Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.107147
Revised: June 24, 2025
Accepted: August 19, 2025
Published online: October 19, 2025
Processing time: 125 Days and 23.9 Hours
Endometriosis affects approximately 10% of reproductive-age women and is frequently associated with chronic pelvic pain. Patients with endometriosis often experience comorbid depression and anxiety, but the underlying mechanisms connecting these conditions are unclear.
To assess the prevalence of depression and anxiety in endometriosis patients and explore neuroimmune mechanisms mediated via inflammatory biomarkers.
A retrospective cohort study was conducted with 200 patients with endometriosis-associated chronic pain from June 2020 to December 2024. Depression and anxiety were assessed using validated psychological instruments. Inflammation biomarkers interleukin (ILs) (IL-6, IL-1β), tumor necrosis factor-alpha, C-reactive protein, and brain-derived neurotrophic factor were measured in serum. Pain severity was assessed using visual analog scales. Correlation and regression analyses were performed to examine relationships between inflammatory markers, pain severity, and psychological outcomes.
Among the 200 patients, 42.5% exhibited clinically significant depression and 51.0% showed anxiety symptoms. Serum levels of IL-6, IL-1β, tumor necrosis factor-alpha, and C-reactive protein were significantly higher in patients with comorbid depression and anxiety compared with those without psychological symptoms (P < 0.001). Brain-derived neurotrophic factor levels were lower in the depression group. Pain severity positively correlated with inflammatory marker levels and with depression and anxiety scores.
Overall, the findings suggest that inflammatory factors mediate a neuroimmune mechanism linking endometriosis-associated chronic pain with depression and anxiety. Therapeutic targets for managing psychological comorbidities in patients with endometriosis through anti-inflammatory interventions should be explored, and an integrated treatment approach addressing both physical and psychological symptoms is emphasized.
Core Tip: Endometriosis-associated chronic pain is frequently accompanied by depression and anxiety, but the underlying mechanisms remain unclear. This study highlights the role of inflammatory biomarkers, including interleukin-6, interleukin-1β, tumor necrosis factor-alpha, C-reactive protein, and brain-derived neurotrophic factor, in mediating these psychological comorbidities. Higher inflammatory marker levels correlate with increased pain severity and psychological distress. These findings suggest a neuroimmune link between endometriosis and mental health conditions, providing potential therapeutic targets. Addressing inflammation may improve both physical and psychological symptoms, emphasizing the need for an integrated treatment approach in managing endometriosis patients.
- Citation: Yang FC, Zhou Y, Zhang SY, Ma R. Depression and anxiety in patients with endometriosis-associated chronic pain: Neuroimmune mechanisms mediated by inflammatory factors. World J Psychiatry 2025; 15(10): 107147
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/107147.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.107147
Endometriosis is a chronic gynecological disorder characterized by the presence of endometrial-like tissue outside the uterus, affecting approximately 10% of women of reproductive age worldwide[1]. The condition is frequently associated with debilitating chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility, significantly impairing quality of life[2]. Beyond the physical symptoms, patients with endometriosis experience a substantial psychological burden, with higher rates of depression and anxiety compared to the general female population and to women with other gynecological conditions[3].
The bidirectional relationship between chronic pain and psychological disorders is well-established in various clinical conditions. However, in endometriosis, this relationship appears particularly complex, as both the physical symptoms and the psychological impact profoundly affect patients’ wellbeing[4]. Studies have reported prevalence rates of depression ranging from 15% to 50% and anxiety from 24% to 65% among women with endometriosis, depending on the assessment methods and population characteristics[5]. Despite this high comorbidity, the underlying mechanisms connecting endometriosis-associated pain with depression and anxiety remain incompletely understood, presenting a significant barrier to developing effective integrated treatments.
Recent advances in psychoneuroimmunology have highlighted the role of inflammatory processes in the pathophysiology of both chronic pain and mood disorders. Endometriosis is characterized by a state of chronic local and systemic inflammation, with elevated levels of proinflammatory cytokines including interleukin (IL)-6, IL-1β, and tumor necrosis factor-alpha (TNF-α)[6]. These inflammatory mediators not only contribute to pain sensitization but may also influence central nervous system function, potentially precipitating or exacerbating depressive and anxious symptoms.
The inflammatory hypothesis of depression posits that proinflammatory cytokines enter the brain and interact with nearly every pathophysiological domain relevant to depression, including neurotransmitter metabolism, neuroendocrine function, and neural plasticity[7]. Similarly, anxiety disorders have been linked to dysregulated inflammatory immune responses. When applied to endometriosis, this hypothesis suggests that the chronic inflammatory state associated with the disease might serve as a biological bridge connecting physical symptoms with psychological manifestations, potentially explaining the high comorbidity observed in clinical practice.
Brain-derived neurotrophic factor (BDNF), a key regulator of neuronal plasticity, has emerged as an important mediator in both pain processing and mood regulation. Altered BDNF levels have been reported in both depression and chronic pain conditions, suggesting its potential role in the comorbidity of these disorders[8]. Specifically in endometriosis, preliminary evidence indicates dysregulated BDNF expression, which may contribute to both pain sensitization and vulnerability to psychological distress.
Another potential mechanism linking endometriosis, pain, and psychological symptoms is the hypothalamic-pituitary-adrenal (HPA) axis, a central component of the stress response system. Chronic pain can lead to HPA axis dysregulation, characterized by abnormal cortisol patterns and altered stress responsivity, which are also hallmarks of depression and anxiety[9,10]. Furthermore, inflammatory cytokines can directly influence HPA axis function, creating a complex interplay between inflammation, stress response, pain perception, and mood regulation.
Despite these advances in understanding potential mechanisms, comprehensive studies investigating the interrelationships between inflammatory markers, pain severity, and psychological symptoms in patients with endometriosis are scarce. Moreover, most existing research has focused on individual aspects of this complex relationship rather than adopting an integrated approach that considers multiple biological and psychological variables simultaneously. This fragmented approach has hampered the development of comprehensive models explaining the neuroimmune mechanisms underlying the comorbidity of endometriosis-associated pain and psychological disorders.
Additionally, the clinical implications of this neuroimmune interplay remain largely unexplored. If inflammatory factors mediate the relationship between chronic pain and psychological symptoms in endometriosis, this would suggest that anti-inflammatory interventions might simultaneously address both physical and psychological manifestations of the disease. Furthermore, identifying specific inflammatory profiles associated with psychological comorbidities in endometriosis could potentially facilitate personalized treatment approaches targeting the most relevant pathological mechanisms in individual patients.
The present study aims to address these knowledge gaps by investigating the prevalence and severity of depression and anxiety in patients with endometriosis-associated chronic pain and exploring the potential neuroimmune mechanisms mediating this relationship.
This retrospective cohort study was conducted from June 1, 2020 to December 31, 2024 at Tongji University Affiliated Oriental Hospital and the Obstetrics and Gynecology Center of Shanghai Tenth People’s Hospital. The research plan has been approved by the Institutional Ethics Committee of Shanghai Tenth People’s Hospital, approval No. SHSY-IEC-6.0/25K132/P01 and conducted in accordance with the Helsinki Declaration. And signed a written informed consent form.
Participant recruitment and selection: Participants were recruited in a gynecology outpatient clinic setting from patients with confirmed diagnoses of endometriosis and chronic pelvic pain. Diagnoses of endometriosis were established through laparoscopic visualization and histological confirmation as per the European Society of Human Reproduction and Embryology guidelines. Chronic pelvic pain was defined as non-menstrual pelvic pain (at least 6 months of duration) severe enough to cause functional disability or require medical or surgical treatment.
The inclusion criteria were: (1) Women aged 18-45 years; (2) Surgically confirmed endometriosis; (3) Presence of chronic pelvic pain; (4) Ability to understand and complete the psychological assessment tools; and (5) Willingness to provide blood samples for biomarker analysis. The exclusion criteria comprised: (1) Pregnancy or breastfeeding; (2) Other chronic pain conditions unrelated to endometriosis; (3) Autoimmune or inflammatory diseases; (4) Active infection or malignancy; (5) Psychiatric disorders diagnosed before the onset of endometriosis symptoms; (6) Current use of antidepressants, anxiolytics, or other psychotropic medications; (7) Use of anti-inflammatory drugs within 2 weeks before blood sampling; and (8) Receiving hormone therapy within 3 months before assessment.
Sample size was determined based on previous similar studies and power calculations, considering a moderate effect size for the association between inflammatory markers and psychological outcomes. A minimum sample size of 194 participants was required to achieve 80% power at a significance level of 0.05. We recruited 200 participants to account for potential dropouts or incomplete data.
Demographic and clinical data collection: Demographic and clinical information was collected using comprehensive structured interviews and medical record review. Data gathered included age, education level, marital status, employment status, body mass index, disease duration, stage of endometriosis according to the revised American Society for Reproductive Medicine classification, previous treatments, and comorbidities.
Pain assessment: Pain severity was evaluated using a 10-cm visual analog scale, with 0 representing “no pain” and 10 representing “worst possible pain”. Participants rated their average pain intensity over the previous 4 weeks. Additionally, the Brief Pain Inventory was used to assess pain interference with daily activities. The McGill Pain Questionnaire was used to evaluate qualitative aspects of pain, including sensory, affective, and evaluative dimensions. Pain catastrophizing was assessed using the Pain Catastrophizing Scale, which measures rumination, magnification, and helplessness related to pain experiences.
Psychological assessment: Depression was assessed using the 21-item Beck Depression Inventory-II (BDI-II), a widely validated instrument for measuring depressive symptoms. Scores range from 0 to 63, with higher scores indicating more severe depression. The following cut-off values were used: 0-13 (minimal depression), 14-19 (mild depression), 20-28 (moderate depression), and 29-63 (severe depression).
Anxiety was measured using the State-Trait Anxiety Inventory (STAI), which consists of two 20-item scales assessing state anxiety (current) and trait anxiety (general tendency). Each item is rated on a 4-point scale, with total scores ranging from 20 to 80 for each scale. Higher scores indicate greater anxiety levels. A cut-off score of 40 or above was used to identify clinically significant anxiety.
Sleep quality was evaluated using the Pittsburgh Sleep Quality Index, a 19-item self-report questionnaire assessing sleep quality and disturbances over the previous month. The Pittsburgh Sleep Quality Index generates seven component scores and a global score ranging from 0 to 21, with higher scores indicating poorer sleep quality.
Quality of life was assessed using the Endometriosis Health Profile-30, a disease-specific instrument measuring the impact of endometriosis on various aspects of life, including pain, control and powerlessness, emotional well-being, social support, and self-image.
Assessment timing and procedures: All clinical and psychological assessments were performed during the follicular phase of the menstrual cycle (days 3-7) to minimize the influence of cyclic hormonal fluctuations on symptoms and biomarkers. Participants completed the questionnaires in a quiet, private environment after receiving standardized instructions from a research assistant. The assessments took approximately 60-90 minutes to complete.
Blood sample collection and processing: Blood samples were collected from all participants after an overnight fasting period of at least 8 hours. Venipuncture was performed between 08:00 am and 10:00 am to minimize diurnal variations in inflammatory markers and hormones. Approximately 20 mL of blood was collected from each participant using standard venipuncture techniques and collected in appropriate tubes (serum separator tubes for inflammatory markers and BDNF; ethylenediaminetetraacetic acid tubes for genetic analyses).
Samples were allowed to coagulate at room temperature for 30 minutes, then centrifuged at 3000 r/minute for 15 minutes at 4 °C. The resulting serum was aliquoted into cryovials and stored at -80 °C until batch analysis to minimize inter-assay variability. All samples were analyzed within 6 months of collection.
Inflammatory marker analysis: Serum levels of inflammatory markers were measured using ELISA kits following the manufacturers’ protocols. IL-6 Levels were quantified using a high-sensitivity ELISA kit (R&D Systems, Minneapolis, MN, United States) with a detection range of 0.2-10 pg/mL and intra- and inter-assay coefficients of variation (CVs) of < 7% and < 10%, respectively. IL-1β levels were measured using a high-sensitivity ELISA kit (R&D Systems) with a detection range of 0.125-8 pg/mL and intra- and inter-assay CVs of < 8% and < 11%, respectively. TNF-α levels were quantified using a high-sensitivity ELISA kit (R&D Systems) with a detection range of 0.5-32 pg/mL and intra- and inter-assay CVs of < 8% and < 10%, respectively. C-reactive protein (CRP) levels were measured using a high-sensitivity immunoturbidimetric assay (Roche Diagnostics, Basel, Switzerland) with a detection limit of 0.15 mg/L and intra- and inter-assay CVs of < 2% and < 3%, respectively. BDNF levels were quantified using an ELISA kit (Promega, Madison, WI, United States) with a detection range of 7.8-500 pg/mL and intra and inter-assay CVs of < 6.5% and < 8.5%, respectively. All assays were performed in duplicate, and the average value was used for analysis. Standard curves were generated for each plate, and samples with values outside the detection range were retested after appropriate dilution.
Hormonal analysis: Serum levels of estradiol, progesterone, and cortisol were measured to account for potential hormonal influences on inflammatory markers and psychological symptoms. Estradiol and progesterone were measured using chemiluminescent immunoassays (ADVIA Centaur XP, Siemens Healthineers, Forchheim, Germany). Cortisol was measured using a solid-phase competitive chemiluminescent enzyme immunoassay (IMMULITE 2000, Siemens Healthineers).
Statistical analyses were performed using SPSS version 27.0 (IBM, Armonk, NY, United States). Descriptive statistics were calculated for all variables. Continuous data were expressed as means and SDs or medians and interquartile ranges, depending on the distribution. Categorical data were expressed as frequencies and percentages. Participants were classified based on the presence of depression (BDI-II score ≥ 14) and anxiety (STAI-state or STAI-trait score ≥ 40). Comparisons between groups were performed using independent samples t-tests or Mann-Whitney U tests for continuous variables and χ2 or Fisher’s exact tests for categorical variables, as appropriate. Bivariate correlations between inflammatory markers, BDNF, pain measures, and psychological symptoms were examined using Pearson’s or Spearman’s correlation coefficients, depending on the distribution. Multiple linear regression analyses were performed to identify predictors of depression and anxiety symptoms, with BDI-II and STAI scores as dependent variables. Independent variables included demographic factors, pain measures, inflammatory markers, BDNF, and hormone levels. Stepwise selection procedures were used to identify the most parsimonious models. Assumptions of linearity, independence, homoscedasticity, and normality of residuals were verified for all regression models.
A total of 200 patients with endometriosis-associated chronic pain participated in the study. The mean age of participants was 32.7 ± 6.4 years (range: 18-45 years). Table 1 presents the demographic and clinical characteristics of the study population overall and stratified by the presence of depression and anxiety. Out of all participants, 85 (42.5%) had clinically significant depression (BDI-II score ≥ 14) and 102 (51.0%) had clinically significant anxiety (STAI-state or STAI-trait score ≥ 40). Sixty-seven (33.5%) participants had both depression and anxiety.
| Characteristic | All participants | With depression | Without depression | P value | With anxiety | Without anxiety | P value |
| Age (year), mean ± SD | 32.7 ± 6.4 | 33.2 ± 6.1 | 32.3 ± 6.6 | 0.325 | 32.5 ± 6.3 | 32.9 ± 6.5 | 0.652 |
| BMI (kg/m2), mean ± SD | 24.6 ± 4.3 | 25.3 ± 4.6 | 24.1 ± 4.0 | 0.047a | 25.0 ± 4.5 | 24.2 ± 4.0 | 0.182 |
| Education level | 0.089 | - | 0.134 | ||||
| Secondary or lower | 42 (21.0) | 23 (27.1) | 19 (16.5) | 26 (25.5) | 16 (16.3) | ||
| College | 114 (57.0) | 43 (50.6) | 71 (61.7) | 53 (52.0) | 61 (62.2) | ||
| Postgraduate | 44 (22.0) | 19 (22.4) | 25 (21.7) | 23 (22.5) | 21 (21.4) | ||
| Marital status | 0.762 | - | 0.518 | ||||
| Single | 62 (31.0) | 25 (29.4) | 37 (32.2) | 34 (33.3) | 28 (28.6) | ||
| Married | 122 (61.0) | 52 (61.2) | 70 (60.9) | 59 (57.8) | 63 (64.3) | ||
| Divorced/separated | 16 (8.0) | 8 (9.4) | 8 (7.0) | 9 (8.8) | 7 (7.1) | ||
| Employment status | 0.018a | - | 0.042a | ||||
| Employed | 138 (69.0) | 51 (60.0) | 87 (75.7) | 63 (61.8) | 75 (76.5) | ||
| Unemployed | 46 (23.0) | 25 (29.4) | 21 (18.3) | 29 (28.4) | 17 (17.3) | ||
| Student | 16 (8.0) | 9 (10.6) | 7 (6.1) | 10 (9.8) | 6 (6.1) | ||
| Disease duration (year), mean ± SD | 6.8 ± 4.5 | 7.5 ± 4.7 | 6.3 ± 4.3 | 0.059 | 7.2 ± 4.6 | 6.4 ± 4.3 | 0.195 |
| rASRM stage | 0.007b | - | 0.013a | ||||
| Stage I (minimal) | 28 (14.0) | 8 (9.4) | 20 (17.4) | 10 (9.8) | 18 (18.4) | ||
| Stage II (mild) | 42 (21.0) | 13 (15.3) | 29 (25.2) | 17 (16.7) | 25 (25.5) | ||
| Stage III (moderate) | 76 (38.0) | 34 (40.0) | 42 (36.5) | 42 (41.2) | 34 (34.7) | ||
| Stage IV (severe) | 54 (27.0) | 30 (35.3) | 24 (20.9) | 33 (32.4) | 21 (21.4) | ||
| Pain severity (VAS), mean ± SD | 6.4 ± 2.1 | 7.3 ± 1.8 | 5.7 ± 2.0 | < 0.001c | 7.0 ± 1.9 | 5.7 ± 2.0 | < 0.001c |
| Previous treatments | |||||||
| Analgesics | 178 (89.0) | 78 (91.8) | 100 (87.0) | 0.283 | 93 (91.2) | 85 (86.7) | 0.320 |
| Hormonal therapy | 142 (71.0) | 64 (75.3) | 78 (67.8) | 0.250 | 76 (74.5) | 66 (67.3) | 0.261 |
| Surgery | 168 (84.0) | 74 (87.1) | 94 (81.7) | 0.307 | 88 (86.3) | 80 (81.6) | 0.364 |
| Infertility | 62 (31.0) | 32 (37.6) | 30 (26.1) | 0.078 | 37 (36.3) | 25 (25.5) | 0.098 |
The mean BDI-II score for all participants was 16.8 ± 10.5. Based on the BDI-II cut-off values, 115 participants (57.5%) had minimal depression, 43 (21.5%) had mild depression, 28 (14.0%) had moderate depression, and 14 (7.0%) had severe depression. The mean STAI-state and STAI-trait scores were 41.3 ± 12.6 and 43.5 ± 11.8, respectively. The psychological assessment results are summarized in Table 2.
| Assessment | All participants | With depression | Without depression | P value | With anxiety | Without anxiety | P value |
| BDI-II, mean ± SD | 16.8 ± 10.5 | 26.4 ± 8.3 | 9.6 ± 5.1 | < 0.001 | 22.3 ± 10.6 | 11.0 ± 7.2 | < 0.001 |
| Depression severity | < 0.001 | ||||||
| Minimal (0-13) | 115 (57.5) | 0 (0.0) | 115 (100.0) | - | 38 (37.3) | 77 (78.6) | - |
| Mild (14-19) | 43 (21.5) | 43 (50.6) | 0 (0.0) | - | 30 (29.4) | 13 (13.3) | - |
| Moderate (20-28) | 28 (14.0) | 28 (32.9) | 0 (0.0) | - | 22 (21.6) | 6 (6.1) | - |
| Severe (29-63) | 14 (7.0) | 14 (16.5) | 0 (0.0) | - | 12 (11.8) | 2 (2.0) | - |
| STAI-state, mean ± SD | 41.3 ± 12.6 | 48.7 ± 11.4 | 35.9 ± 10.4 | < 0.001 | 51.2 ± 9.1 | 31.0 ± 6.8 | < 0.001 |
| STAI-trait, mean ± SD | 43.5 ± 11.8 | 50.2 ± 10.4 | 38.6 ± 10.2 | < 0.001 | 52.4 ± 8.5 | 34.3 ± 7.3 | < 0.001 |
| PSQI global, mean ± SD | 7.9 ± 3.8 | 9.8 ± 3.5 | 6.5 ± 3.4 | < 0.001 | 9.3 ± 3.7 | 6.4 ± 3.3 | < 0.001 |
| Poor sleep quality (PSQI > 5) | 138 (69.0) | 74 (87.1) | 64 (55.7) | < 0.001 | 84 (82.4) | 54 (55.1) | < 0.001 |
| PCS, mean ± SD | 22.4 ± 12.6 | 30.1 ± 11.7 | 16.8 ± 9.9 | < 0.001 | 28.3 ± 12.1 | 16.3 ± 10.0 | < 0.001 |
| EHP-30 core, mean ± SD | 42.6 ± 19.7 | 54.8 ± 16.9 | 33.8 ± 16.8 | < 0.001 | 51.5 ± 18.2 | 33.3 ± 16.7 | < 0.001 |
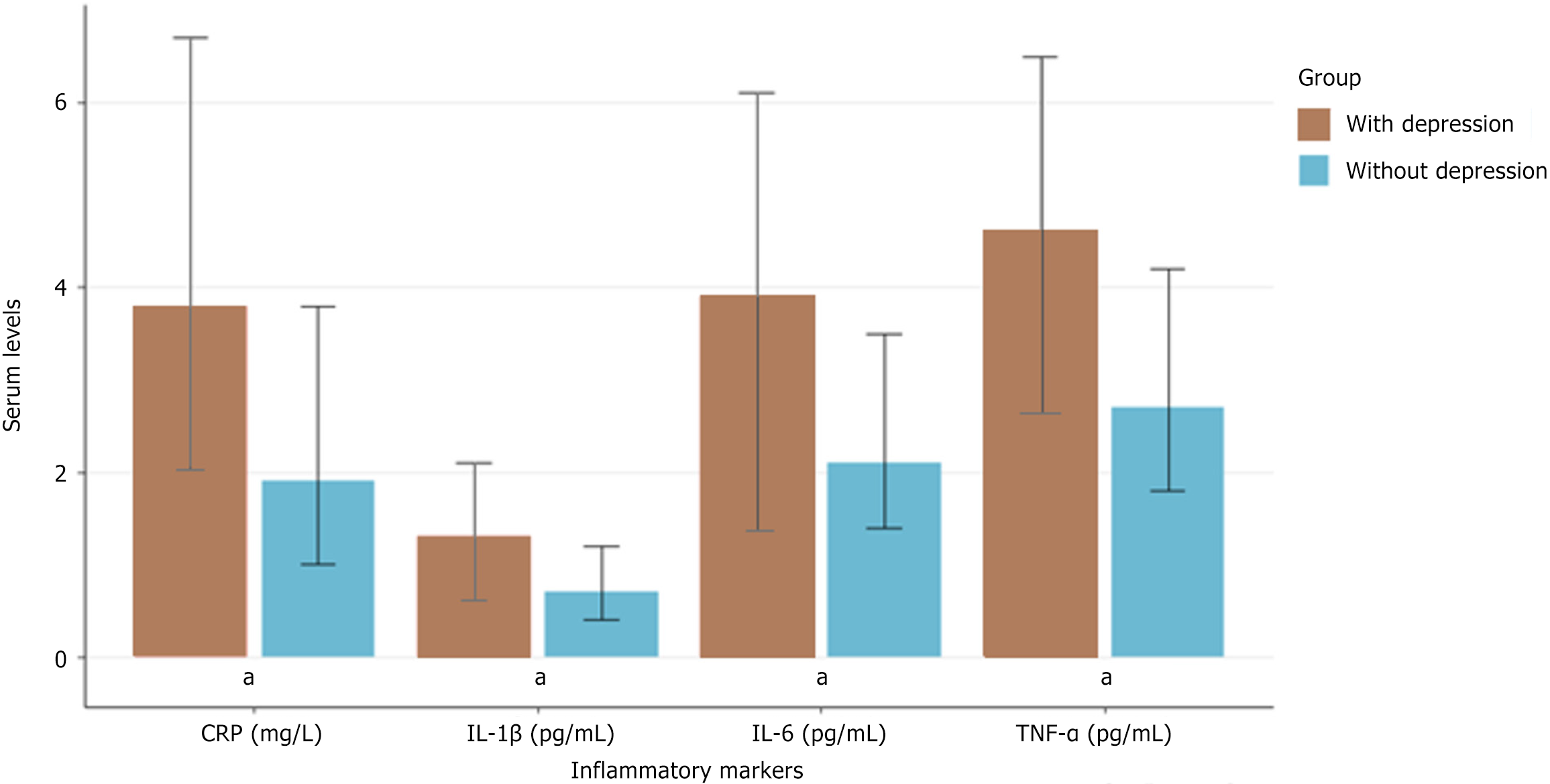
Serum levels of inflammatory markers (IL-6, IL-1β, TNF-α, and CRP) and BDNF were compared between participants with and without depression or anxiety (Table 3). Participants with depression had significantly higher levels of IL-6, IL-1β, TNF-α, and CRP, and lower levels of BDNF compared with those without depression (P < 0.001). Similarly, participants with anxiety showed elevated inflammatory markers and reduced BDNF levels compared with those without anxiety.
| Biomarker | All participants | With depression | Without depression | P value | With anxiety | Without anxiety | P value |
| IL-6 (pg/mL), median (IQR) | 2.8 (1.6-4.7) | 3.9 (2.4–6.1) | 2.1 (1.4-3.5) | < 0.001 | 3.6 (2.1-5.8) | 2.2 (1.4-3.6) | < 0.001 |
| IL-1β (pg/mL), median (IQR) | 0.9 (0.5-1.6) | 1.3 (0.7-2.1) | 0.7 (0.4-1.2) | < 0.001 | 1.2 (0.6-1.9) | 0.7 (0.4-1.2) | < 0.001 |
| TNF-α (pg/mL), median (IQR) | 3.4 (2.1-5.3) | 4.6 (2.8-6.5) | 2.7 (1.8-4.2) | < 0.001 | 4.2 (2.6-6.1) | 2.8 (1.8-4.4) | <0.001 |
| CRP (mg/L), median (IQR) | 2.6 (1.3-5.1) | 3.8 (1.9-6.7) | 1.9 (1.0-3.8) | < 0.001 | 3.4 (1.7-6.2) | 2.0 (1.1-3.9) | < 0.001 |
| BDNF (ng/mL), mean ± SD | 24.3 ± 8.4 | 20.7 ± 7.6 | 26.9 ± 8.0 | < 0.001 | 21.4 ± 7.8 | 27.3 ± 8.1 | < 0.001 |
Figure 1 illustrates the comparison of inflammatory marker levels between participants with and without depression. Significantly elevated levels of all inflammatory markers were found in patients with depression compared with those without depression (P < 0.001), with the most pronounced difference observed for IL-6.
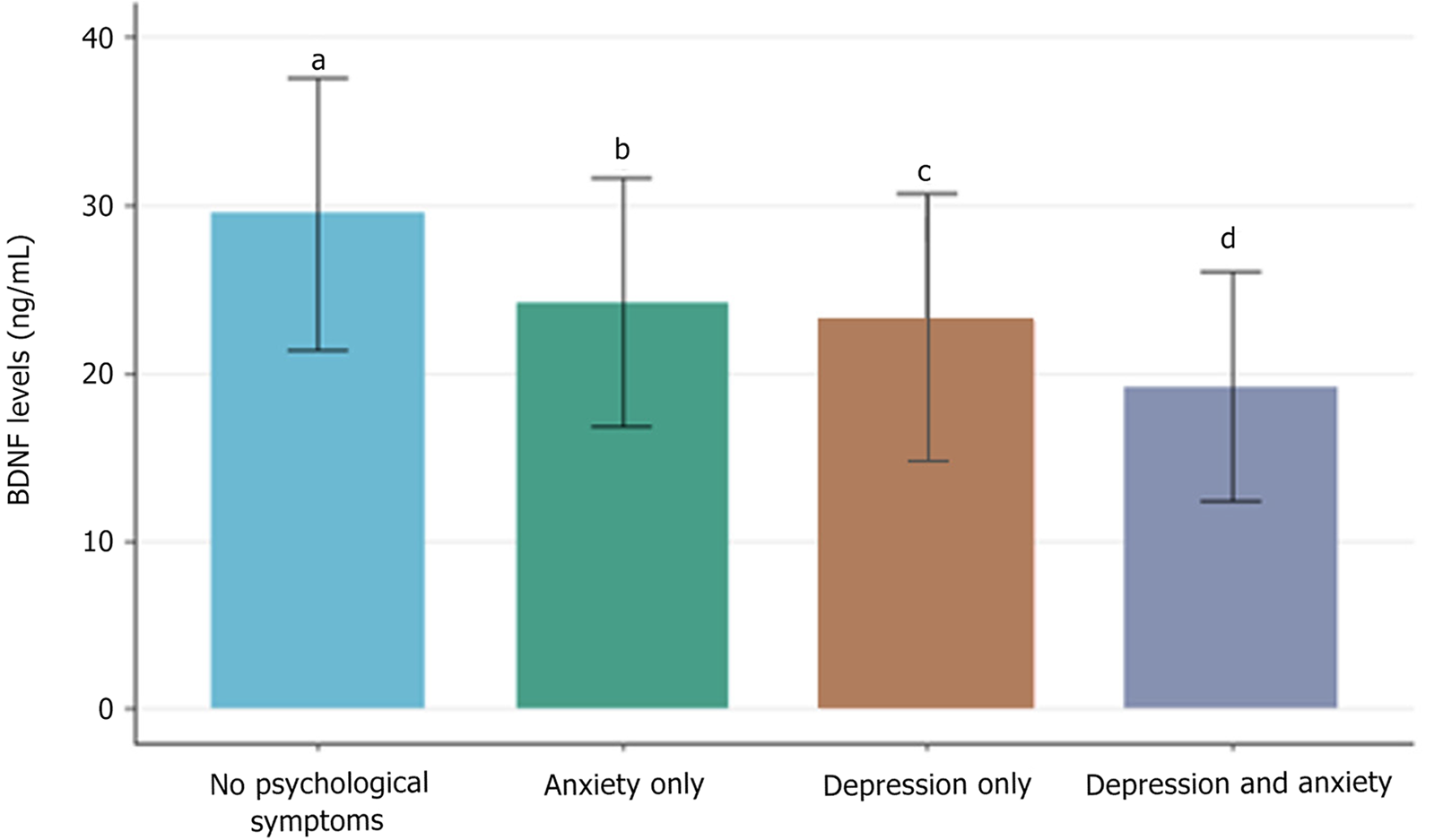
Figure 2 presents the comparison of BDNF levels between different psychological symptom groups. BDNF levels were lowest in participants with both depression and anxiety, intermediate in those with either depression or anxiety alone, and highest in those without psychological symptoms.
Correlation analyses revealed significant associations between pain severity, inflammatory marker levels, BDNF level, and psychological symptoms (Table 4). Pain severity as measured by visual analog scale showed moderate positive correlations with depression and anxiety scores, as well as levels of inflammatory markers. Additionally, inflammatory marker levels positively correlated with depression and anxiety scores, while BDNF level showed negative correlations with both pain severity and psychological symptoms.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1 Pain severity (VAS) | - | - | - | - | - | - | - | - | - |
| 2 BDI-II | 0.52a | - | - | - | - | - | - | - | - |
| 3 STAI-state | 0.48a | 0.65a | - | - | - | - | - | - | - |
| 4 STAI-trait | 0.45a | 0.68a | 0.76a | - | - | - | - | - | - |
| 5 IL-6 | 0.43a | 0.48a | 0.41a | 0.39a | - | - | - | - | - |
| 6 IL-1β | 0.37a | 0.42a | 0.36a | 0.34a | 0.58a | - | - | - | - |
| 7 TNF-α | 0.40a | 0.45a | 0.38a | 0.36a | 0.62a | 0.54a | - | - | - |
| 8 CRP | 0.35a | 0.40a | 0.33a | 0.31a | 0.49a | 0.42a | 0.45a | - | - |
| 9 BDNF | -0.38a | -0.46a | -0.43a | -0.44a | -0.36a | -0.32a | -0.34a | -0.28a | - |
Multiple linear regression analyses were conducted to identify significant predictors of depression and anxiety symptoms (Table 5). For depression (BDI-II scores), pain severity, and IL-6, TNF-α, and BDNF levels emerged as significant independent predictors after adjusting for demographic and clinical variables. Similarly, pain severity and IL-6 and BDNF levels were significant predictors of anxiety (STAI-state scores), along with sleep quality.
| Variable | B | SE | β | P value | 95%CI |
| Dependent variable: BDI-II | |||||
| Constant | 24.63 | 5.42 | - | < 0.001 | 13.95-35.31 |
| Age | 0.06 | 0.09 | 0.04 | 0.497 | -0.12 to 0.24 |
| BMI | 0.14 | 0.13 | 0.06 | 0.289 | -0.12 to 0.40 |
| Disease duration | 0.15 | 0.13 | 0.07 | 0.245 | -0.10 to 0.40 |
| rASRM stage | 0.83 | 0.62 | 0.08 | 0.180 | -0.39 to 2.05 |
| Pain severity (VAS) | 1.32 | 0.31 | 0.27 | < 0.001 | 0.71-1.93 |
| IL-6 | 0.85 | 0.32 | 0.17 | 0.009 | 0.22-1.48 |
| IL-1β | 0.94 | 0.69 | 0.09 | 0.174 | -0.42 to 2.30 |
| TNF-α | 0.78 | 0.31 | 0.16 | 0.013 | 0.17-1.39 |
| CRP | 0.21 | 0.19 | 0.07 | 0.269 | -0.16 to 0.58 |
| BDNF | -0.26 | 0.07 | -0.21 | < 0.001 | -0.40 to -0.12 |
| PSQI global | 0.49 | 0.16 | 0.18 | 0.003 | 0.17 to -0.81 |
| R2 = 0.57, adjusted R2 = 0.54, F (11-188) = 22.61, P < 0.001 | |||||
| Dependent variable: STAI-state | |||||
| Constant | 51.84 | 7.61 | - | < 0.001 | 36.83-66.85 |
| Age | 0.03 | 0.13 | 0.01 | 0.831 | -0.22 to 0.28 |
| BMI | 0.17 | 0.18 | 0.06 | 0.351 | -0.19 to 0.53 |
| Disease duration | 0.18 | 0.18 | 0.06 | 0.310 | -0.17 to 0.53 |
| rASRM stage | 0.91 | 0.87 | 0.06 | 0.297 | -0.81 to 2.63 |
| Pain severity (VAS) | 1.79 | 0.43 | 0.30 | < 0.001 | 0.94-2.64 |
| IL-6 | 0.94 | 0.45 | 0.15 | 0.038 | 0.05-1.83 |
| IL-1β | 0.78 | 0.97 | 0.06 | 0.423 | -1.13 to 2.69 |
| TNF-α | 0.76 | 0.43 | 0.13 | 0.080 | -0.09 to 1.61 |
| CRP | 0.12 | 0.26 | 0.03 | 0.646 | -0.39 to 0.63 |
| BDNF | -0.32 | 0.10 | -0.21 | 0.001 | -0.52 to -0.12 |
| PSQI Global | 0.84 | 0.23 | 0.25 | < 0.001 | 0.39-1.29 |
| R2 = 0.51, adjusted R2 = 0.48, F (11-188) = 17.83, P < 0.001 | |||||
This study investigated the prevalence of depression and anxiety in patients with endometriosis-associated chronic pain and explored the potential neuroimmune mechanisms mediated by inflammatory factors. Our findings indicated high rates of psychological comorbidities in this population, with 42.5% of participants experiencing clinically significant depression and 51.0% experiencing anxiety. These rates are considerably higher than those reported in the general female population, where the prevalence of depression and anxiety disorders typically ranges from 5% to 20%[11]. Our results align with previous studies highlighting the substantial psychological burden associated with endometriosis, particularly when accompanied by chronic pain[12].
The observed associations between disease severity, pain intensity, and psychological symptoms underscore the complex interplay between the physical and psychological aspects of endometriosis. In this study, patients with more advanced stages of endometriosis (revised American Society for Reproductive Medicine stages III and IV) had significantly higher rates of depression and anxiety compared with those with minimal or mild disease. However, pain severity emerged as a stronger predictor of psychological symptoms than disease stage, suggesting that the subjective experience of pain, rather than objective disease markers, may be more relevant to psychological well-being in this population. This finding is consistent with previous research indicating that pain severity often correlates poorly with laparoscopic findings in endometriosis, highlighting the multidimensional nature of pain perception[13].
A key contribution of this study is the comprehensive analysis of inflammatory markers in relation to both pain and psychological symptoms. Our results demonstrated significantly elevated levels of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) and CRP in participants with endometriosis who experienced depression or anxiety compared with those without psychological symptoms. These findings support the inflammatory hypothesis of depression and anxiety, which posits that inflammation plays a causal role in the pathophysiology of mood disorders[14]. The observed correlations between inflammatory markers and psychological symptoms, independent of pain severity, suggest that inflammation may represent a biological pathway linking endometriosis with psychological disturbances.
IL-6 emerged as a particularly relevant marker, showing the strongest associations with both depression and anxiety symptoms. IL-6 is a pleiotropic cytokine with diverse biological functions, including regulation of immune responses, acute-phase reactions, and neural processes. Elevated IL-6 Levels have been consistently reported in cases of both endometriosis and depression, suggesting a potential common inflammatory pathway[15]. In the central nervous system, IL-6 can influence neurotransmitter metabolism, neuroendocrine function, and neural plasticity, all of which are implicated in the pathophysiology of mood disorders. Our mediation analyses further supported the role of IL-6 as a significant mediator in the relationship between pain and psychological symptoms, suggesting that IL-6-targeted interventions might simultaneously address physical and psychological manifestations of endometriosis.
The low BDNF levels observed in participants with depression and anxiety represent another important finding. BDNF, a member of the neurotrophin family, plays crucial roles in neuronal survival, differentiation, and synaptic plasticity. Reduced BDNF levels have been consistently reported in depression and have been linked to impaired neuroplasticity and structural brain changes[16]. In this study, BDNF level showed a negative correlation with both inflammatory markers and psychological symptoms, suggesting that inflammation might contribute to depression and anxiety in endometriosis, partially via downregulation of BDNF expression. This finding aligns with the neurotrophic hypothesis of depression, which posits that reduced BDNF expression contributes to neuronal atrophy in brain regions involved in emotional regulation.
Several mechanisms may explain the observed relationships between inflammation, pain, and psychological symptoms in endometriosis. First, peripherally produced inflammatory cytokines can enter cerebral tissues via multiple routes, including passage through a leaky blood–brain barrier, active transport across the barrier, and activation of vagal afferents[17]. Once these cytokines enter the central nervous system, they can alter neurotransmitter metabolism, affecting serotonin, dopamine, and glutamate systems, which are implicated in mood regulation. Second, inflammatory cytokines can activate the hypothalamic–pituitary–adrenal axis, leading to glucocorticoid resistance and altered stress responses, which are characteristic features of depression. Third, chronic inflammation can induce oxidative stress and reduce BDNF expression, potentially compromising neuronal integrity and plasticity in brain regions involved in emotional processing[18].
The association between poor sleep quality and psychological symptoms in this cohort deserves special attention. Sleep disturbances were reported by 69% of participants and were more prevalent among those with depression (87.1%) and anxiety (82.4%). Sleep quality was identified as an independent predictor of both depression and anxiety in regression analyses. These findings suggest that sleep disturbances may represent an important mechanism linking endometriosis with psychological symptoms, potentially via inflammatory processes. Previous research[19] has demonstrated bidirectional relationships between sleep, inflammation, and mood, with sleep disruption promoting inflammatory responses and exacerbating psychological distress.
This study has several strengths, including its prospective design, large sample size, comprehensive assessment of psychological symptoms and biological markers, and sophisticated statistical analyses exploring potential mediating mechanisms. Nonetheless, some limitations must be acknowledged. First, the cross-sectional design precludes drawing causal inferences regarding inflammation, pain, and psychological symptoms; thus, longitudinal studies are needed to clarify temporal relationships. Second, we measured only circulating inflammatory markers, which may not fully reflect central nervous system inflammation. Future investigations using cerebrospinal fluid analyses or neuroimaging could offer more direct evidence of neuroimmune mechanisms. Third, this study focused on a limited range of inflammatory markers, and assessing a broader spectrum - including anti-inflammatory cytokines and specialized pro-resolving mediators - would provide a more comprehensive depiction of the inflammatory milieu[20]. Genetic and epigenetic factors that may influence both endometriosis susceptibility and the risk of depression and anxiety[21] were not addressed. In addition, unmeasured variables such as childhood adversity, personality traits, and social support could impact both inflammatory pathways and psychological vulnerability[22], suggesting the need for more inclusive biopsychosocial models. Further research should also examine how various treatments - such as hormonal therapies, surgery, and analgesics - affect inflammatory markers and psychological outcomes[23]. Finally, intervention studies targeting inflammatory pathways (e.g., COX-2 inhibitors, TNF-α antagonists, IL-6 receptor antagonists) and integrating non-pharmacological strategies with anti-inflammatory effects (e.g., mindfulness, exercise, dietary modifications) are warranted to optimize pain and psychological symptom management[24,25]. Prospective longitudinal cohort studies are needed to confirm temporal links.
This study provides evidence for a neuroimmune mechanism linking endometriosis-associated chronic pain with depression and anxiety, mediated by inflammatory factors. The high prevalence of psychological comorbidities observed in this population highlights the need for integrated care approaches addressing both physical and psychological dimensions of endometriosis. The identified associations between inflammatory markers, pain, and psychological symptoms suggest potential therapeutic targets for managing psychological comorbidities in endometriosis patients through anti-inflammatory interventions.
| 1. | Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K, Saridogan E, Tomassetti C, van Hanegem N, Vulliemoz N, Vermeulen N; ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum Reprod Open. 2022;2022:hoac009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 900] [Article Influence: 225.0] [Reference Citation Analysis (0)] |
| 2. | As-Sanie S, Black R, Giudice LC, Gray Valbrun T, Gupta J, Jones B, Laufer MR, Milspaw AT, Missmer SA, Norman A, Taylor RN, Wallace K, Williams Z, Yong PJ, Nebel RA. Assessing research gaps and unmet needs in endometriosis. Am J Obstet Gynecol. 2019;221:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 3. | Pope CJ, Sharma V, Sharma S, Mazmanian D. A Systematic Review of the Association Between Psychiatric Disturbances and Endometriosis. J Obstet Gynaecol Can. 2015;37:1006-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | van Barneveld E, Manders J, van Osch FHM, van Poll M, Visser L, van Hanegem N, Lim AC, Bongers MY, Leue C. Depression, Anxiety, and Correlating Factors in Endometriosis: A Systematic Review and Meta-Analysis. J Womens Health (Larchmt). 2022;31:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Rasp E, Saavalainen L, But A, Gissler M, Härkki P, Heikinheimo O, Rönö K. Psychiatric disorders and mortality due to external causes following diagnosis of endometriosis at a young age: a longitudinal register-based cohort study in Finland. Am J Obstet Gynecol. 2024;230:651.e1-651.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Mccallion A, Sisnett DJ, Zutautas KB, Hayati D, Spiess KG, Aleksieva S, Lingegowda H, Koti M, Tayade C. Endometriosis through an immunological lens: a pathophysiology based in immune dysregulation. Explor Immunol. 2022;2:454-483. [DOI] [Full Text] |
| 7. | Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1671] [Cited by in RCA: 2702] [Article Influence: 270.2] [Reference Citation Analysis (0)] |
| 8. | Sonmez EO, Uguz F, Sahingoz M, Sonmez G, Kaya N, Camkurt MA, Gokmen Z, Basaran M, Gezginc K, Erdem SS, Dulger HH, Tasyurek E. Effect of Maternal Depression on Brain-derived Neurotrophic Factor Levels in Fetal Cord Blood. Clin Psychopharmacol Neurosci. 2019;17:308-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Koninckx PR, Ussia A, Adamyan L, Tahlak M, Keckstein J, Wattiez A, Martin DC. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best Pract Res Clin Obstet Gynaecol. 2021;71:14-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Miller AH, Haroon E, Felger JC. Therapeutic Implications of Brain-Immune Interactions: Treatment in Translation. Neuropsychopharmacology. 2017;42:334-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 11. | Friedrich MJ. Depression Is the Leading Cause of Disability Around the World. JAMA. 2017;317:1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Li B, Zhou Y, Wang Y, Han X, Zhang S, He Z, Ouyang L. Does Endometriosis Disturb Mental Health and Quality of Life? A Systematic Review and Meta-Analysis. Gynecol Obstet Invest. 2021;86:315-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Rocha TP, Andres MP, Carmona F, Baracat EC, Abrão MS. Deep Endometriosis: the Involvement of Multiple Pelvic Compartments Is Associated with More Severe Pain Symptoms and Infertility. Reprod Sci. 2023;30:1668-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Vöckel J, Markser A, Wege L, Wunram HL, Sigrist C, Koenig J. Pharmacological anti-inflammatory treatment in children and adolescents with depressive symptoms: A systematic-review and meta-analysis. Eur Neuropsychopharmacol. 2024;78:16-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Zhang Q, Li T, Xu M, Islam B, Wang J. Application of Optogenetics in Neurodegenerative Diseases. Cell Mol Neurobiol. 2024;44:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Castrén E, Monteggia LM. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol Psychiatry. 2021;90:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 17. | Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 894] [Cited by in RCA: 870] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 18. | Orsolini L, Pompili S, Tempia Valenta S, Salvi V, Volpe U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? Int J Mol Sci. 2022;23:1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 19. | Holmer BJ, Lapierre SS, Jake-Schoffman DE, Christou DD. Effects of sleep deprivation on endothelial function in adult humans: a systematic review. Geroscience. 2021;43:137-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Ghai V, Jan H, Shakir F, Haines P, Kent A. Diagnostic delay for superficial and deep endometriosis in the United Kingdom. J Obstet Gynaecol. 2020;40:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 21. | Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol. 2015;25:1532-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 22. | Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, Raine-Fenning N. The social and psychological impact of endometriosis on women's lives: a critical narrative review. Hum Reprod Update. 2013;19:625-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 330] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 23. | Gallagher JS, DiVasta AD, Vitonis AF, Sarda V, Laufer MR, Missmer SA. The Impact of Endometriosis on Quality of Life in Adolescents. J Adolesc Health. 2018;63:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 492] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 25. | Evans S, Fernandez S, Olive L, Payne LA, Mikocka-Walus A. Psychological and mind-body interventions for endometriosis: A systematic review. J Psychosom Res. 2019;124:109756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/