Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.107123
Revised: June 18, 2025
Accepted: August 4, 2025
Published online: October 19, 2025
Processing time: 128 Days and 23.2 Hours
Neurodevelopmental delays encompass a wide range of conditions that impair cognitive, motor, and social functioning, often increasing the risk of psychiatric comorbidities. Children with these delays frequently present with disorders such as attention-deficit/hyperactivity disorder (ADHD), anxiety, and behavioral disturbances, which can significantly affect development and quality of life. While genetic predisposition has been linked to these comorbidities, growing evidence highlights the role of environmental factors, including prenatal and early-life stressors. However, the interaction between genetic susceptibility and environmental influences remains poorly understood. Identifying specific genetic va
To investigate the combined effects of genetic and environmental factors on psy
This retrospective cohort study included 80 children with confirmed neurodevelopmental delays and 40 age- and sex-matched typically developing controls. Comprehensive clinical and psychiatric evaluations, genetic testing (chromosomal microarray analysis and targeted next-generation sequencing), and environmental exposure assessments were conducted. Statistical analyses explored associations between genetic variants and psychiatric comorbidities, environmental risk fac
Children with neurodevelopmental delays exhibited significantly higher rates of psychiatric comorbidities (70.0%) compared to controls (15.0%), with ADHD (42.5%), anxiety disorders (28.8%), and behavioral disorders (23.8%) being the most common. Pathogenic genetic variants were identified in specific pathways associated with distinct psychiatric presentations: Glutamatergic signaling variants were linked to anxiety disorders (odds ratio = 3.8), dopaminergic system variants to ADHD (odds ratio = 4.2), and synaptic function variants to both behavioral and anxiety disorders. Environmental factors, particularly prenatal maternal stress, early childhood adversity, and family dysfunction were strong predictors of psychiatric outcomes (β = 0.42). Significant gene-environment interactions were identified, indicating that environmental exposure can moderate the effects of genetic risks on psychiatric outcomes.
Psychiatric comorbidities in children with neurodevelopmental delays are significantly influenced by both genetic and environmental factors, with complex interactions between the two. These findings underscore the need for integrated assessments and targeted interventions addressing both biological and environmental contributors to improve outcomes in this vulnerable population.
Core Tip: This study highlights the significant roles of both genetic and environmental factors in the development of psychiatric comorbidities among children with neurodevelopmental delays. Key findings include the identification of specific genetic variants associated with disorders such as attention-deficit/hyperactivity disorder and anxiety, as well as the influence of environmental stressors, including prenatal maternal stress and early childhood adversity. The complex interactions between genes and the environment underscore the need for integrated assessment approaches and targeted interventions to improve outcomes in this vulnerable population.
- Citation: Li L, Song LJ, Liu XL, Wang ZF. Roles of genetic and environmental factors in psychiatric comorbidities among children with neurodevelopmental delays. World J Psychiatry 2025; 15(10): 107123
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/107123.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.107123
Neurodevelopmental delays represent a significant public health concern, affecting approximately 15% of children globally[1]. These conditions, characterized by impairments in brain and central nervous system development, typically manifest as difficulties in motor function, language acquisition, social interaction, or cognitive development. The etiology of neurodevelopmental delays is complex and multifactorial, involving intricate interactions between genetic predispositions and environmental influences[2].
A particularly challenging aspect of neurodevelopmental delays is the high prevalence of psychiatric comorbidities observed in affected children. Research indicates that up to 70% of children with neurodevelopmental delays experience at least one psychiatric comorbidity during childhood or adolescence[3]. These often include attention-deficit/hyperacti
The genetic basis of neurodevelopmental disorders has been extensively studied, with numerous genetic variants identified in association with specific developmental delays. Copy number variations (CNVs), single nucleotide polymor
However, the genetic mechanisms underlying psychiatric comorbidities in neurodevelopmental disorders remain unclear. Emerging evidence indicates that shared genetic vulnerabilities may predispose individuals to both neurodevelopmental delays and specific psychiatric conditions. For instance, variations in dopaminergic and serotonergic pathways have been implicated in both ADHD and anxiety disorders, suggesting common neurobiological mechanisms[7]. Under
Additionally, environmental factors play a critical role in the development of psychiatric comorbidities among children with neurodevelopmental delays. Prenatal exposures to toxins, maternal stress, and nutritional deficiencies have been linked to increased risk for both neurodevelopmental delays and psychiatric disorders[8]. Postnatally, factors such as early life adversity, socioeconomic disadvantage, family dysfunction, and inadequate educational support may exacer
The timing of environmental exposures appears to be particularly significant, with evidence suggesting the existence of critical developmental windows during which the brain is especially vulnerable to environmental influences. For example, prenatal exposures during the first trimester may disrupt neural tube formation and early brain organization, while exposures in the third trimester may interfere with synaptic pruning and neural circuit refinement[10]. Similarly, early childhood represents a period of substantial neural plasticity, during which environmental factors can have a profound effect on brain development and subsequent mental health outcomes.
The interplay between genetic and environmental factors - often referred to as gene-environment interaction - further complicates our understanding of psychiatric comorbidities in neurodevelopmental disorders. Certain genetic variants may increase susceptibility to specific environmental stressors, while others may confer resilience to these stressors. For instance, children with particular genetic profiles may be more vulnerable to the adverse effects of maternal stress during pregnancy or early childhood adversity such as maltreatment[11]. In contrast, suppositive environments, such as secure attachment relationships and enriched learning opportunities, may buffer against genetic risks and foster resilience.
Despite increasing recognition of the importance of gene-environment interactions in shaping neurodevelopmental outcomes, research specifically exploring these interactions in the context of psychiatric comorbidities remains limited. The majority of studies have focused on genetic or environmental factors in isolation, with relatively few adopting integrated frameworks that consider both domains simultaneously. This represents a significant gap in the literature, as a comprehensive understanding of the complex interplay between genes and the environment is crucial for developing effective prevention and intervention strategies.
An in-depth understanding of the genetic and environmental contributors to psychiatric comorbidities in children with neurodevelopmental delays carries significant clinical relevance. Identifying specific genetic markers associated with increased risk for certain psychiatric conditions could support early, targeted screening and facilitate the implementation of preventive interventions. Similarly, identifying modifiable environmental risk factors may guide the development of public health policies and family-centered interventions aimed at reducing the prevalence of comorbidity and improving overall outcomes[12].
This study aims to bridge existing gaps in the literature by comprehensively evaluating the combined effect of genetic and environmental factors on the development of psychiatric comorbidities among children with neurodevelopmental delays. Through an integrated approach that examines both domains concurrently, this research aims to elucidate the complex interactions underlying comorbidity patterns and identify potential targets for clinical intervention.
This retrospective study was conducted at the Department of Child and Adolescent Psychiatry, University Medical Center, between January 2023 and December 2024 (Data collected at Children’s Hospital of Shanxi Province; analyzed at University Medical Center). The study protocol was approved by the Institutional Review Board, and written informed consent was obtained from all parents or legal guardians before enrollment. We recruited 80 children with confirmed neurodevelopmental delays (case group) and 40 typically developing children matched for age and sex (control group). Neurodevelopmental delay was defined as a significant impairment in at least one developmental domain-cognitive, language, motor, or social-measured using standardized assessments and confirmed by a multidisciplinary team comprising developmental pediatricians, child neurologists, and child psychologists. Children with severe sensory impairments (such as blindness or deafness), progressive neurological conditions, or a recent significant head injury were excluded. The control group was recruited from general pediatric clinics and local schools, with the absence of developmental delays confirmed through comprehensive screening.
Clinical assessment: All participants underwent a comprehensive clinical assessment conducted by a multidisciplinary team. This assessment included a detailed medical history, physical and neurological examination, and a developmental evaluation. Developmental status was assessed using the Bayley Scales of Infant and Toddler Development (Third Edition) for children under 42 months of age and the Wechsler Preschool and Primary Scale of Intelligence (Fourth Edition) for children aged 42 months and older. Adaptive functioning was evaluated using the Vineland Adaptive Behavior Scales (Third Edition). Developmental delay in specific domains was determined using standardized cutoffs, defined as scores ≥ 1.5 standard deviations below the mean. Clinical diagnoses of neurodevelopmental disorders were made according Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria by experienced clinicians who were blinded to the genetic testing results.
Psychiatric comorbidity assessment: Psychiatric comorbidities were assessed using a combination of standardized instruments and clinical interviews. The Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version was administered to parents of all participants by trained clinicians to identify psychiatric conditions. For children under 6 years of age, the Preschool Age Psychiatric Assessment was additionally used. The Child Behavior Checklist was completed by parents to provide a dimensional assessment of emotional and behavioral problems. Teachers completed the Teacher Report Form to provide cross-informant data on behavioral symptoms in the educational setting. All assessors were blinded to participants’ genetic testing results and environmental exposure status to minimize assessment bias.
Genetic testing: Blood samples were collected from all participants for genetic analyses. Chromosomal microarray analysis was performed using the Affymetrix CytoScan HD platform to detect CNVs. Additionally, targeted next-generation sequencing was conducted using a custom-designed panel comprising 184 genes previously associated with neurodevelopmental disorders and psychiatric conditions. The panel included genes involved in synaptogenesis, neuronal migration, and neurotransmitter systems. Identified variants were classified according to the American College of Medical Genetics and Genomics guidelines as pathogenic, likely pathogenic, variants of uncertain significance, likely benign, or benign. Genetic counseling was provided to all families who received genetic testing results. Laboratory personnel conducting the genetic analyses were blinded to participants’ clinical status and assessment outcomes.
Environmental exposure assessment: Environmental exposures were comprehensively evaluated through structured parent interviews, medical record reviews, and standardized questionnaires. Prenatal exposures assessed included maternal medication use, substance exposure (alcohol, tobacco, or illicit drugs), infections, maternal medical conditions, pregnancy complications, and maternal stress (using the Prenatal Life Events Scale). Perinatal factors documented included gestational age, birth weight, delivery complications, Apgar scores, and neonatal medical events. Postnatal environmental exposures evaluated included early life adversity (using the Childhood Trauma Questionnaire), family functioning (using the Family Assessment Device), socioeconomic status (using the Hollingshead Four-Factor Index), parental mental health (using the Brief Symptom Inventory), and home environment quality (using the Home Obser
All statistical analyses were conducted using SPSS version 28.0, with the Haldane–Anscombe correction applied to account for the 80:40 group-size imbalance. Descriptive statistics were calculated for demographic characteristics, clinical variables, genetic findings, and environmental exposures. Between-group comparisons were performed using independent samples t-tests for continuous variables and χ2 tests for categorical variables. Associations between specific genetic variants and psychiatric comorbidities were analyzed using logistic regression models, adjusting for age, sex, and severity of developmental delay. Environmental risk factors were assessed using hierarchical linear regression, with cumulative environmental adversity scores calculated by summing weighted exposure severity scores across domains.
To examine gene-environment interactions, moderation analyses were conducted using the PROCESS macro for SPSS. Specifically, we tested whether environmental factors moderated the relationship between genetic risk scores and psychiatric comorbidity outcomes. Genetic risk scores were derived from the number of identified pathogenic or likely pathogenic variants in genes associated with specific neurodevelopmental domains. Statistical significance was set at P < 0.05, and Bonferroni correction was applied to account for multiple comparisons. Missing data (< 5% across all variables) were handled using multiple imputation techniques.
The demographic and clinical characteristics of the study participants are presented in Table 1. The mean age of children in the neurodevelopmental delay group was 5.7 ± 2.2 years, with 62.5% being male. The control group was appropriately matched, with a mean age of 5.8 ± 2.1 years and 60.0% male participants. No significant differences were observed between groups regarding race/ethnicity or parental education. As expected, children with neurodevelopmental delays demonstrated significantly lower scores across developmental domains compared to controls.
| Characteristic | Neurodevelopmental delay group (n = 80) | Control group (n = 40) | P value |
| Age (years), mean ± SD | 5.7 ± 2.2 | 5.8 ± 2.1 | 0.814 |
| Sex | 0.783 | ||
| Male | 50 (62.5) | 24 (60.0) | |
| Female | 30 (37.5) | 16 (40.0) | |
| Race/ethnicity | 0.896 | ||
| White | 42 (52.5) | 22 (55.0) | |
| Black | 15 (18.8) | 7 (17.5) | |
| Hispanic | 14 (17.5) | 6 (15.0) | |
| Asian | 6 (7.5) | 4 (10.0) | |
| Other | 3 (3.8) | 1 (2.5) | |
| Parental education | 0.682 | ||
| High school or less | 18 (22.5) | 7 (17.5) | |
| Some college | 28 (35.0) | 13 (32.5) | |
| College graduate | 24 (30.0) | 14 (35.0) | |
| Postgraduate | 10 (12.5) | 6 (15.0) | |
| Developmental domains, mean ± SD | |||
| Cognitive (standard score) | 76.3 ± 14.6 | 104.8 ± 12.4 | < 0.001 |
| Language (standard score) | 72.7 ± 16.2 | 102.3 ± 10.9 | < 0.001 |
| Motor (standard score) | 81.5 ± 15.8 | 103.4 ± 11.6 | < 0.001 |
| Social (standard score) | 74.9 ± 17.3 | 101.5 ± 11.7 | < 0.001 |
| Adaptive (standard score) | 75.2 ± 13.9 | 103.7 ± 12.1 | < 0.001 |
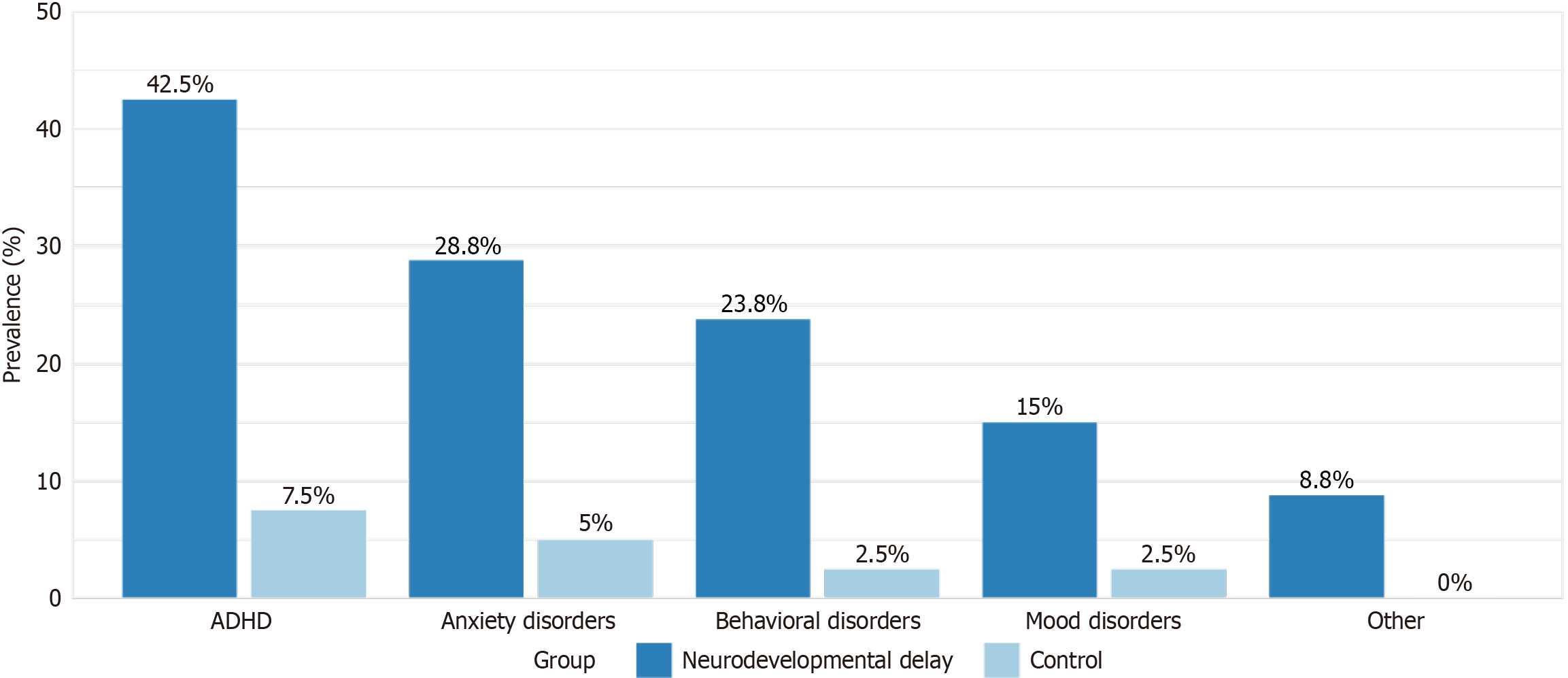
Within the neurodevelopmental delay group, 56 children (70.0%) had at least one psychiatric comorbidity, compared to six children (15.0%) in the control group (P < 0.001). The most common comorbidities in the neurodevelopmental delay group were ADHD (42.5%), anxiety disorders (28.8%), behavioral disorders (23.8%), and mood disorders (15.0%). Figure 1 illustrates the prevalence of psychiatric comorbidities in both groups.
Chromosomal microarray analysis identified pathogenic or likely pathogenic CNVs in 23 children (28.8%) in the neurodevelopmental delay group, compared to one child (2.5%) in the control group (P < 0.001). The most common CNVs included 16p11.2 deletions (n = 4), 22q11.2 deletions (n = 3), and 15q11.2-13.1 duplications (n = 3). Targeted sequencing revealed pathogenic or likely pathogenic sequence variants in 35 children (43.8%) in the neurodevelopmental delay group and three children (7.5%) in the control group (P < 0.001).
We identified significant associations between specific genetic variants and distinct patterns of psychiatric comorbidity (Table 2). Children with pathogenic variants in genes involved in glutamatergic signaling (GRIN2A, GRIN2B, SHANK3) showed higher rates of anxiety disorders (odds ratio = 3.8, 95% confidence interval: 1.7-8.5, P = 0.001). Similarly, variants in dopaminergic system genes (DRD4, DAT1, COMT) were significantly associated with ADHD comorbidity (odds ratio = 4.2, 95% confidence interval: 1.9-9.3, P < 0.001). Children with pathogenic variants in genes related to synaptic function
| Genetic pathway | Genes | Associated psychiatric comorbidity | OR (95%CI) | P value |
| Glutamatergic signaling | GRIN2A, GRIN2B, SHANK3 | Anxiety disorders | 3.8 (1.7-8.5) | 0.001 |
| Dopaminergic system | DRD4, DAT1, COMT | ADHD | 4.2 (1.9-9.3) | < 0.001 |
| Serotonergic system | SLC6A4, TPH2, HTR1A | Mood disorders | 3.1 (1.4-6.9) | 0.006 |
| Synaptic function | NRXN1, NLGN3, NLGN4 | Behavioral disorders | 3.5 (1.5-8.1) | 0.003 |
| Synaptic function | NRXN1, NLGN3, NLGN4 | Anxiety disorders | 2.9 (1.3-6.4) | 0.008 |
| Chromatin remodeling | CHD8, ARID1B | Behavioral disorders | 2.7 (1.2-6.1) | 0.018 |
| Calcium signaling | CACNA1C, CACNA1H | Mood disorders | 3.3 (1.3-8.2) | 0.012 |
Analysis of environmental exposures revealed significant differences between the neurodevelopmental delay and control groups. Children with neurodevelopmental delays had higher rates of prenatal exposures, including maternal medication use (38.8% vs 15.0%, P = 0.006), maternal stress (45.0% vs 22.5%, P = 0.011), and pregnancy complications (43.8% vs 17.5%, P = 0.003). Postnatally, they experienced higher rates of early childhood adversity (32.5% vs 12.5%, P = 0.015) and were more likely to live in families with lower socioeconomic status and elevated rates of parental mental health problems.
Hierarchical regression analysis demonstrated that cumulative environmental adversity significantly predicted the number of psychiatric comorbidities in children with neurodevelopmental delays (β = 0.42, P < 0.001), even after controlling for age, sex, and severity of developmental delay. The environmental factors most strongly associated with psychiatric comorbidities included prenatal maternal stress (β = 0.31, P = 0.004), early childhood adversity (β = 0.35, P = 0.001), and family dysfunction (β = 0.28, P = 0.009).
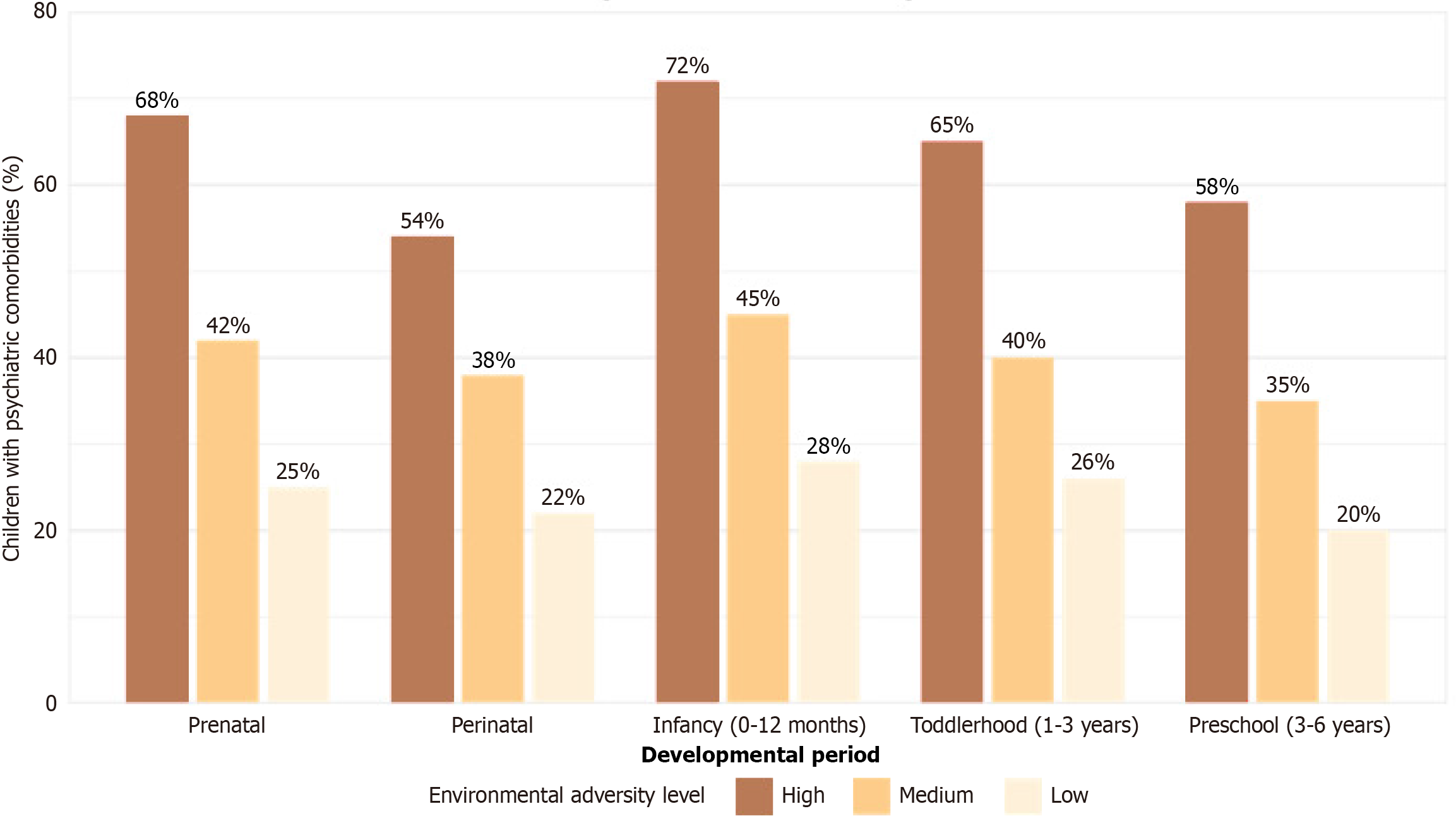
Additionally, the timing of environmental exposures appeared to be critical, with prenatal exposures more strongly associated with neurodevelopmental severity, while early childhood exposures were more predictive of psychiatric comorbidities. Figure 2 illustrates the relationship between the timing of environmental adversity exposure and rates of psychiatric comorbidity.
Our analysis revealed significant gene-environment interactions in the development of psychiatric comorbidities. The effects of genetic variants on psychiatric outcomes were moderated by environmental factors in several domains. For example, the relationship between dopaminergic system gene variants and ADHD was significantly stronger in children exposed to high levels of early life adversity (interaction term: β = 0.32, P = 0.003). Similarly, the association between serotonergic system variants and mood disorders was amplified in the context of family dysfunction (interaction term: β = 0.28, P = 0.011).
In contrast, positive environmental factors appeared to mitigate the impact of genetic risk in some cases. Children carrying risk-related genetic variants but raised in high-quality home environments with supportive parenting practices exhibited lower rates of psychiatric comorbidities compared to those with similar genetic profiles but less favorable environmental conditions.
This retrospective cohort study provides comprehensive evidence for the complex interplay between genetic and environmental factors in the development of psychiatric comorbidities among children with neurodevelopmental delays. Our findings demonstrate that both domains make significant contributions to comorbidity patterns, with relevant impli
The high prevalence of psychiatric comorbidities observed in our neurodevelopmental delay cohort (70.0%) is consistent with previous literature highlighting the substantial comorbidity burden in this population[13]. The predominance of ADHD, anxiety disorders, and behavioral disturbances mirrors patterns reported in earlier studies[14]. However, our integrative approach, examining both genetic and environmental contributors, offers novel insights into the mechanisms underlying these comorbidity patterns.
Our genetic findings revealed significant associations between specific gene pathways and particular psychiatric comorbidities. The relationship between glutamatergic signaling gene variants and anxiety disorders supports emerging evidence implicating glutamate dysregulation in anxiety pathophysiology[15]. Similarly, the association between dopaminergic system variants and ADHD comorbidity is consistent with the established role of dopamine in attention regulation and executive function[16]. These genotype-phenotype correlations highlight shared neurobiological mecha
The identification of pathogenic variants in synaptic function genes associated with both behavioral and anxiety disorders underscores the fundamental role of synaptic development and plasticity in a range of neurodevelopmental and psychiatric phenotypes. Disruptions in synaptogenesis and synaptic pruning may represent a common pathogenic mechanism contributing to the high comorbidity rates observed in neurodevelopmental disorders[17]. This finding emphasizes the value of considering shared neurobiological pathways when conceptualizing comorbidity patterns and designing targeted interventions.
Our environmental exposure analyses demonstrated substantial differences between the neurodevelopmental delay and control groups, with significantly higher rates of both prenatal and postnatal adversity among the former. The strong association between cumulative environmental adversity and psychiatric comorbidity burden underscores the critical influence of environmental factors on mental health outcomes in vulnerable neurodevelopmental populations. These findings align with the growing body of evidence supporting the Developmental Origins of Health and Disease frame
The differential impact of exposure timing on developmental outcomes warrants particular attention. Our observation that prenatal exposures more strongly predicted neurodevelopmental severity, while early childhood adversity was more predictive of psychiatric comorbidities, suggests distinct sensitive periods for different developmental domains. This temporal specificity has essential implications for the timing of preventive interventions and highlights the potential value of targeted support during critical developmental windows[19].
Perhaps most notably, our study demonstrated significant gene-environment interactions in the development of comorbidity, with environmental factors moderating the impact of genetic variants on psychiatric outcomes. The amplified effect of dopaminergic system variants on ADHD symptoms in the context of early life adversity exemplifies how environmental stressors may unmask genetic vulnerabilities or exacerbate their phenotypic expression. These findings support the differential susceptibility model, which suggests that certain genetic variants may confer not only vulnerability to adverse environments but also increased responsiveness to supportive conditions[20].
The protective effect of positive environmental factors-such as high-quality home environments and supportive parenting–against genetic risk offers an encouraging perspective on intervention. Such environmental buffers may foster resilience by mitigating the impact of genetic vulnerabilities, suggesting that family-centered interventions could significantly improve outcomes even among children with substantial genetic risk[21]. This underscores the importance of comprehensive support programs that address family functioning, parenting practices, and the quality of the home environment in clinical management strategies.
The clinical implications of our findings are substantial. The significant contribution of both genetic and environmental factors to comorbidity patterns supports the implementation of integrated assessment approaches in neurodevelopmental clinics. Comprehensive evaluations should include genetic testing, alongside a detailed environmental exposure history and family assessment, to identify both risk and protective factors[22]. This integrated perspective can inform indivi
The specific genotype-phenotype correlations and gene-environment interactions identified in our study may guide more targeted screening and preventive strategies. For example, children with identified genetic variants in glutamatergic or serotonergic pathways may benefit from improved monitoring for anxiety and mood symptoms, particularly when exposed to environmental adversity. Similarly, early intervention programs may be especially beneficial for children with genetic vulnerabilities who are also exposed to high-risk environments[23].
From a public health perspective, our findings underscore the substantial potential of environmental modification in improving outcomes for neurodevelopmentally vulnerable populations. Prenatal care programs that address maternal stress, substance exposure, and nutrition, alongside early childhood interventions that promote positive parenting and reduce adversity, could substantially decrease psychiatric comorbidity burden[24]. The economic and societal benefits of such preventive approaches are likely to be considerable, given the high costs associated with neurodevelopmental disorders and psychiatric comorbidities.
The research implications of our findings include the need for longitudinal studies to examine how gene-environment interactions evolve across development, potentially identifying additional sensitive periods and opportunities for intervention. Moreover, future investigations should explore the neurobiological mechanisms underlying these in
However, some limitations should be considered when interpreting our findings. The retrospective design introduces potential recall bias in environmental exposure assessment, as well as reliance on parent-reported stress assessments; however, we attempted to mitigate this through medical record review and the use of standardized instruments. The modest sample size limited the statistical power to detect smaller effect sizes, particularly in gene-environment in
This study provides compelling evidence for the significant contributions of both genetic and environmental factors to psychiatric comorbidities in children with neurodevelopmental delays. Our findings highlight the intricate interplay between these domains, with specific genetic variants linked to particular comorbidity patterns and environmental factors that both directly influence outcomes and moderate genetic effects. The identification of significant gene-environment interactions supports both the diathesis-stress and differential susceptibility models, offering valuable insights for clinical practice and the development of interventions. These results underscore the importance of integrated assessment approaches that address multiple domains simultaneously, advocating for the implementation of targeted screening protocols and personalized intervention strategies that consider both biological and environmental contri
The authors would like to express their sincere gratitude to all the children and families who participated in this study.
| 1. | Sonuga-Barke EJS, Becker SP, Bölte S, Castellanos FX, Franke B, Newcorn JH, Nigg JT, Rohde LA, Simonoff E. Annual Research Review: Perspectives on progress in ADHD science - from characterization to cause. J Child Psychol Psychiatry. 2023;64:506-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 2. | Doi M, Usui N, Shimada S. Prenatal Environment and Neurodevelopmental Disorders. Front Endocrinol (Lausanne). 2022;13:860110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 3. | Micai M, Fatta LM, Gila L, Caruso A, Salvitti T, Fulceri F, Ciaramella A, D'Amico R, Del Giovane C, Bertelli M, Romano G, Schünemann HJ, Scattoni ML. Prevalence of co-occurring conditions in children and adults with autism spectrum disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2023;155:105436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Mutluer T, Aslan Genç H, Özcan Morey A, Yapici Eser H, Ertinmaz B, Can M, Munir K. Population-Based Psychiatric Comorbidity in Children and Adolescents With Autism Spectrum Disorder: A Meta-Analysis. Front Psychiatry. 2022;13:856208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Zhang Y, Liu X, Guo R, Xu W, Guo Q, Hao C, Ni X, Li W. Biological implications of genetic variations in autism spectrum disorders from genomics studies. Biosci Rep. 2021;41:BSR20210593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Derks EM, Thorp JG, Gerring ZF. Ten challenges for clinical translation in psychiatric genetics. Nat Genet. 2022;54:1457-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 7. | Woodward DJ, Thorp JG, Middeldorp CM, Akóṣílè W, Derks EM, Gerring ZF. Leveraging pleiotropy for the improved treatment of psychiatric disorders. Mol Psychiatry. 2025;30:705-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Cordeiro CN, Tsimis M, Burd I. Infections and Brain Development. Obstet Gynecol Surv. 2015;70:644-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 887] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 10. | Di Gesù CM, Matz LM, Buffington SA. Diet-induced dysbiosis of the maternal gut microbiome in early life programming of neurodevelopmental disorders. Neurosci Res. 2021;168:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | van Ijzendoorn MH, Bakermans-Kranenburg MJ, Ebstein RP. Methylation Matters in Child Development: Toward Developmental Behavioral Epigenetics. Child Dev Perspect. 2011;5:305-310. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Insel TR. Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry. 2009;66:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 357] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Totsika V, Liew A, Absoud M, Adnams C, Emerson E. Mental health problems in children with intellectual disability. Lancet Child Adolesc Health. 2022;6:432-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 14. | Chan N, Fenning RM, Neece CL. Prevalence and Phenomenology of Anxiety in Preschool-Aged Children with Autism Spectrum Disorder. Res Child Adolesc Psychopathol. 2023;51:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Glessner JT, Khan ME, Chang X, Liu Y, Otieno FG, Lemma M, Slaby I, Hain H, Mentch F, Li J, Kao C, Sleiman PMA, March ME, Connolly J, Hakonarson H. Rare recurrent copy number variations in metabotropic glutamate receptor interacting genes in children with neurodevelopmental disorders. J Neurodev Disord. 2023;15:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Rao S, Baranova A, Yao Y, Wang J, Zhang F. Genetic Relationships between Attention-Deficit/Hyperactivity Disorder, Autism Spectrum Disorder, and Intelligence. Neuropsychobiology. 2022;81:484-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, Singh T, Klei L, Kosmicki J, Shih-Chen F, Aleksic B, Biscaldi M, Bolton PF, Brownfeld JM, Cai J, Campbell NG, Carracedo A, Chahrour MH, Chiocchetti AG, Coon H, Crawford EL, Curran SR, Dawson G, Duketis E, Fernandez BA, Gallagher L, Geller E, Guter SJ, Hill RS, Ionita-Laza J, Jimenz Gonzalez P, Kilpinen H, Klauck SM, Kolevzon A, Lee I, Lei I, Lei J, Lehtimäki T, Lin CF, Ma'ayan A, Marshall CR, McInnes AL, Neale B, Owen MJ, Ozaki N, Parellada M, Parr JR, Purcell S, Puura K, Rajagopalan D, Rehnström K, Reichenberg A, Sabo A, Sachse M, Sanders SJ, Schafer C, Schulte-Rüther M, Skuse D, Stevens C, Szatmari P, Tammimies K, Valladares O, Voran A, Li-San W, Weiss LA, Willsey AJ, Yu TW, Yuen RK; DDD Study; Homozygosity Mapping Collaborative for Autism; UK10K Consortium, Cook EH, Freitag CM, Gill M, Hultman CM, Lehner T, Palotie A, Schellenberg GD, Sklar P, State MW, Sutcliffe JS, Walsh CA, Scherer SW, Zwick ME, Barett JC, Cutler DJ, Roeder K, Devlin B, Daly MJ, Buxbaum JD. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1776] [Cited by in RCA: 2073] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 18. | Alves JGB, Alves LV. Early-life nutrition and adult-life outcomes. J Pediatr (Rio J). 2024;100 Suppl 1:S4-S9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Migliore L, Coppedè F. Gene-environment interactions in Alzheimer disease: the emerging role of epigenetics. Nat Rev Neurol. 2022;18:643-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 142] [Reference Citation Analysis (0)] |
| 20. | Gobeil-Bourdeau J, Lemelin J, Letarte M, Laurent A. Interactions between child temperament and family environment in relation to school readiness: Diathesis-stress, differential susceptibility, or vantage sensitivity? Early Child Res Q. 2022;60:274-286. [DOI] [Full Text] |
| 21. | Sonuga-Barke EJS, Kennedy M, Kumsta R, Knights N, Golm D, Rutter M, Maughan B, Schlotz W, Kreppner J. Child-to-adult neurodevelopmental and mental health trajectories after early life deprivation: the young adult follow-up of the longitudinal English and Romanian Adoptees study. Lancet. 2017;389:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 22. | Cattane N, Vernon AC, Borsini A, Scassellati C, Endres D, Capuron L, Tamouza R, Benros ME, Leza JC, Pariante CM, Riva MA, Cattaneo A; European College of Neuropsychopharmacology (ECNP) ImmunoNeuroPsychiatry Thematic Working Group. Preclinical animal models of mental illnesses to translate findings from the bench to the bedside: Molecular brain mechanisms and peripheral biomarkers associated to early life stress or immune challenges. Eur Neuropsychopharmacol. 2022;58:55-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Law ML, Singh J, Mastroianni M, Santosh P. Parent-Mediated Interventions for Infants under 24 Months at Risk for Autism Spectrum Disorder: A Systematic Review of Randomized Controlled Trials. J Autism Dev Disord. 2022;52:2553-2574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329-47 3p following 347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 422] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 25. | Conradt E, Crowell SE, Cicchetti D. Using Development and Psychopathology Principles to Inform the Research Domain Criteria (RDoC) Framework. Dev Psychopathol. 2021;33:1521-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/