Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.107936
Revised: May 7, 2025
Accepted: July 23, 2025
Published online: October 19, 2025
Processing time: 178 Days and 19.7 Hours
The glymphatic system, a recently discovered cerebrospinal fluid-mediated pathway, plays a crucial role in fluid exchange and waste clearance in the brain. Its dysfunction has been implicated in various neurological disorders, including Alzheimer’s disease and traumatic brain injury. Recent studies suggest that alcohol intake has a biphasic effect on the glymphatic system: Low doses of alcohol enhance glymphatic function, whereas high doses lead to glymphatic suppression and cognitive decline, mirroring patterns seen in alcohol-related dementia, providing valuable insights into the dose-dependent effects of alcohol on glymphatic function, but significant gaps persist, particularly regarding the mechanistic understanding and the influence of confounding factors such as sex, age, blood pressure, and wakefulness. Here, we synthesize and critically evaluate the important research findings within this field to gauge its progress and identify new research opportunities. We discuss the specific mechanisms by which alcohol affects the glymphatic system, including how alcohol influences cerebrospinal fluid-interstitial fluid exchange and waste removal. We also discuss the potential of the glymphatic system as a new target, such as through pharmacological or lifestyle interventions aimed at enhancing glymphatic function to treat alcohol use disorder and other neurological disorders associated with glymphatic dysfunction.
Core Tip: The glymphatic system, a critical pathway for fluid exchange and waste clearance in the brain, is linked to neurological disorders when dysfunctional. Alcohol has a biphasic effect: Low doses enhance glymphatic function, while high doses suppress it and cause cognitive decline, mirroring alcohol-related dementia. Studies explore how alcohol affects cerebrospinal fluid-interstitial fluid exchange and waste removal. Despite progress, gaps remain in understanding mechanisms and confounding factors like sex, age, and blood pressure. The significance of this review lies in deepening the understanding of the relationship between alcohol and glymphatic system disorders and offering possible targets for developing new treatments.
- Citation: Lin JY, Zhang HB, Luo L, Li RJ, Wang XG. Glymphatic system dysfunction in alcohol use disorder: Current understanding and future directions. World J Psychiatry 2025; 15(10): 107936
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/107936.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.107936
Alcohol is the most commonly used addictive substance worldwide. Alcohol use disorder (AUD), a prevalent psychiatric condition, is characterized by compulsive alcohol consumption, loss of control over drinking, and withdrawal-related negative emotions[1]. It poses a significant threat to public health, contributing to more than 140000 deaths annually in the United States[2]. The history of mechanistic understanding of AUD is long, complex, and full of controversies. Records from ancient biblical, Egyptian, and Babylonian sources document early recognition of alcohol misuse. By the 18th century, habitual intoxication was moralized as weakness, necessitating punitive re-education (moral model). The 19th century temperance movement reframed AUD as a pharmacological vulnerability, advocating regulatory control. Modern biological frameworks emerged in the 20th century, culminating in Leshner’s “brain disease model”, which recognized AUD as a neurobiological disorder with behavioral and social dimensions[3]. Recently emerging research highlights the glymphatic system in AUD pathophysiology. This system, newly identified as a mechanism responsible for clearing metabolic waste in the brain, can lead to the accumulation of metabolic waste when dysfunctional, thereby affecting cognitive function and potentially exacerbating the progression of neurodegenerative diseases[4,5]. Currently, the effects of alcohol on the glymphatic system have not been fully summarized.
This review examines alcohol’s potential mechanisms of action on glymphatic regulation, aiming to identify novel therapeutic targets for patients with AUD with glymphatic impairment.
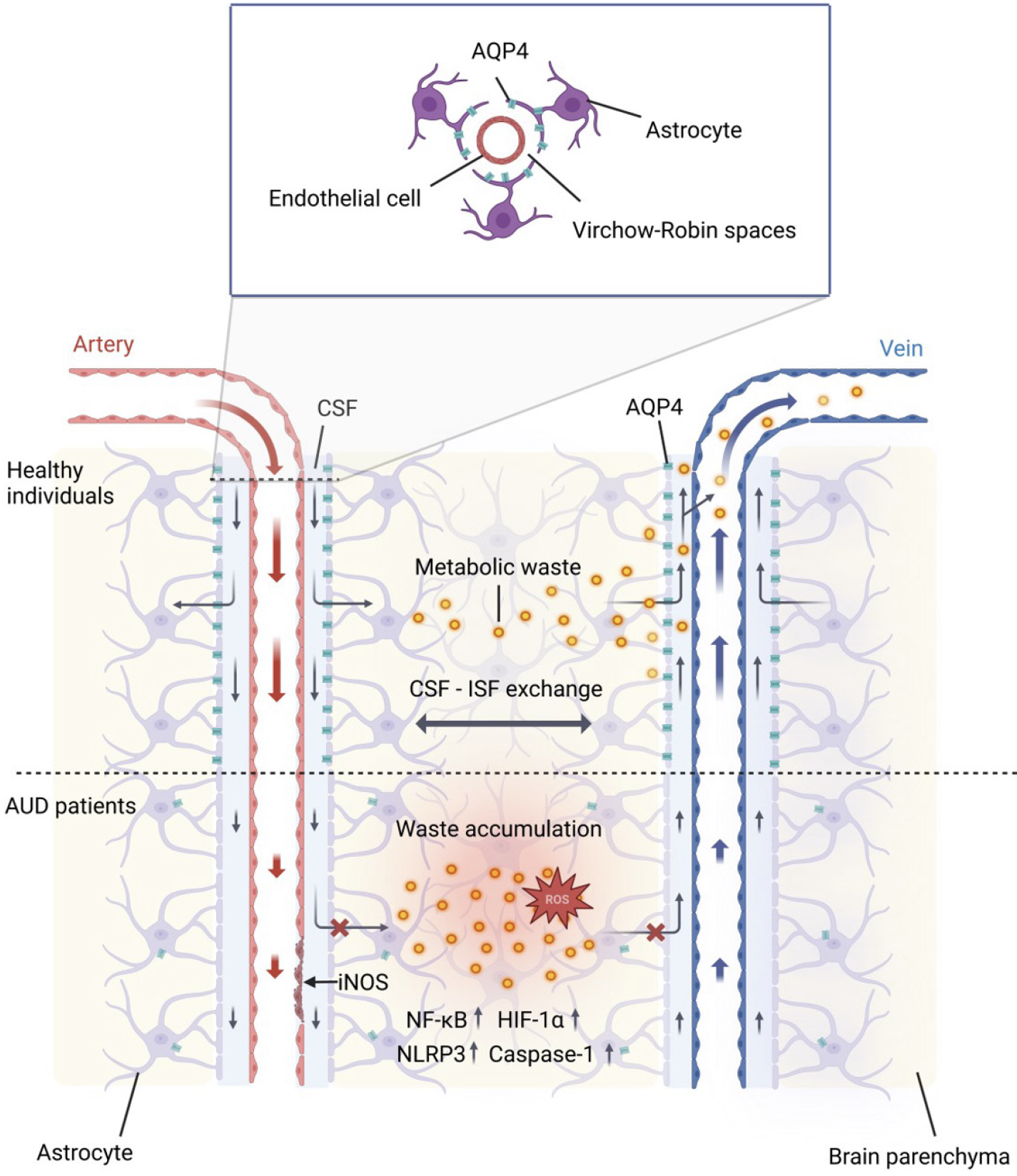
In 2012, Iliff et al[6] discovered that cerebrospinal fluid (CSF) enters the brain parenchyma via perivascular spaces around arteries, and interstitial fluid (ISF) is cleared along paravenous pathways. This system, resembling a brain lymphatic system and reliant on astrocytes, was named the glymphatic system. It comprises three components: CSF inflow pathways (PVSs/Virchow-Robin spaces[7] around arteries), ISF outflow pathways (around veins), and fluid exchange pathways connecting the two, mediated by aquaporin 4 (AQP4) on astrocyte endfeet (Figure 1)[8].
The Virchow-Robin space, formed by endothelial cells, basement membrane, astrocyte endfeet, and extracellular fluid, serves as the CSF entry pathway[9]. As pial arteries penetrate the brain parenchyma, arterial pulsations and AQP4 water channels on astrocyte endfeet drive CSF influx[10-12], where it mixes with ISF containing metabolic waste. Waste-laden fluid is subsequently cleared through three efflux routes: Along cranial/spinal nerve sheaths to lymphatic vessels[13,14], along olfactory nerves to nasal lymphatics[15], or absorption by arachnoid granulations into venous blood[16,17]. By maintaining ISF homeostasis and cerebral metabolic balance via convective CSF transport, the glymphatic system prevents neurotoxic waste accumulation. Dysfunction in this system is implicated in neuroinflammation and neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD).
The dose-effect and time-effect of alcohol on the glymphatic system remain unclear. Lundgaard et al[18] found that both acute and chronic low-dose alcohol exposure improved glymphatic system function without affecting the polarization of AQP4. Additionally, chronic high-dose alcohol exposure reduced glial fibrillary acidic protein (GFAP) expression. He proposed that these improvements may be attributed to low-dose alcohol reducing neuroinflammation and enhancing cerebral arterial pulsatility[18]. In contrast, acute high-dose alcohol exposure significantly inhibited glymphatic function[18,19]. Lundgaard et al[18] attributed this inhibition to reduced cardiac output and pulse pressure caused by acute alcohol intoxication, leading to diminished CSF flow. However, Liu et al[19] suggested that elevated β-endorphin release and reduced cerebrovascular pulsatility following acute alcohol consumption played an important role[19]. Notably, neither acute high-dose nor chronic low-dose exposure affects GFAP or AQP4 expression.
In a mouse model, chronic high-dose alcohol exposure increased GFAP expression, facilitated AQP4 polarization, promoted reactive astrocytosis and enlarged PVS, and inhibited glymphatic function[18-20]. In human clinical samples, chronic high-dose alcohol consumption has been shown to impair the glymphatic system. Histopathological examination of the brains of chronic alcoholics reveals enlargement of Virchow-Robin spaces, which may be associated with personality changes[21]. Consistently, patients exposed to alcohol long term with alcohol-related brain damage demonstrate a significant enlargement PVS in the frontal cortex and basal ganglia, indicating that glymphatic function may be impaired[20]. We recently investigated whether diffusion tensor imaging (DTI) along the PVS (ALPS) index can used as a translational biomarker for glymphatic dysfunction in AUD. The results showed that the AUD group had a lower ALPS index than the control group, and there was a positive correlation between the ALPS index and Montreal Cognitive Assessment/Mini-Mental State Examination scores, suggesting that chronic high-dose alcohol consumption can induce glymphatic impairment and cognitive deficits[22]. However, longitudinal studies are needed to disentangle causal relationships in the future. Consistently, it was found that individuals with AUD exhibited widespread astrocytic reactive proliferation and neuroinflammation[23]. In summary, these studies highlight the dose-dependent and time-dependent effects of alcohol on the glymphatic system. Acute and chronic low-dose alcohol exposure improved glymphatic function, while acute and chronic high-dose exposure inhibited it, albeit through differing mechanisms.
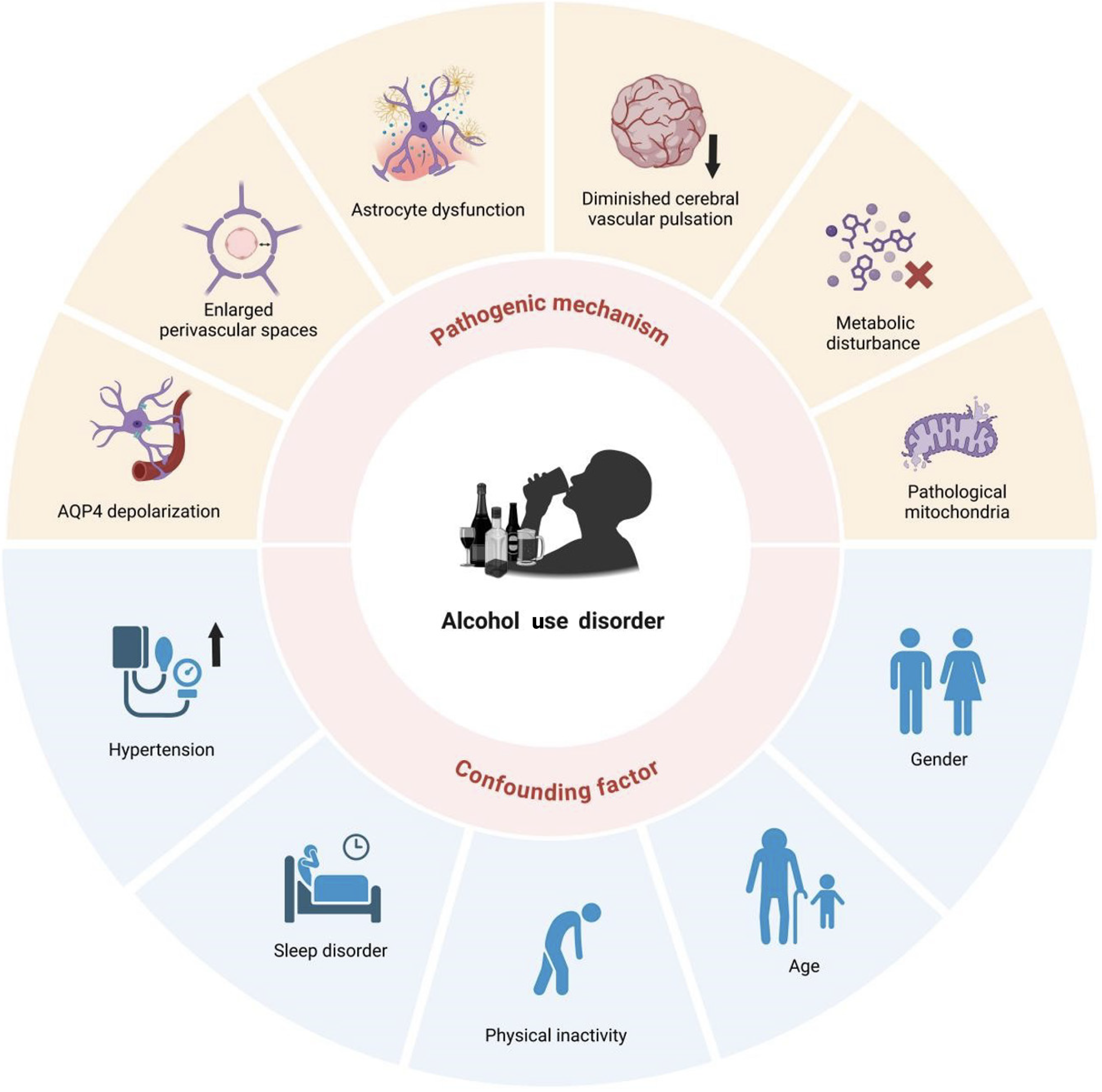
Alcohol induces the dysfunction of the glymphatic system through a variety of mechanisms, including the loss of AQP4 polarization, expansion of the PVS, astrocyte dysfunction, reduced cerebrovascular pulsation, metabolic disturbance, and mitochondrial dysfunction (Figure 2).
Alcohol disrupts the spatial distribution of AQP4 in astrocytes. Chronic alcohol consumption reduces AQP4 polarization, characterized by diminished localization to perivascular foot processes and increased expression in the brain parenchyma. This depolarization causes irreversible impairment of glymphatic fluid flow[18,19]. A study indicates that long-term drinking (intragastric administration of alcohol for 2 months in mice) downregulates AQP4 expression, increased blood-brain barrier permeability, and aggravated cognitive decline[24]. However, another study found that “binge drinking” induces brain edema and neuroinflammation by upregulating AQP4 in astrocytes in the hippocampal-entorhinal cortical[25,26]. These findings indicate that short-term and long-term drinking have different effects on the regulation of AQP4 expression.
Moreover, alcohol’s regulation of astrocytic AQP4 varies across disease conditions. In traumatic brain injury (TBI), pre-injury ethanol exposure increases post-trauma AQP4, particularly in astrocyte endfeet, and activates transcription factors nuclear factor-κB and hypoxia-inducible factor-1α, worsening brain edema and neuroinflammation[27,28]. Conversely, post-trauma alcohol consumption significantly reduces AQP mRNA and protein levels, correlating with improved brain edema[29]. These opposing outcomes likely reflect differences in alcohol exposure timing (pre-injury vs post-injury) and the dynamic regulatory interplay in disease states, necessitating further mechanistic investigation. In summary, alcohol’s effects on AQP4 are highly complex and influenced by dosage, duration, and pathological conditions.
Alcohol directly impairs glymphatic function by disrupting astrocyte activity. Chronic alcohol consumption promotes reactive astrocyte proliferation, a hallmark of central nervous system injury, which is associated with neuroinflammation, glymphatic dysfunction, and AQP4 depolarization[18,21,30,31]. Mechanistically, alcohol activates astrocytic connexin 43 hemichannels and pannexin 1 channels, driving the release of inflammatory cytokine, ATP, and glutamate[32]. Moreover, alcohol upregulates cyclooxygenase-2 and inducible nitric oxide synthase expression via nuclear factor kappa B activation[33]. Additionally, alcohol promotes mitochondrial reactive oxygen species-mediated NOD-like receptor protein 1/caspase-1 pathway activation and interleukin 1β/interleukin 18 release in astrocytes[34]. Alcohol also induces cell death. Moderate alcohol doses significantly increase GFAP mRNA level, suggesting exacerbated neuronal damage[35]. Furthermore, alcohol triggers astrocytic oxidative damage via mechanisms such as cytochrome P4502E1 upregulation, reactive oxygen species induction, and glutathione depletion[36].
The Virchow-Robin space is critical for exchanging substances between CSF and brain parenchyma. PVS dilation can impede CSF flow and disrupt the glymphatic function[37]. Consistently, clinical studies show that patients with alcohol-related brain damage have significantly enlarged PVS in the frontal cortex and basal ganglia[20]. Histopathological examinations of chronic alcohol abusers’ brains also reveal Virchow-Robin space enlargement across various brain regions[21]. A cohort study also identified alcohol consumption as a risk factor for PVS expansion in the centrum semiovale of patients with spontaneous cerebral hemorrhage[38]. Animal models corroborate these findings, with long-term alcohol-exposed mice exhibiting PVS enlargement and impaired glymphatic drainage[20]. However, Deike et al[39] showed that drinking habits do not affect the PVS volume fraction (PVS volume/brain parenchyma volume). Another epidemiological study involving 5000 healthy middle-aged individuals showed that drinking history does not affect basal ganglia and centrum semiovale PVS enlargement[40]. Therefore, the relationship between alcohol and PVS still requires further investigation.
Cerebral vascular pulsation is a critical driver of glymphatic fluid exchange and waste clearance[41], and it can enhance CSF axial and radial diffusion coefficients[42]. A previous study showed that unilateral internal carotid artery ligation reduced arterial pulsation by approximately 50% and slowed perivascular CSF-ISF exchange. Conversely, systemic adrenergic agonist dobutamine administration increased arterial pulsation by 60%, boosting perivascular the CSF-ISF exchange[10]. Furthermore, it was found that vascular lesions such as arteriosclerosis and perivascular remodeling caused by chronic hypertension altered the cerebral arterial pulsation and impaired the glymphatic function[43]. Additionally, a study showed that carotid artery stenosis reduced vascular pulsation, impaired glymphatic function, and may lead to cognitive impairment[44].
Alcohol consumption impacts arterial pulsation. Acute moderate alcohol intake (2.0 g/kg/day for 4 weeks, intraperitoneal injection) in mice increases β-endorphin release, reduces cerebral vascular pulsation, and impairs glymphatic function[19]. It was found that 0.75 g/kg ethanol consumption increases systolic blood pressure, diastolic blood pressure, and mean blood flow velocity, while significantly reducing the middle cerebral artery pulsation index[45]. Thus, reduced cerebral vascular pulsation may be a key pathway through which alcohol regulates glymphatic function.
Alcohol consumption perturbs homeostatic metabolic processes and may induce systemic metabolic dysregulation. Hepatic ethanol metabolism constitutes the principal source of circulating acetate. Alcohol-derived acetate modulates neuronal histone acetylation through chromatin-bound acetyl-CoA synthetase 2, driving the transcription of synaptic plasticity-related genes, and facilitates ethanol-induced conditioned place preference[46]. Alcohol may also induce brain metabolic disturbance by reducing omega-3 (n-3) fatty acid[47]. Docosahexaenoic acid, the predominant n-3 fatty acid in neural membranes, structurally stabilizes lipid bilayers while modulating membrane protein activity, intracellular signaling, and transcriptional regulation. Recent reports support the protective effects of n-3 fatty acids on the glymphatic system. Chronic unpredictable mild stress induced depression-associated glymphatic impairment in mice, manifesting as decreased cerebral arterial pulsatility, reduced vascular compliance, and perivascular AQP4 depolarization[48]. Notably, supplementation of polyunsaturated fatty acid (PUFA) alleviated neuroinflammation and glymphatic dysfunction in mice, ultimately improving cognitive abilities[48]. Studies indicate that n-3 PUFA improves the brain lymphatic clearance rate in mice possibly by reducing amyloid-β (Aβ) 42 accumulation[49,50]. Additionally, genetic variation in n-3 PUFA biosynthesis genes (glucokinase regulator, fatty acid desaturase 2 enzyme, acyl-CoA oxidase 1) is associated with AUD diagnosis, and externalizing behaviors[51], implicating endogenous lipid metabolism in addiction vulnerability. Thus, alcohol may indirectly disrupt glymphatic function by altering n-3 fatty acid levels.
Alcohol can induce brain mitochondrial dysfunction via multiple mechanisms, thereby impairing glymphatic function. High ethanol exposure alters mitochondrial permeability transition pore component expression, resulting in the impairment of mitochondrial bioenergetics[52]. Alcohol also reduces the activity of mitochondrial complexes I, III, and IV, inhibits Na+/K+-ATPase function, and depletes cardiolipin content, which is critical for maintaining oxidative phosphorylation and coupled respiration[53].
Notably, chronic alcohol exposure dysregulates genes regulating mitochondrial energy metabolism and neurodegeneration pathways linked to neurodegenerative diseases like AD and PD[54]. Given the glymphatic system’s role in neurodegenerative pathology, alcohol-induced mitochondrial damage may exacerbate glymphatic dysfunction, potentially accelerating neuropsychiatric disease progression. Supporting this hypothesis, patients with PD exhibit a significantly reduced ALPS index[55] and increased mitochondrial DNA levels, indicating glymphatic dysfunction is linked to oxidative stress[56]. Similarly, in patients with idiopathic intracranial hypertension, pathological astrocyte endfoot mitochondria significantly increase and are positively correlated with astrocyte reactive proliferation[57]. These studies indicate that mitochondrial dysfunction may cause glymphatic system dysfunction.
Given numerous comorbid or confounding factors also modulate the glymphatic system, we explored the complex interconnections between AUD, the glymphatic system, and confounding factors such as hypertension, sleep disorders, lack of exercise, age, and sex (Figure 2).
Studies indicate a significantly increased risk of hypertension in patients with AUD[58]. Hypertension may impair glymphatic function through specific mechanisms. A study used dynamic contrast-enhanced magnetic resonance imaging to assess the impact of hypertension on brain glymphatic transport kinetics in young/adult spontaneously hypertensive male rats vs age-matched normotensive Wistar-Kyoto rats, and found the glymphatic transport was significantly impaired in spontaneously hypertensive male rats[43]. Another study demonstrated that increased blood pressure increases reflux by reducing arterial wall pulsatility, thereby decreasing PVS net flow[59]. Moreover, a clinical study calculated the ALPS index along blood vessels in hypertension and healthy control groups, finding that the hypertension group exhibit a significantly lower average ALPS index than the control group. The average PVS analysis index in all subjects was significantly negatively correlated with the blood pressure[60]. Consistently, a study found that intensive systolic blood pressure treatment, targeting a reduction to less than 120 mmHg[61], decreases the PVS volume fraction (PVS volume/total regional volume)[62].
Sleep disorders are highly prevalent among individuals with AUD[63]. Alcohol disrupts sleep through mechanisms like damaging the electrophysiological sleep structure, causing insomnia, circadian rhythm abnormalities, short sleep duration, reduced slow-wave sleep duration, and increased respiratory-related sleep disorders[64-66]. Patients with AUD have a higher risk of obstructive sleep apnea, linked to glymphatic dysfunction[67]. Studies also found that patients with obstructive sleep apnea have a significantly lower ALPS index than healthy controls, which was significantly negatively correlated with the apnea-hypopnea index and sleep N period oxygen saturation index[68-70]. Alcohol may disrupt sleep homeostasis, causing sleep fragmentation and affecting glymphatic function[71]. The sleep fragmentation group had a lower ALPS index than the healthy control group, and it was negatively correlated with the Pittsburgh Sleep Quality Index[72], a self-administered questionnaire evaluating sleep quality and patterns over the past month[73]. These findings suggest that AUD may indirectly impairs human glymphatic function through sleep disorders. Thus, treating sleep disorders and AUD is crucial for preventing potential cognitive decline.
Exercise is crucial for glymphatic system regulation[74], and AUD may indirectly affect this system through physical inactivity. Studies have shown that individuals with AUD exhibit a significant decline in physical fitness and impaired motor function, which is specifically manifested in the shortened distance of the 6-minute walk test[75-77]. The benefits of motor behavior on the glymphatic system are well-documented. Exercise enhances glymphatic function and Aβ clearance by inhibiting transient receptor potential vanilloid 4 expression, promoting AQP4 polarization, and regulating astrocyte phenotype[78]. Similarly, swimming training enhances hippocampal glymphatic clearance of Aβ deposition by upregulating laminin subunit alpha 1 and dystrophin 71 transcription, reducing AQP4 depolarization, and enhancing CSF-ISF exchange[79]. Given exercise effectively repairs and improves glymphatic function, physical inactivity in patients with AUD may worsen glymphatic dysfunction.
Sex differences significantly influence AUD susceptibility, driven by variations in sex hormone signaling and neuroanatomical organization[80,81]. A study showed sex-specific mechanisms and functional manifestations of alcohol exposure on astrocytes. In females, alcohol exposure activated astrocytes, increased pro-inflammatory cytokine tumor necrosis factor expression, decreased neuroprotective cytokine transforming growth factor-β1 expression, impaired bioenergetics, and reduced excitatory amino acid uptake. By contrast, male mice exhibit astrocyte inactivation marked by reduced GFAP expression[82]. Thus, sex differences must be considered in studying and treating alcohol-mediated glymphatic dysfunction.
Studies have shown that aging is linked to a sharp decline in subarachnoid CSF and brain parenchyma exchange efficiency in mice. Compared to young mice, aged mice exhibit a 40% reduction in injected Aβ clearance rate, a 27% decrease in cortical small artery vascular wall pulsation, and widespread AQP4 depolarization around penetrating arteries[83]. Moreover, aging affects the balance between glymphatic system inflow and outflow. Human studies reveal an age-dependent imbalance in glymphatic dynamics. Participants over 45 exhibit higher glymphatic inflow but significantly lower outflow compared to younger adults (ages 21-38). This mismatch may result from age-related changes of the arterial pulsation and AQP4 polarization[84].
Although accumulating evidence implicates glymphatic dysfunction in the pathogenesis of AUD, few studies have directly targeted this system for therapeutic intervention. However, emerging research on glymphatic modulation in other neuropsychiatric disorders may provide valuable insights for developing novel interventions against AUD. A previous study found that AUD increases cerebral Aβ deposition[85] and AD risk[86]. Notably, AD progression involves glymphatic impairment, indicating a bidirectional relationship between AUD, AD, and glymphatic dysfunction. For example, studies have shown that the DTI-ALPS index in patients with AD is negatively correlated with positron emission tomography-imaged amyloid and tau deposition and positively correlated with cognitive scores[87-90]. Moreover, similar to AUD, AQP4 mislocation occurs in both AD animal models and patients[91,92]. TBI is a brain functional disorder or pathological change caused by external physical forces[93]. It is frequently accompanied by glymphatic system dysfunction[94]. A study using the DTI-ALPS method assessed glymphatic system activity in patients with TBI. The results showed that patients with TBI had a significantly lower ALPS index than healthy controls. Multivariate analysis indicated that subarachnoid hemorrhage and diffuse axonal injury were associated with a lower ALPS index[95]. Thus, the glymphatic system may be an emerging target for AD and TBI treatment. Recently, various interventions, such as low-intensity ultrasound, electroacupuncture, fingolimod, etc., have been confirmed to improve pathological conditions by regulating glymphatic function (Table 1)[96-105]. These emerging glymphatic-focused approaches are poised to serve as investigative tools in animal models to elucidate the role of glymphatic dysfunction in AUD pathogenesis, and also as promising clinical strategies for translating these mechanistic insights into effective AUD treatments.
| Treatment | Outcome | Mechanism | Ref. |
| High-intensity interval training | Alleviate cognitive dysfunction of STZ-induced AD rats, promote clearance of abnormal tau and Aβ | Shift astrocyte phenotype from A1 to A2, improve AQP4 polarization | [96] |
| Electroacupuncture therapy | Improve cognitive function of SMAP8 AD model mice, mitigate neuropathological damage in brain tissue | Suppress astrocyte reactivity, improve AQP4 polarity | [97] |
| VLIUS | Enhance influx of CSF tracer into perivascular spaces, promote clearance of brain interstitial solutes | TRPV4 in astrocytes activated by VLIUS, induce Ca2+ influx and CaM activation to promote AQP4 polarization | [98] |
| OAB-14 | Improve cognitive impairment of 9-10-month-old APP/PS1 transgenic mice, promote Aβ clearance | Upregulate PPARγ-P2X7r-AQP4 pathway to increase AQP4 expression, promote AQP4 polarization by raising Agustin, SNTA-1 and ABCA1 Levels | [99] |
| L-3-n-butylphthalide | Enhance glymphatic clearance rate of APP/PS1 mice, reduce parenchymal Aβ deposition | Increase brain pulsatility, improve perivascular AQP4 Localization | [100] |
| Yuanzhi powder | Clear excessive Aβ deposits, improve cognitive defects and pathological damage of APP/PS1 mice | Improve AQP4 polarity distribution, upregulate transport function of MLV | [101] |
| Nano-plumber encapsulating pro-DHA and VEGF-C | reverse inflammatory microenvironment, enhance glymphatic system function | Reverse microglial inflammatory phenotype, restore phagocytic ability and protect vasculature, stimulate angiogenesis of meningeal lymphatics | [102] |
| GLP-1R agonists | Improve post-traumatic cognitive function in mice, increase fluid drainage in perivascular spaces | Suppress reactive gliosis, improve AQP4 polarization, reduce axonal injury and neuronal apoptosis | [103] |
| Cannabidiol | Improve motor, memory and cognitive functions, promote clearance of p-tau and Aβ | Improve AQP4 polarization, reduce inflammation, increase cerebral blood flow | [104] |
| Fingolimod | Reduce brain edema of DBI rats, mitigate axonal injury, restore glymphatic function | Improve AQP4 polarization, reduce inflammation, increase cerebral blood flow | [105] |
Alcohol exerts dose-dependent and time-dependent effects on the glymphatic system. In AUD, glymphatic dysfunction exacerbates neuroinflammation, metabolic dysregulation, and neurodegeneration. Current evidence on the effects of alcohol on the glymphatic system predominantly stems from animal studies that adopt administration paradigms discordant with the pathophysiological features of human chronic alcohol intoxication. Future studies should prioritize developing chronic alcohol intoxication animal models with higher translational relevance, such as long-term voluntary alcohol consumption models. To systematically delineate the dose-dependent, temporal, and modality-specific impacts of alcohol exposure on glymphatic dynamics, large-scale longitudinal cohort studies are imperative. These should integrate multimodal neuroimaging approaches to quantify spatiotemporal variations in glymphatic clearance efficiency. Such investigations will advance mechanistic insights into alcohol-associated neurodegenerative processes and inform the development of glymphatic-targeted therapeutic interventions.
| 1. | Koob GF. Alcohol Use Disorder Treatment: Problems and Solutions. Annu Rev Pharmacol Toxicol. 2024;64:255-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 2. | Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from Excessive Alcohol Use - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2024;73:154-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 3. | Nathan PE, Conrad M, Skinstad AH. History of the Concept of Addiction. Annu Rev Clin Psychol. 2016;12:29-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Lohela TJ, Lilius TO, Nedergaard M. The glymphatic system: implications for drugs for central nervous system diseases. Nat Rev Drug Discov. 2022;21:763-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 142] [Reference Citation Analysis (0)] |
| 5. | Sun YR, Lv QK, Liu JY, Wang F, Liu CF. New perspectives on the glymphatic system and the relationship between glymphatic system and neurodegenerative diseases. Neurobiol Dis. 2025;205:106791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2426] [Cited by in RCA: 4059] [Article Influence: 312.2] [Reference Citation Analysis (0)] |
| 7. | Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990;170:111-123. [PubMed] |
| 8. | Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1069] [Cited by in RCA: 1134] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 9. | Bacyinski A, Xu M, Wang W, Hu J. The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy. Front Neuroanat. 2017;11:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190-18199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 952] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 11. | Salman MM, Kitchen P, Halsey A, Wang MX, Törnroth-Horsefield S, Conner AC, Badaut J, Iliff JJ, Bill RM. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain. 2022;145:64-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 189] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 12. | Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol. 1990;52:431-439. [PubMed] |
| 13. | Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 404] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 14. | Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1148] [Cited by in RCA: 1609] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 15. | Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 382] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Simon MJ, Iliff JJ. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta. 2016;1862:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 18. | Lundgaard I, Wang W, Eberhardt A, Vinitsky HS, Reeves BC, Peng S, Lou N, Hussain R, Nedergaard M. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci Rep. 2018;8:2246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Liu Q, Yan L, Huang M, Zeng H, Satyanarayanan SK, Shi Z, Chen D, Lu JH, Pei Z, Yao X, Su H. Experimental alcoholism primes structural and functional impairment of the glymphatic pathway. Brain Behav Immun. 2020;85:106-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Liu H, Meng L, Wang J, Qin C, Feng R, Chen Y, Chen P, Zhu Q, Ma M, Teng J, Ding X. Enlarged perivascular spaces in alcohol-related brain damage induced by dyslipidemia. J Cereb Blood Flow Metab. 2024;44:1867-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Miyakawa T, Hattori E, Shikai I, Shimoji A, Nagatoshi K. Histopathological changes of chronic alcoholism. Folia Psychiatr Neurol Jpn. 1977;31:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Dai X, Gao L, Zhang J, Li X, Yu J, Yu L, Li Y, Zeng M, Wang X, Zhang H. Investigating DTI-ALPS index and its association with cognitive impairments in patients with alcohol use disorder: A diffusion tensor imaging study. J Psychiatr Res. 2024;180:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | García-Dolores F, Hernández-Torres MA, Fuentes-Medel E, Díaz A, Guevara J, Baltazar-Gaytan E, Aguilar-Hernández L, Nicolini H, Morales-Medina JC, González-Cano SI, de la Cruz F, Gil-Velazco A, Tendilla-Beltrán H, Flores G. Atrophy and Higher Levels of Inflammatory-Related Markers in the Posterior Cerebellar Lobe Cortex in Chronic Alcohol Use Disorder: A Cross-Sectional Study. Neuropathol Appl Neurobiol. 2025;51:e70011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 24. | Wei J, Qin L, Fu Y, Dai Y, Wen Y, Xu S. Long-term consumption of alcohol exacerbates neural lesions by destroying the functional integrity of the blood-brain barrier. Drug Chem Toxicol. 2022;45:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Sripathirathan K, Brown J 3rd, Neafsey EJ, Collins MA. Linking binge alcohol-induced neurodamage to brain edema and potential aquaporin-4 upregulation: evidence in rat organotypic brain slice cultures and in vivo. J Neurotrauma. 2009;26:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Collins MA, Tajuddin N, Moon KH, Kim HY, Nixon K, Neafsey EJ. Alcohol, phospholipase A2-associated neuroinflammation, and ω3 docosahexaenoic acid protection. Mol Neurobiol. 2014;50:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Katada R, Nishitani Y, Honmou O, Mizuo K, Okazaki S, Tateda K, Watanabe S, Matsumoto H. Expression of aquaporin-4 augments cytotoxic brain edema after traumatic brain injury during acute ethanol exposure. Am J Pathol. 2012;180:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Wu W, Tian R, Hao S, Xu F, Mao X, Liu B. A pre-injury high ethanol intake in rats promotes brain edema following traumatic brain injury. Br J Neurosurg. 2014;28:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Wang T, Chou DY, Ding JY, Fredrickson V, Peng C, Schafer S, Guthikonda M, Kreipke C, Rafols JA, Ding Y. Reduction of brain edema and expression of aquaporins with acute ethanol treatment after traumatic brain injury. J Neurosurg. 2013;118:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Hayes DM, Deeny MA, Shaner CA, Nixon K. Determining the threshold for alcohol-induced brain damage: new evidence with gliosis markers. Alcohol Clin Exp Res. 2013;37:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | LeComte MD, Shimada IS, Sherwin C, Spees JL. Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. Proc Natl Acad Sci U S A. 2015;112:8726-8731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Gómez GI, Alvear TF, Roa DA, Farias-Pasten A, Vergara SA, Mellado LA, Martinez-Araya CJ, Prieto-Villalobos J, García-Rodríguez C, Sánchez N, Sáez JC, Ortíz FC, Orellana JA. Cx43 hemichannels and panx1 channels contribute to ethanol-induced astrocyte dysfunction and damage. Biol Res. 2024;57:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 33. | Blanco AM, Pascual M, Valles SL, Guerri C. Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-kappa B. Neuroreport. 2004;15:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Alfonso-Loeches S, Ureña-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci. 2014;8:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 35. | Fletcher TL, Shain W. Ethanol-induced changes in astrocyte gene expression during rat central nervous system development. Alcohol Clin Exp Res. 1993;17:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Montoliu C, Sancho-Tello M, Azorin I, Burgal M, Vallés S, Renau-Piqueras J, Guerri C. Ethanol increases cytochrome P4502E1 and induces oxidative stress in astrocytes. J Neurochem. 1995;65:2561-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond). 2017;131:2257-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 286] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 38. | Wang X, Feng H, Wang Y, Zhou J, Zhao X. Enlarged Perivascular Spaces and Cerebral Small Vessel Disease in Spontaneous Intracerebral Hemorrhage Patients. Front Neurol. 2019;10:881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Deike K, Decker A, Scheyhing P, Harten J, Zimmermann N, Paech D, Peters O, Freiesleben SD, Schneider LS, Preis L, Priller J, Spruth E, Altenstein S, Lohse A, Fliessbach K, Kimmich O, Wiltfang J, Bartels C, Hansen N, Jessen F, Rostamzadeh A, Düzel E, Glanz W, Incesoy EI, Butryn M, Buerger K, Janowitz D, Ewers M, Perneczky R, Rauchmann BS, Teipel S, Kilimann I, Goerss D, Laske C, Munk MH, Spottke A, Roy N, Wagner M, Roeske S, Heneka MT, Brosseron F, Ramirez A, Dobisch L, Wolfsgruber S, Kleineidam L, Yakupov R, Stark M, Schmid MC, Berger M, Hetzer S, Dechent P, Scheffler K, Petzold GC, Schneider A, Effland A, Radbruch A. Machine Learning-Based Perivascular Space Volumetry in Alzheimer Disease. Invest Radiol. 2024;59:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 40. | Yamasaki T, Ikawa F, Ichihara N, Hidaka T, Matsuda S, Ozono I, Chiku M, Kitamura N, Hamano T, Horie N, Akiyama Y, Yamaguchi S, Tomimoto H, Suzuki M. Factors associated with the location of perivascular space enlargement in middle-aged individuals undergoing brain screening in Japan. Clin Neurol Neurosurg. 2022;223:107497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 41. | Kedarasetti RT, Drew PJ, Costanzo F. Arterial pulsations drive oscillatory flow of CSF but not directional pumping. Sci Rep. 2020;10:10102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 42. | Han G, Jiao B, Zhang Y, Wang Z, Liang C, Li Y, Hsu YC, Bai R. Arterial pulsation dependence of perivascular cerebrospinal fluid flow measured by dynamic diffusion tensor imaging in the human brain. Neuroimage. 2024;297:120653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 43. | Mortensen KN, Sanggaard S, Mestre H, Lee H, Kostrikov S, Xavier ALR, Gjedde A, Benveniste H, Nedergaard M. Impaired Glymphatic Transport in Spontaneously Hypertensive Rats. J Neurosci. 2019;39:6365-6377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 44. | Li M, Kitamura A, Beverley J, Koudelka J, Duncombe J, Lennen R, Jansen MA, Marshall I, Platt B, Wiegand UK, Carare RO, Kalaria RN, Iliff JJ, Horsburgh K. Impaired Glymphatic Function and Pulsation Alterations in a Mouse Model of Vascular Cognitive Impairment. Front Aging Neurosci. 2021;13:788519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Stendel R, Irnich B, al Hassan AA, Heidenreich J, Pietilae T. The influence of ethanol on blood flow velocity in major cerebral vessels. A prospective and controlled study. Alcohol. 2006;38:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Mews P, Egervari G, Nativio R, Sidoli S, Donahue G, Lombroso SI, Alexander DC, Riesche SL, Heller EA, Nestler EJ, Garcia BA, Berger SL. Alcohol metabolism contributes to brain histone acetylation. Nature. 2019;574:717-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 47. | Serrano M, Rico-Barrio I, Grandes P. The effect of omega-3 fatty acids on alcohol-induced damage. Front Nutr. 2023;10:1068343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 48. | Liu X, Hao J, Yao E, Cao J, Zheng X, Yao D, Zhang C, Li J, Pan D, Luo X, Wang M, Wang W. Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain Behav Immun. 2020;89:357-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 49. | Zhang E, Wan X, Yang L, Wang D, Chen Z, Chen Y, Liu M, Zhang G, Wu J, Han H, Fan Z. Omega-3 Polyunsaturated Fatty Acids Alleviate Traumatic Brain Injury by Regulating the Glymphatic Pathway in Mice. Front Neurol. 2020;11:707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Ren H, Luo C, Feng Y, Yao X, Shi Z, Liang F, Kang JX, Wan JB, Pei Z, Su H. Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017;31:282-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Aliev F, Barr PB, Davies AG, Dick DM, Bettinger JC. Genes regulating levels of ω-3 long-chain polyunsaturated fatty acids are associated with alcohol use disorder and consumption, and broader externalizing behavior in humans. Alcohol Clin Exp Res. 2022;46:1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Tapia-Rojas C, Carvajal FJ, Mira RG, Arce C, Lerma-Cabrera JM, Orellana JA, Cerpa W, Quintanilla RA. Adolescent Binge Alcohol Exposure Affects the Brain Function Through Mitochondrial Impairment. Mol Neurobiol. 2018;55:4473-4491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Reddy VD, Padmavathi P, Kavitha G, Saradamma B, Varadacharyulu N. Alcohol-induced oxidative/nitrosative stress alters brain mitochondrial membrane properties. Mol Cell Biochem. 2013;375:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Liu M, Guo S, Huang D, Hu D, Wu Y, Zhou W, Song W. Chronic Alcohol Exposure Alters Gene Expression and Neurodegeneration Pathways in the Brain of Adult Mice. J Alzheimers Dis. 2022;86:315-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Gumeler E, Aygun E, Tezer FI, Saritas EU, Oguz KK. Assessment of glymphatic function in narcolepsy using DTI-ALPS index. Sleep Med. 2023;101:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Chen HL, Chen PC, Lu CH, Tsai NW, Yu CC, Chou KH, Lai YR, Taoka T, Lin WC. Associations among Cognitive Functions, Plasma DNA, and Diffusion Tensor Image along the Perivascular Space (DTI-ALPS) in Patients with Parkinson's Disease. Oxid Med Cell Longev. 2021;2021:4034509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 57. | Eide PK, Hasan-Olive MM, Hansson HA, Enger R. Increased occurrence of pathological mitochondria in astrocytic perivascular endfoot processes and neurons of idiopathic intracranial hypertension. J Neurosci Res. 2021;99:467-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | Tseng PY, Sung FC, Muo CH, Lan YC, Hser YI, Chien SH, Wang JY. Risk of diabetes and hypertension in a population with alcohol use disorders. BMC Public Health. 2024;24:868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 59. | Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9:4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 741] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 60. | Kikuta J, Kamagata K, Takabayashi K, Taoka T, Yokota H, Andica C, Wada A, Someya Y, Tamura Y, Kawamori R, Watada H, Naganawa S, Aoki S. An Investigation of Water Diffusivity Changes along the Perivascular Space in Elderly Subjects with Hypertension. AJNR Am J Neuroradiol. 2022;43:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 61. | Berlowitz DR, Foy CG, Kazis LE, Bolin LP, Conroy MB, Fitzpatrick P, Gure TR, Kimmel PL, Kirchner K, Morisky DE, Newman J, Olney C, Oparil S, Pajewski NM, Powell J, Ramsey T, Simmons DL, Snyder J, Supiano MA, Weiner DE, Whittle J; SPRINT Research Group. Effect of Intensive Blood-Pressure Treatment on Patient-Reported Outcomes. N Engl J Med. 2017;377:733-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 62. | Kern KC, Nasrallah IM, Bryan RN, Reboussin DM, Wright CB. Intensive systolic blood pressure treatment remodels brain perivascular spaces: A secondary analysis of the Systolic Pressure Intervention Trial (SPRINT). Neuroimage Clin. 2023;40:103513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Piekarski D, Sullivan EV, Pfefferbaum A, Zahr NM. Poor subjective sleep predicts compromised quality of life but not cognitive impairment in abstinent individuals with Alcohol Use Disorder. Alcohol. 2022;103:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 64. | He S, Hasler BP, Chakravorty S. Alcohol and sleep-related problems. Curr Opin Psychol. 2019;30:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 65. | Laniepce A, Segobin S, André C, Bertran F, Boudehent C, Lahbairi N, Maillard A, Mary A, Urso L, Vabret F, Cabé N, Pitel AL, Rauchs G. Distinct Sleep Alterations in Alcohol Use Disorder Patients with and without Korsakoff's Syndrome: Relationship with Episodic Memory. J Clin Med. 2023;12:2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 66. | Knapp CM, Ciraulo DA, Datta S. Mechanisms underlying sleep-wake disturbances in alcoholism: focus on the cholinergic pedunculopontine tegmentum. Behav Brain Res. 2014;274:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Ko J, Lim JH, Kim DB, Joo MJ, Jang YS, Park EC, Shin J. Association between alcohol use disorder and risk of obstructive sleep apnea. J Sleep Res. 2024;33:e14128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Lee HJ, Lee DA, Shin KJ, Park KM. Glymphatic system dysfunction in obstructive sleep apnea evidenced by DTI-ALPS. Sleep Med. 2022;89:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 69. | Roy B, Nunez A, Aysola RS, Kang DW, Vacas S, Kumar R. Impaired Glymphatic System Actions in Obstructive Sleep Apnea Adults. Front Neurosci. 2022;16:884234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 70. | Xiong Z, Bai M, Wang Z, Wang R, Tian C, Wang L, Nie L, Zeng X. Resting-state fMRI network efficiency as a mediator in the relationship between the glymphatic system and cognitive function in obstructive sleep apnea hypopnea syndrome: Insights from a DTI-ALPS investigation. Sleep Med. 2024;119:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 71. | Thakkar MM, Sharma R, Sahota P. Alcohol disrupts sleep homeostasis. Alcohol. 2015;49:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 72. | Saito Y, Hayakawa Y, Kamagata K, Kikuta J, Mita T, Andica C, Taoka T, Uchida W, Takabayashi K, Tuerxun R, Mahemuti Z, Yoshida S, Kitagawa T, Arai T, Suzuki A, Sato K, Nishizawa M, Akashi T, Shimoji K, Wada A, Aoki S. Glymphatic system impairment in sleep disruption: diffusion tensor image analysis along the perivascular space (DTI-ALPS). Jpn J Radiol. 2023;41:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 73. | Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1184] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 74. | Yin M, Pu T, Wang L, Marshall C, Wu T, Xiao M. Astroglial water channel aquaporin 4-mediated glymphatic clearance function: A determined factor for time-sensitive treatment of aerobic exercise in patients with Alzheimer's disease. Med Hypotheses. 2018;119:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Vancampfort D, Kimbowa S, Hallgren M, Mugisha J. Physical activity and physical fitness in community patients with alcohol use disorders versus matched healthy controls: cross-sectional data from Uganda. Pan Afr Med J. 2022;41:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Vancampfort D, Vandael H, Hallgren M, Van Damme T. Test-retest reliability and correlates of the 6-min walk test in people with alcohol use disorders. Physiother Res Int. 2021;26:e1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Hallgren M, Vancampfort D, Nguyen TT, Ekblom-Bak E, Wallin P, Andersson G, Lundin A. Physical Activity, Sedentary Behavior, and Cardiorespiratory Fitness in Hazardous and Non-Hazardous Alcohol Consumers. Am J Health Promot. 2021;35:669-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Li M, Xu J, Li L, Zhang L, Zuo Z, Feng Y, He X, Hu X. Voluntary wheel exercise improves glymphatic clearance and ameliorates colitis-associated cognitive impairment in aged mice by inhibiting TRPV4-induced astrocytic calcium activity. Exp Neurol. 2024;376:114770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, Pei Z, Xu GQ, Lan Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front Mol Neurosci. 2017;10:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 278] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 80. | Flores-Bonilla A, Richardson HN. Sex Differences in the Neurobiology of Alcohol Use Disorder. Alcohol Res. 2020;40:04. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 81. | Erol A, Ho AM, Winham SJ, Karpyak VM. Sex hormones in alcohol consumption: a systematic review of evidence. Addict Biol. 2019;24:157-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 82. | Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Andrew MR, Wiren KM. Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain Pathol. 2016;26:433-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 1125] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 84. | Han G, Zhou Y, Zhang K, Jiao B, Hu J, Zhang Y, Wang Z, Lou M, Bai R. Age- and time-of-day dependence of glymphatic function in the human brain measured via two diffusion MRI methods. Front Aging Neurosci. 2023;15:1173221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 85. | Flanigan MR, Royse SK, Cenkner DP, Kozinski KM, Stoughton CJ, Himes ML, Minhas DS, Lopresti B, Butters MA, Narendran R. Imaging beta-amyloid (Aβ) burden in the brains of middle-aged individuals with alcohol-use disorders: a [(11)C]PIB PET study. Transl Psychiatry. 2021;11:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 86. | Wang G, Li DY, Vance DE, Li W. Alcohol Use Disorder as a Risk Factor for Cognitive Impairment. J Alzheimers Dis. 2023;94:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 87. | Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, Kishimoto T, Naganawa S. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol. 2017;35:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 708] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 88. | Huang SY, Zhang YR, Guo Y, Du J, Ren P, Wu BS, Feng JF; Alzheimer's Disease Neuroimaging Initiative, Cheng W, Yu JT. Glymphatic system dysfunction predicts amyloid deposition, neurodegeneration, and clinical progression in Alzheimer's disease. Alzheimers Dement. 2024;20:3251-3269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 126] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 89. | Zhang X, Wang Y, Jiao B, Wang Z, Shi J, Zhang Y, Bai X, Li Z, Li S, Bai R, Sui B. Glymphatic system impairment in Alzheimer's disease: associations with perivascular space volume and cognitive function. Eur Radiol. 2024;34:1314-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 90. | Hsu JL, Wei YC, Toh CH, Hsiao IT, Lin KJ, Yen TC, Liao MF, Ro LS. Magnetic Resonance Images Implicate That Glymphatic Alterations Mediate Cognitive Dysfunction in Alzheimer Disease. Ann Neurol. 2023;93:164-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 171] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 91. | Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, Alper SL, Lundgaard I, Nedergaard M, Kahle KT. Glymphatic System Impairment in Alzheimer's Disease and Idiopathic Normal Pressure Hydrocephalus. Trends Mol Med. 2020;26:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 297] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 92. | Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, Ahmed Z, Fisher A, Meftah S, Murray TK, Ottersen OP, Nagelhus EA, O'Neill MJ, Wells JA, Lythgoe MF. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain. 2020;143:2576-2593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 429] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 93. | Menon DK, Schwab K, Wright DW, Maas AI; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91:1637-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1086] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 94. | Wang X, Deng L, Liu X, Cheng S, Zhan Y, Chen J. Relationship between glymphatic system dysfunction and cognitive impairment in patients with mild-to-moderate chronic traumatic brain injury: an analysis of the analysis along the perivascular space (ALPS) index. Quant Imaging Med Surg. 2024;14:9246-9257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 95. | Park JH, Bae YJ, Kim JS, Jung WS, Choi JW, Roh TH, You N, Kim SH, Han M. Glymphatic system evaluation using diffusion tensor imaging in patients with traumatic brain injury. Neuroradiology. 2023;65:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 96. | Feng S, Wu C, Zou P, Deng Q, Chen Z, Li M, Zhu L, Li F, Liu TC, Duan R, Yang L. High-intensity interval training ameliorates Alzheimer's disease-like pathology by regulating astrocyte phenotype-associated AQP4 polarization. Theranostics. 2023;13:3434-3450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 97. | Liang PZ, Li L, Zhang YN, Shen Y, Zhang LL, Zhou J, Wang ZJ, Wang S, Yang S. Electroacupuncture Improves Clearance of Amyloid-β through the Glymphatic System in the SAMP8 Mouse Model of Alzheimer's Disease. Neural Plast. 2021;2021:9960304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Wu CH, Liao WH, Chu YC, Hsiao MY, Kung Y, Wang JL, Chen WS. Very Low-Intensity Ultrasound Facilitates Glymphatic Influx and Clearance via Modulation of the TRPV4-AQP4 Pathway. Adv Sci (Weinh). 2024;11:e2401039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 99. | Zhang X, Cao R, Zhu C, Yang L, Zheng N, Ji W, Liu P, Chi T, Ji X, Zheng Z, Chen G, Zou L. Mechanism of anti-AD action of OAB-14 by enhancing the function of glymphatic system. Neurochem Int. 2023;105633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 100. | Zhang B, Li W, Zhuo Y, Xiang H, Li W, Liu H, Xie L, Gao Q, Tan S. L-3-n-Butylphthalide Effectively Improves the Glymphatic Clearance and Reduce Amyloid-β Deposition in Alzheimer's Transgenic Mice. J Mol Neurosci. 2021;71:1266-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Li J, Hao Y, Wang S, Li W, Yue S, Duan X, Yang Y, Li B. Yuanzhi powder facilitated Aβ clearance in APP/PS1 mice: Target to the drainage of glymphatic system and meningeal lymphatic vessels. J Ethnopharmacol. 2024;319:117195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 102. | Tong S, Xie L, Xie X, Xu J, You Y, Sun Y, Zhou S, Ma C, Jiang G, Ma F, Wang Z, Gao X, Chen J. Nano-Plumber Reshapes Glymphatic-Lymphatic System to Sustain Microenvironment Homeostasis and Improve Long-Term Prognosis after Traumatic Brain Injury. Adv Sci (Weinh). 2023;10:e2304284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 103. | Lv C, Han S, Sha Z, Liu M, Dong S, Zhang C, Li Z, Zhang K, Lu S, Xu Z, Bie L, Jiang R. Cerebral glucagon-like peptide-1 receptor activation alleviates traumatic brain injury by glymphatic system regulation in mice. CNS Neurosci Ther. 2023;29:3876-3888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 104. | Dong S, Zhao H, Nie M, Sha Z, Feng J, Liu M, Lv C, Chen Y, Jiang W, Yuan J, Qian Y, Wan H, Gao C, Jiang R. Cannabidiol Alleviates Neurological Deficits After Traumatic Brain Injury by Improving Intracranial Lymphatic Drainage. J Neurotrauma. 2024;41:e2009-e2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 105. | Feng D, Liu T, Zhang X, Xiang T, Su W, Quan W, Jiang R. Fingolimod improves diffuse brain injury by promoting AQP4 polarization and functional recovery of the glymphatic system. CNS Neurosci Ther. 2024;30:e14669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/