Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.107298
Revised: May 13, 2025
Accepted: July 18, 2025
Published online: October 19, 2025
Processing time: 180 Days and 16.1 Hours
Postoperative delirium (POD), an acute neuropsychiatric complication in elderly surgical patients, manifests as attention and cognitive disturbances that may last 24-72 hours after surgery, potentially progressing to dementia. Transcranial direct current stimulation (tDCS), a non-invasive neuromodulation technique, enhances cortical excitability and cognitive function by modulating brain networks and synaptic plasticity. Elderly patients undergoing major laparoscopic surgery face elevated POD risks due to prolonged anesthesia and pneumoperitoneum-induced cerebral hypoperfusion. This study investigates whether pre-anesthesia tDCS can reduce POD incidence in this population.
To investigate the effect of preoperative tDCS on reducing the incidence of POD in elderly patients undergoing major laparoscopic surgery.
In this study, we enrolled 220 elderly patients who underwent major laparoscopic surgery between April 2024 and December 2024. Patients were randomly assigned to the active-tDCS (group A) and sham-tDCS (group S) groups. A single session of tDCS or sham stimulation was administered 30 minutes before anesthesia induction. The primary outcome was the incidence of POD during within 3 days postoperatively.
A total of 201 patients were included in the final analysis, with 100 patients in group A and 101 in group S. The incidence of POD within 3 days postoperatively was 7.0% in group A, which was significantly lower than 22.8% in group S. On postoperative day 1, the Self-Rating Anxiety Scale and Self-Rating Depression Scale scores sig
A single session of preoperative tDCS can reduce the incidence of POD in elderly patients undergoing major laparoscopic surgery and can also reduce postoperative anxiety and depression in these patients.
Core Tip: A single session of preoperative transcranial direct current stimulation (tDCS) significantly reduces the incidence of postoperative delirium (POD) in elderly patients undergoing major laparoscopic surgery, with a POD rate of 7.0% in the tDCS group compared to 22.8% in the sham group. In addition, tDCS significantly reduces postoperative anxiety and depression but not pain. These findings uphold preoperative tDCS as a promising intervention for preventing POD and enhancing psychological recovery in elderly surgical patients.
- Citation: Yang X, Wei YF, Ye CH, Tian WD, Li XY, Hu YJ, Chen LP. Effect of preoperative transcranial direct current stimulation on postoperative delirium in elderly patients following laparoscopic surgery. World J Psychiatry 2025; 15(10): 107298
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/107298.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.107298
Postoperative delirium (POD) is an acute central nervous system syndrome and is a common postoperative complication in elderly patients, often associated with surgical trauma. POD typically occurs in a paroxysmal manner within 24 hours to 72 hours after surgery. It is characterized by disturbances in attention, perception, awareness, cognitive function, and consciousness, with significant fluctuations in both the timing and severity of episodes[1]. In elderly patients, POD can impair cognitive function, and in severe cases, it may directly lead to dementia, with severe implications for patients, their families, and society[2,3]. Therefore, effective prevention of POD is of clinical importance. Current research indicates that nonpharmacological interventions can reduce the incidence of POD[4].
Transcranial direct current stimulation (tDCS) is a noninvasive neuromodulation technique that regulates cortical excitability by applying weak direct current to specific brain areas through two or more electrodes placed on the scalp[5]. Anodal stimulation typically increases cortical excitability, whereas cathodal stimulation decreases it. Research has shown that 15 minutes of tDCS can induce sustained changes in cortical excitability that last over 60 minutes, resulting in improved cognitive and motor functions in healthy elderly individuals[6,7], reduced age-related cognitive decline, and reversal of age-related functional brain activity and connectivity changes[8]. Its mechanisms of action may involve changes at multiple levels[9,10], ranging from cellular processes to the regulation of brain network functions. These include altering cortical excitability[5], improving local cerebral blood flow[11], increasing synaptic plasticity[12], modulating intercortical brain network connectivity[13], and influencing brain neurotransmitters[14].
Older age (≥ 65 years) is an independent risk factor for cognitive decline[15], which can be further exacerbated by surgery and anesthesia. In particular, major laparoscopic surgery is associated with prolonged surgical duration, and the pneumoperitoneum required for it can impair the autoregulatory function of the brain (e.g., blood flow, tissue oxygenation, and regional cerebral oxygen saturation), thereby increasing the incidence of POD[16,17]. Therefore, we aimed to investigate the role of a single session of tDCS before anesthesia induction in reducing the incidence of POD among elderly patients undergoing major laparoscopic surgery.
This was a single-center, double-blind, randomized controlled trial conducted at the Affiliated Hospital of Xuzhou Medical University from April 2024 to December 2024. The study protocol was approved by the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University, approval No. XYFY2024-KL026-01 and registered with the Chinese Clinical Trial Registry, registration No. ChiCTR2400087975. All participants signed a written informed consent form and voluntarily participated in this study. The study is reported according to the CONSORT statement for nonpharmacological treatment randomized trials.
The study population included patients aged ≥ 65 years with an American Society of Anesthesiologists (ASA) classification of II or III who were scheduled for elective laparoscopic surgery (with an expected surgery duration of > 2 hours). The following were the exclusion criteria: (1) Patients with severe neurological or psychiatric disorders or those currently taking antipsychotic medications; (2) Patients with cognitive impairment as assessed by the Mini-Mental State Examination (MMSE) (illiterate ≤ 17, primary school ≤ 20, and junior high school and above ≤ 23); (3) Patients with communication difficulties; (4) Patients who refused to sign the informed consent form; (5) Patients with contraindications for tDCS (e.g., intracranial metal foreign bodies and pacemakers); (6) Patients with severe cardiovascular/cerebrovascular diseases or liver/kidney insufficiency; and (7) Patients with preoperative hemoglobin < 7 g/dL.
Patients who were initially included but met any of the following criteria during the course of the study were eliminated from the study: (1) Patients who were postoperatively transferred to the intensive care unit (ICU); (2) Those who withdrew from the study at any assessment stage; and (3) Those whose surgical procedure was altered or who experienced anesthesia or surgical complications (e.g., allergies and massive bleeding). All participants were informed of the overall study process.
Patients were randomly assigned to the active-tDCS group (group A) or the sham-tDCS group (group S) in a 1:1 ratio on the basis of a computer-generated random number sequence. The allocation information was sealed in an envelope, and only the medical staff administering the tDCS were aware of the group assignments. Both patients and the researchers involved in subsequent follow-ups and data analyses were blinded to the group allocation.
tDCS was initiated 30 minutes before anesthesia induction. The equipment was provided by Jiangxi Huaheng Jingxing Medical Technology Co. Ltd., model MBM-I, as shown in Supplementary Figure 1. First, saline-soaked sponge electrodes were used, with the anode placed at the F3 region (determined using the 10-20 electroencephalogram electrode placement method) and the cathode placed 1-2 cm above the right eyebrow. Group A patients received a constant current stimulation of 2 mA for 20 minutes, with the current gradually increasing during the first 30 seconds and gradually decreasing during the final 30 seconds. Group S patients did not receive constant current stimulation during the 19-minute middle phase; they were given only the gradual increase during the first 30 seconds and the gradual decrease during the last 30 seconds. In a previous study[18], participants receiving tDCS could not accurately identify whether they were administered real or sham stimulation. This suggests that the inclusion of a sham stimulation group can effectively maintain blinding in double-blind studies. Patients were closely monitored throughout the procedure, and any adverse events were recorded. If necessary, the stimulation could be stopped at any time.
Anesthesia induction was performed using 0.01 mg/kg midazolam, 0.3 mg/kg etomidate, 0.6 mg/kg rocuronium, and 0.5 μg/kg sufentanil. A bilateral transversus abdominis plane block and rectus sheath block were performed with 40 mL of 0.375% ropivacaine. Intraoperatively, anesthesia was maintained with a propofol infusion at 3-6 mg/(kg × hour), remifentanil infusion at 0.1-0.25 μg/(kg × minute), and sevoflurane at 1%, keeping the bispectral index between 40 and 60. Additional muscle relaxants were given as needed. Intraoperative blood pressure was maintained within 20% of the baseline. Respiratory parameters were set to a tidal volume of 6-8 mL/kg and respiratory rate of 12-16 breaths/minute, and the partial pressure of end tidal carbon dioxide (PetCO2) was maintained at 35-45 mmHg. The body temperature was kept above 36 °C during the procedure.
Postoperatively, all patients were transferred to the post-anesthesia care unit (PACU) and antagonized with 0.5 mg flumazenil and 2 mg/kg sugammadex sodium. Postoperative pain management was self-administered by patients through patient-controlled intravenous analgesia. The solution comprised 2 μg/kg sufentanil and 6 mg ondansetron and was diluted to 110 mL, with a background dose of 2 mL/hour, a single dose of 0.5 mL, and a lockout interval of 15 minutes. Patients were transferred from the PACU to the ward only when their Steward score was > 4 and they exhibited stable respiratory and circulatory function. After returning to the ward, if the patient’s Numeric Rating Scale (NRS) score was > 6, dezocine was administered intramuscularly as rescue analgesia.
The primary outcome measure is the incidence of POD within 3 days postoperatively. In the PACU, the Confusion Assessment Method (CAM) for the ICU (CAM-ICU) was used for delirium assessments, whereas in the ward, the CAM was used. The CAM was developed by Inouye et al[19] in the United States on the basis of the diagnostic criteria for delirium in the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised. Its design features make it particularly suitable for use by non-psychiatric clinicians and nursing staff. The CAM-ICU is an adapted version of the CAM with modified attention assessment components. This method shows high sensitivity and specificity, proving to be both reliable and clinically effective[19]. The investigators assessed the patients for delirium at the time of preparations for patients’ transfer back to the ward (T1), in the evening of the day of surgery (T2); the morning (T3) and afternoon (T4) of postoperative day 1; the morning (T5) and afternoon (T6) of postoperative day 2; and the morning (T7) and afternoon (T8) of postoperative day 3. If POD was identified at T8, follow-up was continued for an additional day until the delirium symptoms were resolved. Secondary outcome measures included postoperative pain score (NRS), depression score (Self-Rating Depression Scale), and anxiety score (Self-Rating Anxiety Scale) at T4, along with the recording of rescue analgesia usage, types of POD, length of hospital stay, and any adverse events. During the assessments, the patient’s family members were also interviewed to avoid missing any key information. The study timeline is shown in Supplementary Figure 2.
Sample size was calculated using the PASS 15.0 software. Based on previous literature, the incidence of POD in elderly patients undergoing laparoscopic major surgery (surgery time > 2 hours) was 24.8%[20]. Pre-experimental data indicated that the incidence of POD could be reduced to 10% after preoperative tDCS intervention. With a significance level of α = 0.05 and a power of 1-β = 0.8, the required sample size was determined to be 200 patients. Considering a 10% dropout rate, the planned recruitment was 220 patients (n = 110).
Statistical analysis was performed using IBM SPSS 27.0 and R 4.4.1 software. Categorical data were expressed as rates and percentages and analyzed using the χ² or Fisher’s exact test. Normally distributed continuous data were presented as mean ± SD and compared between groups using independent samples t-test. Non-normally distributed continuous data were expressed as median and interquartile range and analyzed using the Mann-Whitney U test. The cumulative incidence of POD was analyzed using Kaplan-Meier survival analysis and log-rank test. Logistic regression was used to analyze the risk factors for POD. Subgroup analysis was performed to explore the differences in intervention effects across various subgroups. The mediation effect of depression and anxiety on POD was analyzed using R software. Two-tailed tests were conducted, with α = 0.05. A P value of < 0.05 was considered statistically significant.
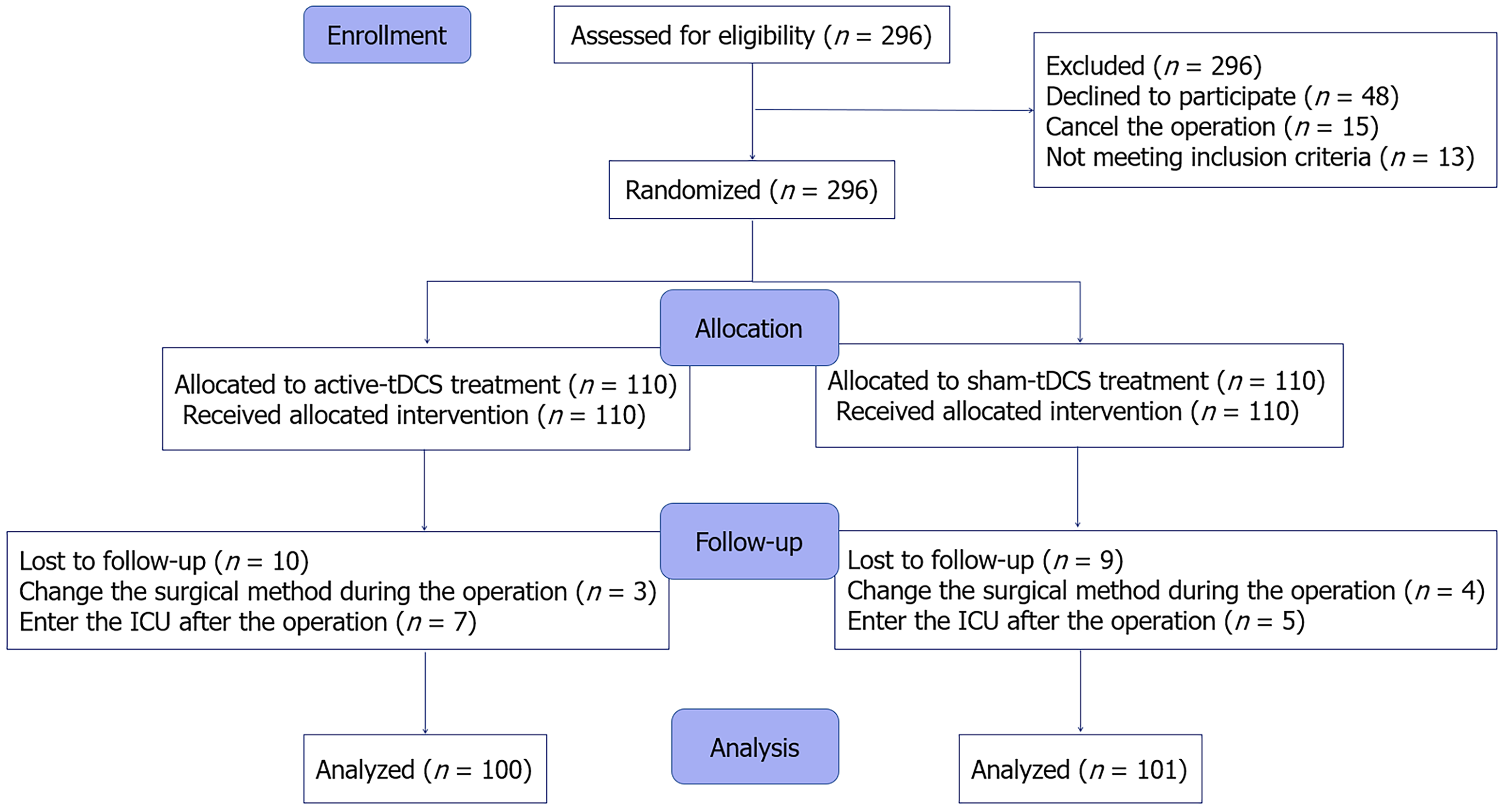
Between April 2024 and December 2024, 296 elderly patients scheduled for laparoscopic major surgery were screened. Among them, 48 patients refused to participate, 13 did not meet the inclusion criteria (5 patients had preoperative hemoglobin levels < 7 g/L, 4 patients had severe liver or kidney dysfunction, 3 had preoperative MMSE scores below the standard, and 1 had difficulty communicating), and 15 had canceled their planned operations. Consequently, 220 patients were enrolled in the study and were randomly assigned to group A or group S in a 1:1 ratio. Ten patients in group A were eliminated (7 patients were admitted to the ICU postoperatively and 3 had a change in the surgical procedure), and 9 patients in group S were eliminated (5 were admitted to the ICU postoperatively and 4 had a change in the surgical procedure). Ultimately, 201 patients were included in the final analysis (100 in group A and 101 in group S). The entire study flow is shown in Figure 1.
The baseline data and clinical characteristics of the two groups are shown in Table 1. The two groups did not significantly differ in terms of age, sex distribution, the type of surgery, the ASA classification, preoperative depression score, anxiety score, sleep score, comorbidity index, and frailty score. The groups also did not significantly differ in terms of surgical and anesthesia duration and use of anesthetic drugs (Supplementary Table 1).
| Characteristics | Sham-tDCS (n = 101) | Active-tDCS (n = 100) | P value |
| Sex | 0.332 | ||
| Male | 74 (73.3) | 67 (67.0) | |
| Female | 27 (26.7) | 33 (33.0) | |
| Age (year), median (IQR) | 72 (69-76) | 72 (69-75) | 0.877 |
| BMI (kg/m2), mean ± SD | 22.79 ± 2.67 | 22.43 ± 2.52 | 0.332 |
| ASA score (%) | 0.472 | ||
| II | 81 (80.2) | 76 (76.0) | |
| III | 20 (19.8) | 24 (24.0) | |
| History of smoking | 36 (35.6) | 40 (40.0) | 0.524 |
| History of anesthesia | 33 (32.7) | 34 (34.0) | 0.842 |
| MMSE score, median (IQR) | 26 (25-28) | 26 (25-28) | 0.144 |
| Hemoglobin (g/L), median (IQR) | 125 (111.5-139) | 123 (115.5-132) | 0.680 |
| Albumin (g/L), median (IQR) | 39.14 (3.64) | 39.25 (3.52) | 0.827 |
| Self-Rating Depression Scale, median (IQR) | 28 (26-29) | 28 (26.25-30) | 0.353 |
| Self-Rating Anxiety Scale, median (IQR) | 28 (27-30) | 28.5 (27-30.75) | 0.104 |
| Athens Insomnia Scale, median (IQR) | 1 (1-2) | 1 (1-2.75) | 0.797 |
| Age-adjusted Charlson Comorbidity Index, median (IQR) | 3 (3-4) | 3 (2-3) | 0.327 |
| Education level | 0.657 | ||
| Illiteracy | 17 (16.8) | 21 (21.0) | |
| Elementary school | 45 (44.6) | 43 (43.0) | |
| Middle school | 28 (27.7) | 28 (28.0) | |
| High school | 10 (9.9) | 7 (7.0) | |
| College graduate | 1 (1.0) | 1 (1.0) | |
| Frailty | 0.204 | ||
| Robust | 46 (45.5) | 58 (58.0) | |
| Prefrail | 38 (37.6) | 30 (30.0) | |
| Frail | 17 (16.8) | 12 (12.0) | |
| Comorbidities | 0.728 | ||
| Hypertension | 40 (39.6) | 44 (44.0) | |
| Diabetes | 24 (23.8) | 19 (19.0) | |
| Stroke | 24(23.8) | 22 (22.0) | |
| Coronary heart disease | 4 (4.0) | 5 (5.0) | |
| Type of operation | 0.867 | ||
| Stomach | 44 (43.6) | 44 (44.0) | |
| Rectum | 23 (22.8) | 28 (28.0) | |
| Colon | 15 (14.9) | 11 (11.0) | |
| Liver | 13 (12.9) | 11 (11.0) | |
| Pancreaticoduodenum | 6 (5.9) | 6 (6.0) | |
The incidence of POD within 3 days was significantly lower in group A than in group S (7.0% vs 22.8%; relative risk 0.830, 95% confidence interval: 0.737-0.935) (P = 0.002) (Table 2). However, the two groups did not significantly differ regarding the duration of POD (P = 0.432) and the types of delirium (P = 0.829). The groups also did not significantly differ in terms of NRS scores, use of rescue analgesia, or length of hospital stay at the T4 time point. However, anxiety scores (P = 0.045) and depression scores (P = 0.024) differed significantly between the two groups.
| Characteristics | Sham-tDCS (n = 101) | Active-tDCS (n = 100) | P value |
| Postoperative delirium | 23 (22.8) | 7 (7.0) | 0.002 |
| Days with delirium, median (IQR) | 2 (1-2) | 2 (1.25-2) | 0.432 |
| Type of delirium | 0.829 | ||
| Hypoactive | 15 | 4 | |
| Hyperactive | 4 | 1 | |
| Mixed | 4 | 2 | |
| Self-Rating Anxiety Scale, median (IQR) | 27 (24.5-28) | 26 (24-27) | 0.045 |
| Self-Rating Depression Scale, median (IQR) | 27 (26-29) | 26 (25-28) | 0.024 |
| Numeric Rating Scale score, median (IQR) | 4 (3-5) | 4 (3-4) | 0.243 |
| Use of salvage analgesics | 18 (17.8) | 14 (14.0) | 0.459 |
| Duration of hospitalization, median (IQR), day | 14 (12-18) | 15 (13-18) | 0.445 |
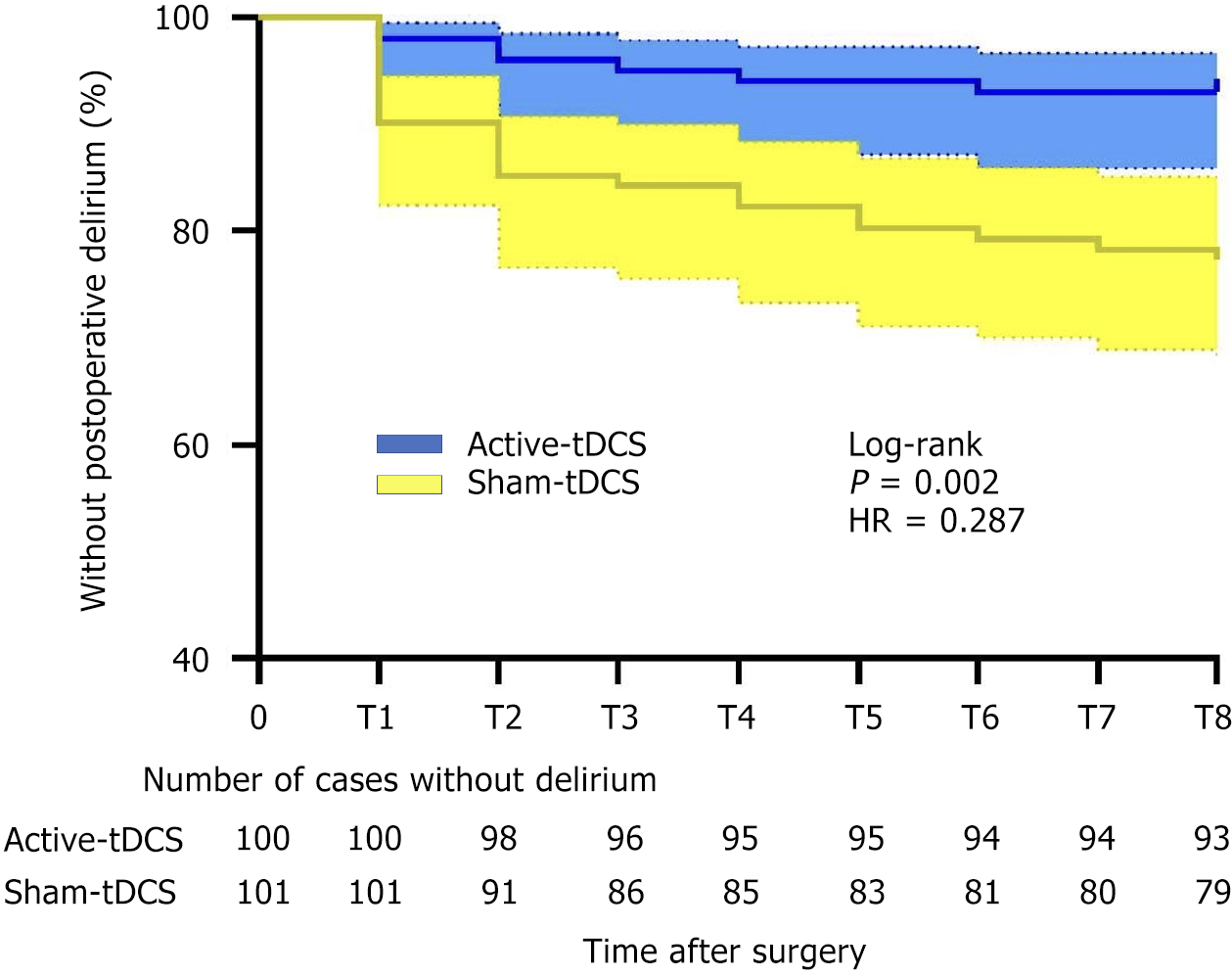
To compare the incidence of POD at different time points, we used Kaplan-Meier curves to analyze the relationship between the incidence of POD and time in both groups. The overall incidence of POD was significantly lower in group A than in group S (log-rank P = 0.002; hazard ratio = 0.287, 95% confidence interval: 0.140-0.586; Figure 2).
Based on previous research, we included 10 potential factors associated with the occurrence of POD in univariate logistic regression analysis[21,22]. These factors included the intervention group, age, the ASA classification, the MMSE score, the sleep score, smoking history, frailty status, surgery duration, preoperative hemoglobin, and albumin levels. Statistical analysis showed that the P values of the intervention group, age, the MMSE score, frailty status, and preoperative albumin levels were < 0.1. Then, we included these five factors in a multivariate logistic regression and found that the P values of the tDCS intervention, the MMSE score, and frailty status were < 0.05, indicating statistical significance (Table 3).
| Characteristics | Univariable analysis unadjusted OR (95%CI) | P value | Multivariable analysis adjusted OR (95%CI) | P value |
| Group | 0.255 (0.104-0.627) | 0.003 | 0.284 (0.111-0.727) | 0.009 |
| Age | 2.889 (1.177-7.089) | 0.021 | 1.994 (0.758-5.241) | 0.162 |
| ASA | 1.665 (0.701-3.954) | 0.248 | - | - |
| MMSE | 0.253 (0.098-0.650) | 0.004 | 0.275 (0.102-0.739) | 0.010 |
| History of smoking | 1.803 (0.826-3.938) | 0.139 | - | - |
| FRAIL | 3.918 (1.596-9.616) | 0.003 | 2.838 (1.091-7.382) | 0.032 |
| Hemoglobin1 | 1.443 (0.627-3.319) | 0.388 | - | - |
| Albumin | 0.474 (0.213-1.057) | 0.068 | 0.599 (0.251-1.430) | 0.248 |
| AIS | 2.000 (0.726-5.512) | 0.180 | - | - |
| Duration of surgery | 1.686 (0.709-4.009) | 0.237 | - | - |
To further explore the efficacy of tDCS under different demographic characteristics, we conducted subgroup analyses incorporating age, the ASA classification, frailty, anemia, albumin, and educational level. The results indicated no significant differences in the therapeutic effects of tDCS across subgroups (Supplementary Figure 3).
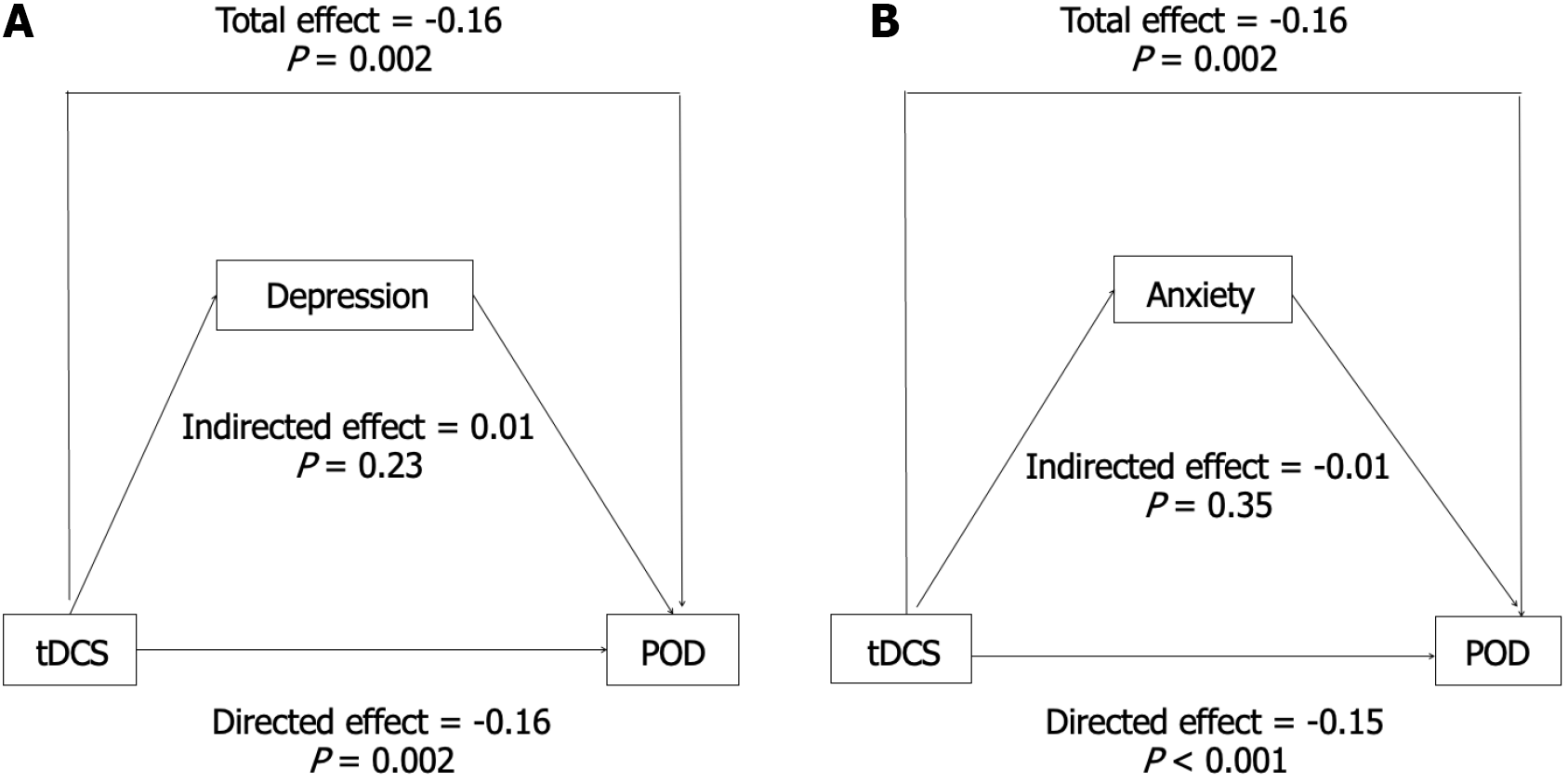
To further explore the potential mechanisms of action of tDCS on POD, we used depression and anxiety scores as mediators in the equation model and conducted mediation effect analysis using R software. Path coefficients for the three variables are shown in Figure 3. tDCS had a direct impact on POD, and neither depression nor anxiety scores served as mediators. Specifically, the mediation effect of anxiety and depression scores accounted for 4.0% and 4.9% of the total effect, respectively (Supplementary Table 2).
This study observed the occurrence of complications within 3 days postoperatively. No significant differences were found between group A and group S. In addition, pain at the stimulation site was identified as the most common adverse reaction to tDCS intervention. The incidence of pain at the stimulation site was 15% in group A and 3% in group S, which was a significant difference (P = 0.003; Supplementary Table 3). However, the pain intensity at the stimulation site was mild, well tolerated by patients, and disappeared immediately after the stimulation was completed.
POD is among the most common postoperative complications and is particularly prevalent in elderly patients. It can directly impact the cognitive function of elderly patients, increasing the risk of postoperative cognitive dysfunction. The likelihood of developing postoperative cognitive dysfunction is 14 times higher in patients with POD than in those without POD[23]. This study used the noninvasive technique of tDCS, which was administered 30 minutes before general anesthesia induction. The results showed that compared with sham stimulation, tDCS significantly reduced the incidence of POD and reduced depressive and anxious emotions on postoperative day 1 in elderly patients undergoing laparoscopic major surgery.
Previous studies have shown that the occurrence of POD is associated with various factors, such as advanced age, surgical trauma, the ASA classification, and preoperative cognitive status[21,22]. Among these, advanced age is an independent risk factor[15]. Research has found that in elderly patients, the brain volume, cortical thickness, and white matter integrity are reduced and the connectivity of brain functional networks is decreased[24]. Poor baseline cognitive status is a strong predictor of perioperative neurocognitive disorder[25]. Gillis et al[26] suggested that preoperative rehabilitation can enhance preoperative physiological reserves, improve surgical tolerance, and promote postoperative recovery[26]. It has been reported that a single session of tDCS can temporarily reverse age-related cognitive decline and functional brain activity changes[8]. These changes are speculated to result from synaptic plasticity changes[12] and regulation of brain network connectivity caused by tDCS[13], which collectively reduce the incidence of POD in elderly patients undergoing lower limb joint replacement[27]. This finding is consistent with our study wherein a single tDCS intervention performed 30 minutes before anesthesia induction still significantly reduced the incidence of POD in elderly patients undergoing major laparoscopic surgery compared to the control group.
However, subsequent multivariate logistic regression analyses revealed that age was not the key factor influencing the occurrence of POD. We believe the reasons may be as follows: (1) The sample size was small, and specifically, there was minimal age variation among patients, thus, the difference caused by age factors was not significant in statistical analyses; and (2) The effect of age on POD is closely related to the degeneration of the central nervous system, which is reflected in cognitive decline and lower MMSE scores. In this study, MMSE scores were among the key factors influencing the occurrence of POD. Furthermore, our subgroup analysis did not show any interaction effect of tDCS efficacy across different subgroups. However, there may be a potential therapeutic advantage in the subgroup with lower MMSE scores and lower education levels, which needs to be further explored in future trials with larger sample sizes.
It is well-known that elderly patients may experience varying degrees of cognitive decline, and the likelihood and severity of cognitive impairment increase with age[28]. The MMSE is a commonly used clinical tool to assess cognitive function, and lower MMSE scores are typically indicative of cognitive impairment[29]. As previously noted, we identified lower preoperative MMSE scores as a risk factor for POD in this study. We consider that surgical and anesthetic trauma may more easily cause further damage to the already frail central nervous system, even though no significant between-group differences in terms of surgical duration and anesthetic medication were identified in our study.
Gastrointestinal surgery patients are often preoperatively frail, and severe preoperative frailty is closely associated with poor clinical outcomes[30]. Gracie et al[31] suggested that preoperative frailty significantly impacts the occurrence of POD and predicts poorer clinical outcomes. Our results also upheld preoperative frailty as a risk factor for POD.
We also found that the depression and anxiety scores on postoperative day 1 were significantly lower in group A than in group S, which is consistent with previous research[32]. Numerous studies have suggested that depression and anxiety result from an imbalance in the left and right dorsolateral prefrontal cortex (DLPFC) and abnormal functioning of the amygdala[33,34]. In the present study, the anodal stimulation applied to the left DLPFC increased cortical excitability and modulated synaptic plasticity, which may be the potential therapeutic effect[35]. Chen et al[35] suggested that preoperative depression and anxiety states significantly increase the incidence of POD; however, upon including depression and anxiety in the mediation analysis, we found that tDCS reduced the incidence of POD directly and not via lowering depression and anxiety scores.
Previous studies have shown that tDCS can alleviate pain symptoms and improve cognitive function in patients[27,36]. However, we identified no significant differences in NRS scores between the two groups. We speculate that this may be due to insufficient stimulation parameters and inadequate number of stimulations in our study and the fact that all patients had adequate postoperative pain relief. In addition, this could also be related to the stimulation site we selected.
In this study, we chose the left DLPFC as the anodal site for tDCS stimulation. The DLPFC is a key component of the frontoparietal network. It is primarily involved in working memory, attention, and executive function and plays a key role in cognitive differences during aging[37,38]. Specifically, the left DLPFC is mainly responsible for working memory, cognitive flexibility, and memory retrieval. Previous research has confirmed using functional magnetic resonance imaging (fMRI) that anodal tDCS increases the general connectivity of the functional networks related to the target brain regions[39]. Few previous studies have investigated the effects of tDCS on POD. To our knowledge, only Tao et al[27] found that a single session of tDCS, using the same stimulation parameters as used by us in the present study, reduced the incidence of POD. This is our reason behind selecting the left DLPFC as the anodal stimulation target. However, due to experimental limitations, we did not use fMRI to observe the specific effects of tDCS. In the future, we can select different stimulation sites to compare the effects of administering tDCS at various stimulation sites on POD in elderly patients.
This study had several limitations. First, this was a single-center study, and protocols and practices of the center may have influenced the outcomes. In the future, we may perform a multicenter study with a large population. Second, the subjects of this study were elderly patients undergoing laparoscopic surgery lasting > 2 hours, which led to a high incidence of POD. Third, we excluded patients who were transferred to the ICU postoperatively and those whose surgical approach was changed during surgery, which does not reflect the clinical characteristics of the study population and may have impacted the incidence of POD[40]. Fourth, we did not use objective measures like imaging techniques (e.g., fMRI and electroencephalography) and biomarkers to further explore the mechanism of tDCS. Fifth, although POD is most likely to occur within the first 3 days postoperatively, the follow-up period in this study may have missed some outcomes. Sixth, although we maintained the PetCO2 within the target range of 35-45 mmHg, neither PetCO2 values nor intra-abdominal pressure were statistically analyzed. This oversight may have resulted in an unaccounted confounding factor.
Although our study shows the potential efficacy of tDCS in reducing POD, we acknowledge the importance of evaluating its clinical feasibility for broader implementation. Regarding operational costs, tDCS devices are relatively affordable compared to other neuromodulation technologies (e.g., transcranial magnetic stimulation or invasive stimulation systems). Moreover, tDCS is characterized by its operational simplicity and portability. The technique can be effectively mastered by non-specialist medical personnel after a brief training, making it particularly suitable for perioperative implementation. We hope that future studies can involve multiple medical institutions, expand the sample size, diversify the types of surgeries, integrate neuroimaging techniques, and further explore the protective effects of tDCS on perioperative cognitive function.
We found that a single session of tDCS before surgery can reduce the incidence of POD. This may represent a new noninvasive technique for protecting perioperative cognitive function in elderly patients; however, further clinical trials are needed to validate our conclusions.
We are thankful to the general surgeons and nursing staff of the Affiliated Hospital of Xuzhou Medical University.
| 1. | Vasunilashorn SM, Dillon ST, Marcantonio ER, Libermann TA. Application of Multiple Omics to Understand Postoperative Delirium Pathophysiology in Humans. Gerontology. 2023;69:1369-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, Cherubini A, Jones C, Kehlet H, MacLullich A, Radtke F, Riese F, Slooter AJ, Veyckemans F, Kramer S, Neuner B, Weiss B, Spies CD. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 745] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 3. | Zhang H, Lu Y, Liu M, Zou Z, Wang L, Xu FY, Shi XY. Strategies for prevention of postoperative delirium: a systematic review and meta-analysis of randomized trials. Crit Care. 2013;17:R47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Peden CJ, Miller TR, Deiner SG, Eckenhoff RG, Fleisher LA; Members of the Perioperative Brain Health Expert Panel. Improving perioperative brain health: an expert consensus review of key actions for the perioperative care team. Br J Anaesth. 2021;126:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 5. | Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, Vanneste S, Ziemann U, Garcia-Larrea L, Paulus W. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128:56-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 1267] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 6. | Nitsche MA, Fregni F. Transcranial Direct Current Stimulation - An Adjuvant Tool for the Treatment of Neuropsychiatric Diseases? Curr Psychiatry Rev. 2007;3:222-232. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Summers JJ, Kang N, Cauraugh JH. Does transcranial direct current stimulation enhance cognitive and motor functions in the ageing brain? A systematic review and meta- analysis. Ageing Res Rev. 2016;25:42-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Meinzer M, Lindenberg R, Antonenko D, Flaisch T, Flöel A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J Neurosci. 2013;33:12470-12478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 9. | Medeiros LF, de Souza IC, Vidor LP, de Souza A, Deitos A, Volz MS, Fregni F, Caumo W, Torres IL. Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry. 2012;3:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Cheng JY, Wang Z. [Progress in mechanism of transcranial direct current stimulation]. Shanghai Jiao Tong Daxue Xuebao. 2022;42:952-957. [DOI] [Full Text] |
| 11. | Mielke D, Wrede A, Schulz-Schaeffer W, Taghizadeh-Waghefi A, Nitsche MA, Rohde V, Liebetanz D. Cathodal transcranial direct current stimulation induces regional, long-lasting reductions of cortical blood flow in rats. Neurol Res. 2013;35:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1248] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 13. | Polanía R, Nitsche MA, Paulus W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp. 2011;32:1236-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 333] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 14. | Lebedev VP, Malygin AV, Kovalevski AV, Rychkova SV, Sisoev VN, Kropotov SP, Krupitski EM, Gerasimova LI, Glukhov DV, Kozlowski GP. Devices for noninvasive transcranial electrostimulation of the brain endorphinergic system: application for improvement of human psycho-physiological status. Artif Organs. 2002;26:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Yashwashi T, Kaman L, Kajal K, Dahiya D, Gupta A, Meena SC, Singh K, Reddy A. Effects of low- and high-pressure carbon dioxide pneumoperitoneum on intracranial pressure during laparoscopic cholecystectomy. Surg Endosc. 2020;34:4369-4373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Asaad OM. Different ventilation techniques and hemodynamic optimization to maintain regional cerebral oxygen saturation (rScO(2)) during laparoscopic bariatric surgery: a prospective randomized interventional study. J Anesth. 2018;32:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Russo R, Wallace D, Fitzgerald PB, Cooper NR. Perception of comfort during active and sham transcranial direct current stimulation: a double blind study. Brain Stimul. 2013;6:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3380] [Cited by in RCA: 3601] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 20. | Lee C, Lee CH, Lee G, Lee M, Hwang J. The effect of the timing and dose of dexmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: A double blind randomized controlled study. J Clin Anesth. 2018;47:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Sadeghirad B, Dodsworth BT, Schmutz Gelsomino N, Goettel N, Spence J, Buchan TA, Crandon HN, Baneshi MR, Pol RA, Brattinga B, Park UJ, Terashima M, Banning LBD, Van Leeuwen BL, Neerland BE, Chuan A, Martinez FT, Van Vugt JLA, Rampersaud YR, Hatakeyama S, Di Stasio E, Milisen K, Van Grootven B, van der Laan L, Thomson Mangnall L, Goodlin SJ, Lungeanu D, Denhaerynck K, Dhakharia V, Sampson EL, Zywiel MG, Falco L, Nguyen AV, Moss SJ, Krewulak KD, Jaworska N, Plotnikoff K, Kotteduwa-Jayawarden S, Sandarage R, Busse JW, Mbuagbaw L. Perioperative Factors Associated With Postoperative Delirium in Patients Undergoing Noncardiac Surgery: An Individual Patient Data Meta-Analysis. JAMA Netw Open. 2023;6:e2337239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 22. | He M, Zhu Z, Jiang M, Liu X, Wu R, Zhou J, Chen X, Liu C. Risk Factors for Postanesthetic Emergence Delirium in Adults: A Systematic Review and Meta-analysis. J Neurosurg Anesthesiol. 2024;36:190-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Han L, Dong MM, Sun QC, Han Y, Cao JL. [Research progress of biomarkers of postoperative cognitive dysfunction]. Guoji Mazuixue Yu Fusu Zazhi. 2019;40:602-609. [DOI] [Full Text] |
| 24. | Onoda K, Ishihara M, Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci. 2012;24:2186-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 25. | O'Gara BP, Gao L, Marcantonio ER, Subramaniam B. Sleep, Pain, and Cognition: Modifiable Targets for Optimal Perioperative Brain Health. Anesthesiology. 2021;135:1132-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 26. | Gillis C, Ljungqvist O, Carli F. Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br J Anaesth. 2022;128:434-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 27. | Tao M, Zhang S, Han Y, Li C, Wei Q, Chen D, Zhao Q, Yang J, Liu R, Fang J, Li X, Zhang H, Liu H, Cao JL. Efficacy of transcranial direct current stimulation on postoperative delirium in elderly patients undergoing lower limb major arthroplasty: A randomized controlled trial. Brain Stimul. 2023;16:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 28. | Huang F, Zhang M, Wang S. Changes in cognitive function among older adults: A latent profile transition analysis. Arch Gerontol Geriatr. 2019;80:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Pradier C, Sakarovitch C, Le Duff F, Layese R, Metelkina A, Anthony S, Tifratene K, Robert P. The mini mental state examination at the time of Alzheimer's disease and related disorders diagnosis, according to age, education, gender and place of residence: a cross-sectional study among the French National Alzheimer database. PLoS One. 2014;9:e103630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Cooper Z, Rogers SO Jr, Ngo L, Guess J, Schmitt E, Jones RN, Ayres DK, Walston JD, Gill TM, Gleason LJ, Inouye SK, Marcantonio ER. Comparison of Frailty Measures as Predictors of Outcomes After Orthopedic Surgery. J Am Geriatr Soc. 2016;64:2464-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Gracie TJ, Caufield-Noll C, Wang NY, Sieber FE. The Association of Preoperative Frailty and Postoperative Delirium: A Meta-analysis. Anesth Analg. 2021;133:314-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 32. | Li C, Tao M, Chen D, Wei Q, Xiong X, Zhao W, Tan W, Yang J, Han Y, Zhang H, Zhang S, Liu H, Cao JL. Transcranial Direct Current Stimulation for Anxiety During Laparoscopic Colorectal Cancer Surgery: A Randomized Clinical Trial. JAMA Netw Open. 2024;7:e246589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 34. | Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 681] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 35. | Chen A, An E, Yan E, Saripella A, Khullar A, Misati G, Alhamdah Y, Englesakis M, Mah L, Tartaglia C, Chung F. Prevalence of preoperative depression and adverse outcomes in older patients undergoing elective surgery: A systematic review and meta-analysis. J Clin Anesth. 2024;97:111532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 36. | Borckardt JJ, Romagnuolo J, Reeves ST, Madan A, Frohman H, Beam W, George MS. Feasibility, safety, and effectiveness of transcranial direct current stimulation for decreasing post-ERCP pain: a randomized, sham-controlled, pilot study. Gastrointest Endosc. 2011;73:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Shaw EE, Schultz AP, Sperling RA, Hedden T. Functional Connectivity in Multiple Cortical Networks Is Associated with Performance Across Cognitive Domains in Older Adults. Brain Connect. 2015;5:505-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Li HJ, Hou XH, Liu HH, Yue CL, Lu GM, Zuo XN. Putting age-related task activation into large-scale brain networks: A meta-analysis of 114 fMRI studies on healthy aging. Neurosci Biobehav Rev. 2015;57:156-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 39. | Kim K, Sherwood MS, McIntire LK, McKinley RA, Ranganath C. Transcranial Direct Current Stimulation Modulates Connectivity of Left Dorsolateral Prefrontal Cortex with Distributed Cortical Networks. J Cogn Neurosci. 2021;33:1381-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Ito K, Suka Y, Nagai M, Kawasaki K, Yamamoto M, Koike D, Nomura Y, Tanaka N, Kawaguchi Y. Lower risk of postoperative delirium using laparoscopic approach for major abdominal surgery. Surg Endosc. 2019;33:2121-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/