, Xu P, Zhu W, Chen Z, Tao X, Liu J, Xue Z, Zhu T, Jiang P. Changes of Cytokines in Children With Tic Disorder. Front Neurol. 2021;12:800189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 34. | Zhong MX, Zhang D, Liu Y. [Research progress in the mechanism of Chinese medicine in thetreatment of tic disorders based on network pharmacology]. Zhongguo Zhongxiyi Jiehe Erkexue. 2024;16:305-308. [DOI] [Full Text] |
| 35. | Liu P, Cheng J, Ma S, Zhou J. Paeoniflorin attenuates chronic constriction injury-induced neuropathic pain by suppressing spinal NLRP3 inflammasome activation. Inflammopharmacology. 2020;28:1495-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Hu B, Xu G, Zhang X, Xu L, Zhou H, Ma Z, Shen X, Zhu J, Shen R. Paeoniflorin Attenuates Inflammatory Pain by Inhibiting Microglial Activation and Akt-NF-κB Signaling in the Central Nervous System. Cell Physiol Biochem. 2018;47:842-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Zhao F, Peng C, Li H, Chen H, Yang Y, Ai Q, Chen N, Liu F. Paeoniae Radix Rubra extract attenuates cerebral ischemia injury by inhibiting ferroptosis and activating autophagy through the PI3K/Akt signalling pathway. J Ethnopharmacol. 2023;315:116567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 38. | Xiao G, Tang R, Yang N, Chen Y. Review on pharmacological effects of gastrodin. Arch Pharm Res. 2023;46:744-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 39. | Wang H, Huang H, Jiang N, Zhang Y, Lv J, Liu X. Tenuifolin ameliorates chronic restraint stress-induced cognitive impairment in C57BL/6J mice. Phytother Res. 2022;36:1402-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/