Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.105932
Revised: July 2, 2025
Accepted: July 21, 2025
Published online: October 19, 2025
Processing time: 128 Days and 23.6 Hours
First-generation antipsychotics demonstrate certain therapeutic benefits in schizophrenia; however, they often fail to significantly address negative symptoms. Thus, continued exploration is essential to refine these treatments.
To examine lurasidone plus sulpiride influence on treatment efficacy, psychiatric symptoms, and quality of life in patients with schizophrenia.
A total of 110 patients with schizophrenia, admitted between October 2021 and October 2024, were recruited for this study. The control group (n = 50) received sulpiride alone. Conversely, the observation group (n = 60) was treated with a combination of lurasidone and sulpiride. A series of assessments were conducted to compare the two groups. These included evaluating treatment efficacy; re
Overall treatment efficacy was significantly higher in the observation group than in the control group. The total incidence of adverse events was comparable between the two groups. After treatment, the scores for positive symptoms, negative symptoms, and general psychopathological symptoms on the PANSS in the observation group were significantly reduced compared to pretreatment levels, and were also lower than those in the control group. Additionally, RBANS and PSP scores in the observation group significantly increased post-treatment and were notably higher than in the control group. Regarding the quality of life, SQLS scores in the psychosocial, symptoms, and side effects and motivation and energy dimensions in the observation group were significantly lower after treatment than both baseline levels and those in the control group. Furthermore, post-treatment levels of IL-6 and IL-17 in the observation group were significantly reduced and lower than those in the control group, whereas the PRL level was significantly elevated.
The combination of lurasidone and sulpiride can effectively enhance treatment efficacy, alleviate psychiatric symptoms, and improve quality of life in patients with schizophrenia, supporting its broader clinical use.
Core Tip: Although first-generation antipsychotics have limited effectiveness against the negative symptoms of schizophrenia, sulpiride combined with lurasidone may offer superior results. This study suggests that, compared with sulpiride monotherapy, the combined approach enhances treatment efficacy safely, with patients experiencing fewer psychiatric symptoms, improved cognitive and social functioning, and better quality of life. It also helps normalize abnormal levels of interleukin 6 (IL-6), IL-17, and prolactin.
- Citation: Zhao JJ, Han CY, Xu GX, Zhou M, Jin ZM. Effect of lurasidone plus sulpiride on treatment efficacy, psychiatric manifestations, and quality of life among patients with schizophrenia. World J Psychiatry 2025; 15(10): 105932
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/105932.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.105932
Schizophrenia, a heterogeneous syndrome, has an etiology intertwined with multiple factors, including chronic stress, low-grade systemic inflammation, and gut microbiota dysbiosis. Its underlying pathological mechanisms are closely associated with disruptions in synaptic signal transduction and impairments in cerebral neural plasticity[1,2]. Epidemiological data indicate a predilection for this disorder among men. Alarmingly, the lifetime risk of suicide among patients with schizophrenia can reach up to 26.8%, and their life expectancy is shortened by approximately 10-25 years compared with the general population[3-5]. Patients with schizophrenia commonly present with a spectrum of symptoms. Positive symptoms, such as hallucinations, peculiar behaviors, and delusions, are frequently observed. Concurrently, negative symptoms, including apathy, pronounced social withdrawal, and excessive somnolence, are also prevalent. They are often accompanied by cognitive deficits, such as impairment in executive function impairments, speech abnormalities, and disruptions in short-term memory. Collectively, these manifestations significantly impair patients’ daily routines, social interactions, and professional undertakings[6].
Antipsychotic medications remain the cornerstones of treatment. First-generation antipsychotics have proven effective in alleviating positive symptoms. However, their capacity to mitigate negative symptoms remains relatively constrained. Moreover, they are frequently associated with various side effects, which can adversely affect treatment adherence[7,8]. Conversely, second-generation antipsychotics have emerged as a more favorable option, effectively addressing the limitations of their predecessors. They provide significant clinical benefits, including improved management of negative symptoms and a reduced incidence of neurological side effects, thereby enhancing the overall treatment experience for patients[9].
This study primarily aimed to determine the effects of lurasidone plus sulpiride on the treatment efficacy, alleviation of psychiatric symptoms, and quality of life in patients with schizophrenia. Currently, research on this specific combination therapy remains limited. Therefore, this study is intended to provide more refined clinical guidance to support informed treatment decisions. Sulpiride, exerts a selective inhibitory action on dopaminergic receptors. This pharmacological property renders it an effective therapeutic option for treating various central nervous system disorders, such as schizophrenia, depression, and other psychotic conditions[10]. Moreover, a study explored the combination of sulpiride with cell-penetrating peptides, a strategy that has shown promise in augmenting its antidepressant efficacy while concurrently reducing the serum levels of prolactin (PRL)[11]. However, despite its therapeutic utility, sulpiride, as a first-generation antipsychotic, often necessitates the incorporation of additional medications to maximize overall efficacy[12]. Lurasidone, a second-generation antipsychotic, is essentially a benzisothiazole derivative that has a strong affinity for dopamine D2, 5-hydroxytryptamine 2A (5-HT2A), 5-HT7, 5-HT1A, and norepinephrine α2C receptors, and can reduce antagonistic interactions in various dopaminergic pathways, thus ensuring a high degree of tolerability[13]. Its antagonism of 5-HT2A receptors also allows it to effectively inhibit the serotonergic excitation of cortical pyramidal cells, thus helping to alleviate the positive symptoms characteristic of schizophrenia[14]. Notably, a study also indicated that lurasidone may effectively improve the quality of life in patients with schizophrenia[15].
Inclusion criteria: (1) Patients were required to meet the diagnostic criteria for schizophrenia as per the Schizophrenia Pharmacotherapy Guidelines[16]; and (2) Patients should not have received any antipsychotic treatment within the preceding month and must have complete clinical records.
Exclusion criteria: (1) Individuals with coexisting mental disorders of other types; (2) History of craniocerebral trauma; (3) Alcohol dependence; (4) Hypersensitivity to the medications used in this trial; (5) Cognitive, language, or auditory impairments; (6) Severe encephalopathy; (7) Epilepsy; (8) Poisoning; (9) Severe endocrine disorders; and (10) Severe hepatic and renal dysfunction.
In strict accordance with these inclusion and exclusion criteria, 110 patients with schizophrenia admitted between October 2021 and October 2024 were retrospectively enrolled. Patients were categorized based on the administered therapy in a non-randomized manner, with 50 cases in the control group (sulpiride treatment) and 60 in the observation group (lurasidone plus sulpiride).
The sample size was calculated to adequately power between-group mean comparisons. Parameters included Δ (effect size) = 10%, σ (standard deviation) = 15%, α = 0.05 (two-tailed), and power (1-β) = 80%. The initial calculation yielded 36 per group. However, after considering potential participant withdrawal (estimated at 20% attrition), the initial estimate of 36 participants per group was increased to 45.
The control group received sulpiride. The initial dosage was set at 100 mg per administration, three times daily (8:00, 14:00, and 20:00, postprandial). After 7 days of treatment, the dosage was increased to 300 mg per administration, also three times daily.
The observation group received lurasidone plus sulpiride, with the sulpiride regimen following the same protocol as the control group. Lurasidone tablets were orally administered at a dosage of 40 mg once daily after dinner. The treatment course for both groups was eight consecutive weeks.
Treatment efficacy: Efficacy was gauged based on changes in positive and negative syndrome scale (PANSS) scores. After treatment, a reduction of > 75% in the PANSS score was designated as a “cure”; a decrease of 50%-75% was classified as a “significant improvement”; a decline of 25%-50% was considered an “improvement”; and cases failing to meet these thresholds were labeled as “ineffective”. The overall response rate was calculated as the proportion of patients achieving complete remission or significant improvement.
Adverse events: The occurrences of side effects, including fatigue, xerostomia, insomnia, anorexia, and headache, were monitored and documented in both groups following treatment. The cumulative incidence of these adverse events was subsequently calculated.
Psychiatric symptom assessment: The PANSS is a psychometrically validated rating scale that systematically assesses the severity of characteristic symptoms in schizophrenia. It comprises three components: (1) Positive symptoms (7-49 points); (2) Negative symptoms (7-49 points); and (3) General psychopathological symptoms (16-112 points). Higher scores on this scale indicated more severe psychiatric manifestations.
Cognitive and social function assessment: Neurocognitive assessment employed the repeatable battery for the assessment of neuropsychological status (RBANS) with five domains, namely, immediate memory, visuospatial ability, language, attention, delayed memory (total score 40-160); higher scores indicate better cognitive function. Social functioning was evaluated using the Chinese personal and social performance scale (PSP), a validated measure examining four functional domains (work/study, interpersonal relationships, and self-care) on a 0-100 scale, with higher values denoting superior social functionality.
Quality of life evaluation: The schizophrenia quality of life scale (SQLS) was employed to assess the quality of life. The SQLS is divided into three domains: (1) Psychosocial (15 items); (2) Symptoms and side effects (8 items); and (3) Motivation and energy (7 items). Each SQLS subscale assigns scores ranging from 0 to 100 points. Lower scores indicate better quality of life.
Serum marker analysis: Before and following the treatment, 2 mL peripheral venous blood was collected from patients in a fasting state. After centrifugation, the serum was isolated. The levels of interleukin (IL-6), IL-17, and PRL were determined using the enzyme-linked immunosorbent assay.
Statistical Package for the Social Sciences 20.0 (IBM SPSS, Armonk, NY, United States) and GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, United States) were used for comprehensive data analyses. Categorical data, such as sex, are presented as the frequency/count and percentage [n (%)], and compared using the χ² test. Quantitative data such as age and disease duration are expressed as the mean ± standard error of the mean. Between-group comparisons used the independent samples t-test; within-group comparisons before and after treatment, were assessed using the paired t-test. Statistical significance was set at a threshold of P < 0.05.
No significant disparities were observed in the general characteristics, including sex, age, disease duration, body mass index, years of education, concomitant medications, tobacco use, and regular exercise habits, between the control and observation groups (P > 0.05; Table 1).
| Indicators | Control group (n = 50) | Observation group (n = 60) | χ²/t | P value |
| Sex (male/female) | 30/20 | 31/29 | 0.767 | 0.876 |
| Age (years) | 54.46 ± 7.22 | 52.20 ± 8.61 | 1.474 | 0.144 |
| Disease course (years) | 6.26 ± 2.00 | 6.05 ± 2.60 | 0.467 | 0.641 |
| Body mass index (kg/m2) | 25.12 ± 2.18 | 24.45 ± 2.20 | 1.597 | 0.113 |
| Years of education (years) | 10.80 ± 2.73 | 10.00 ± 3.15 | 1.408 | 0.162 |
| Concomitant medications (yes/no) | 12/38 | 10/50 | 0.917 | 0.338 |
| Tobacco use (yes/no) | 18/32 | 15/45 | 1.571 | 0.210 |
| Regular exercise habits (yes/no) | 10/40 | 8/52 | 0.886 | 0.347 |
The overall treatment efficacy rate in the control group was 76.00%, whereas in the observation group, it reached 93.33%. The observation group demonstrated a significantly higher overall efficacy rate than the control group (P < 0.05; Table 2).
| Indicators | Control group (n = 50) | Observation group (n = 60) | χ² | P value |
| Cure | 15 (30.00) | 20 (33.33) | ||
| Improvement | 23 (46.00) | 36 (60.00) | ||
| Ineffectiveness | 12 (24.00) | 4 (6.67) | ||
| Overall efficacy | 38 (76.00) | 56 (93.33) | 6.592 | 0.010 |
Safety-related data indicated that the cumulative incidence of adverse events including fatigue, xerostomia, insomnia, anorexia, and headache in the observation group was 10.00%. These data were comparable to the 16.00% observed in the control group (P > 0.05; Table 3).
| Indicators | Control group (n = 50) | Observation group (n = 60) | χ² | P value |
| Fatigue | 2 (4.00) | 1 (1.67) | ||
| Xerostomia | 1 (2.00) | 1 (1.67) | ||
| Insomnia | 1 (2.00) | 2 (3.33) | ||
| Anorexia | 1 (2.00) | 0 (0.00) | ||
| Headache | 3 (6.00) | 2 (3.33) | ||
| Total | 8 (16.00) | 6 (10.00) | 0.884 | 0.347 |
The PANSS was used to analyze the psychiatric symptoms in both groups. Before treatment, no significant differences were detected in the PANSS scores for positive, negative, and general psychopathological symptoms between the two groups (P > 0.05). After treatment, a significant reduction in PANSS scores was noted in both groups (P < 0.05). Moreover, the PANSS scores of the observation group were significantly lower than those of the control group across all symptom categories (P < 0.05; Table 4).
| Indicators | Control group (n = 50) | Observation group (n = 60) | t value | P value |
| Positive symptoms | ||||
| Before treatment | 23.24 ± 6.22 | 21.22 ± 7.68 | 1.495 | 0.138 |
| After treatment | 14.46 ± 2.92a | 10.92 ± 2.63b | 6.685 | < 0.001 |
| Negative symptoms | ||||
| Before treatment | 29.86 ± 10.60 | 30.42 ± 7.84 | 0.318 | 0.751 |
| After treatment | 25.00 ± 5.41a | 18.78 ± 5.48b | 5.962 | < 0.001 |
| General psychopathological symptoms | ||||
| Before treatment | 60.26 ± 13.15 | 61.33 ± 13.50 | 0.121 | 0.904 |
| After treatment | 43.68 ± 12.99a | 35.95 ± 10.46b | 3.457 | < 0.001 |
The cognitive and social functions of the two groups were evaluated using the RBANS and PSP, respectively. Before treatment, no significant discrepancies were observed in the RBANS and PSP scores between the two groups (P > 0.05). A significant increase in both scores was observed after treatment (P < 0.05). Moreover, across all aspects, the scores of the observation group were markedly higher than those of the control group (P < 0.05; Table 5).
| Indicators | Control group (n = 50) | Observation group (n = 60) | t value | P value |
| Repeatable battery for the assessment of neuropsychological status (points) | ||||
| Before treatment | 79.36 ± 13.19 | 78.07 ± 12.39 | 0.528 | 0.599 |
| After treatment | 87.72 ± 10.04a | 95.80 ± 11.09b | 3.971 | < 0.001 |
| Personal and social performance scale (points) | ||||
| Before treatment | 49.54 ± 4.52 | 48.80 ± 6.65 | 0.668 | 0.505 |
| After treatment | 76.94 ± 5.50a | 84.17 ± 7.54b | 5.642 | < 0.001 |
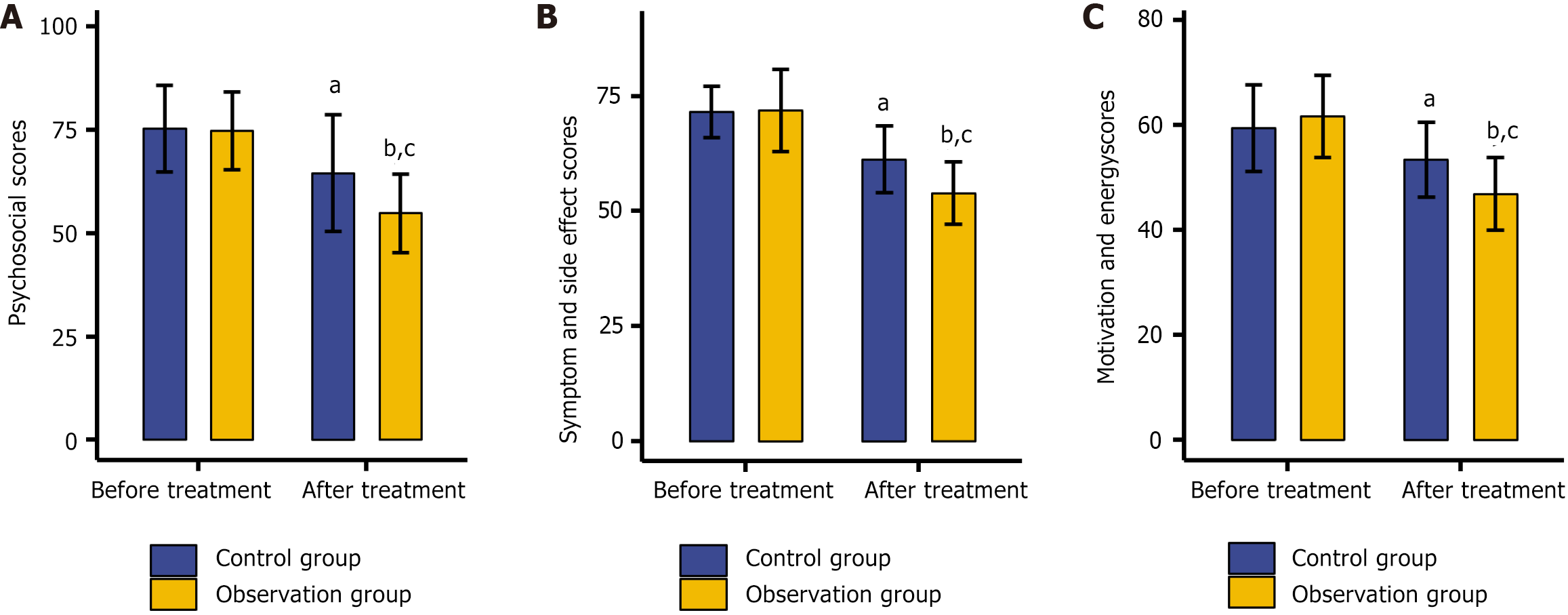
The SQLS was employed to assess the quality of life of the two groups. Before treatment, there were no significant intergroup differences in scores related to psychosocial aspects, symptoms and side effects, or motivation and energy (P > 0.05). After treatment, all these scores declined significantly (P < 0.05), with the observation group showing significantly lower scores in each category compared with the control group (P < 0.05; Figure 1).
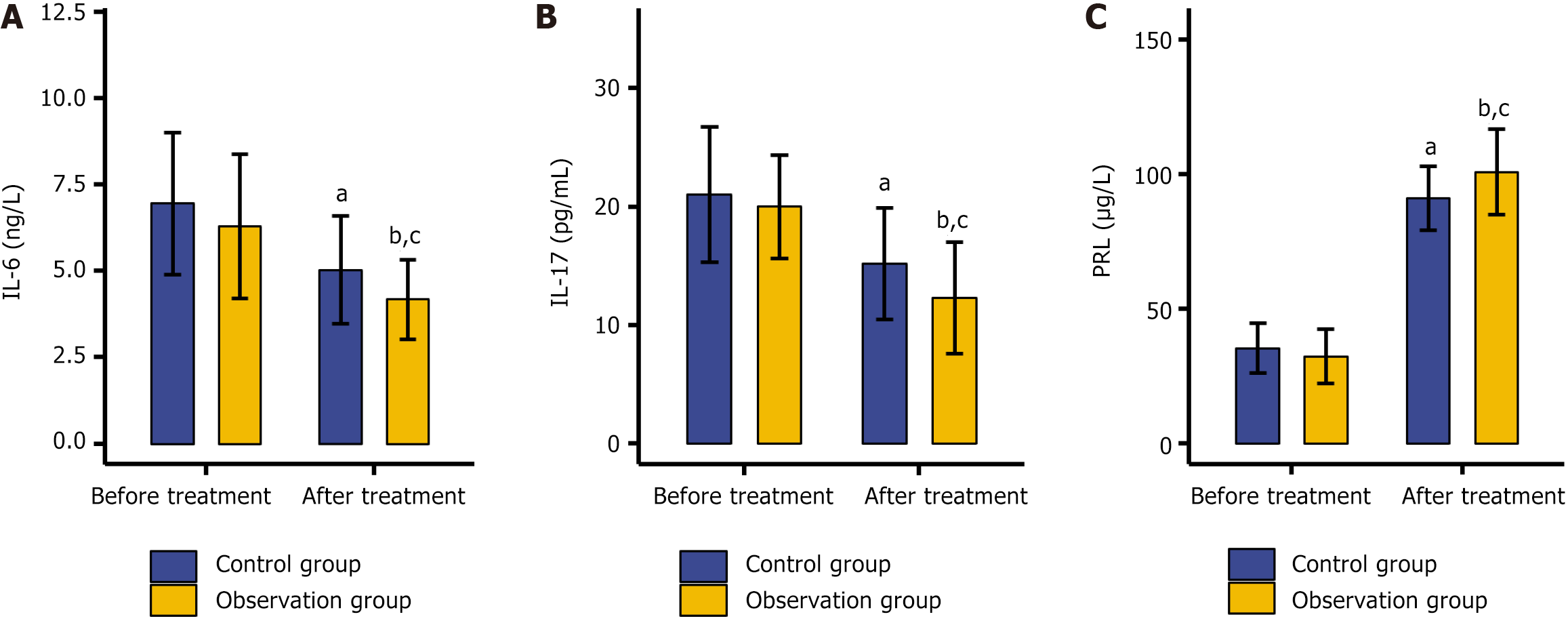
The serum biomarkers of the two groups, namely, IL-6, IL-17, and PRL, were examined. Before treatment, no significant disparities were detected in the levels of these biomarkers between the two groups (P > 0.05). After treatment, a remarkable shift was observed: The concentrations of IL-6 and IL-17 decreased significantly, whereas the level of PRL increased substantially (P < 0.5). Notably, compared with the control group, the observation group exhibited lower levels of IL-6 and IL-17, along with a higher PRL level (P < 0.5; Figure 2).
In this study, a retrospective analysis was conducted on 110 patients with schizophrenia to compare the clinical efficacy and safety of lurasidone plus sulpiride compared to sulpiride monotherapy. The results revealed that this combination therapy increased the efficacy rate from 76.00% to 93.33%, indicating that it may optimize therapeutic outcomes in patients with schizophrenia. Regarding safety, the combination therapy did not significantly increase the incidence of adverse events, including fatigue, xerostomia, insomnia, anorexia, and headache (10.00% vs 16.00%). In a 22-month double-blind placebo-controlled trial, Correll et al[17] reported that only 14.7% of patients discontinued taking lurasidone because of adverse reactions, a figure (the original) remarkably similar to our data. Lurasidone’s limited interference with weight gain, blood sugar, lipids, and PRL levels explains why it seldom increases adverse reactions in schizophrenia therapy[18]. Additionally, existing evidence underscores its long-term therapeutic safety and tolerability—benefits that possibly stem from its negligible effects on metabolic parameters and weight fluctuations[19]. In terms of psychiatric symptoms, the utilization of lurasidone plus sulpiride for patients with schizophrenia led to a more pronounced alleviation of positive, negative, and general psychopathological symptoms. In the study by Loebel et al[20], lurasidone significantly improved symptoms across five dimensions: (1) Positive symptoms; (2) Negative symptoms; (3) Thinking disorder; (4) Hostility/excitement; and (5) Depression/anxiety in patients with schizophrenia, which is similar to our observations. A study indicated that the mitigation of negative symptoms in patients with schizophrenia by lurasidone is, in part, attributable to its promotion of dopamine release in the prefrontal cortex[21]. Meanwhile, the antagonistic actions of lurasidone on dopamine and 5-HT receptors are pivotal in alleviating the clinical symptoms of patients with schizophrenia. The synergistic combination of lurasidone and sulpiride is particularly advantageous, as it enables the integration of the distinctive benefits of both agents, thereby optimizing the therapeutic outcomes[22]. The enhanced treatment efficacy observed with this combination could be ascribed to their differing pharmacological pathways, facilitating a multi-targeted effect that leads to significant symptom relief[10,13,14].
Furthermore, the administration of lurasidone plus sulpiride to patients with schizophrenia significantly bolsters their cognitive and social functions, evidenced by a more substantial elevation in RBANS and PSP scores under the combination protocol compared with monotherapy. In patients with schizophrenia, the advancement of the disease course, coupled with the progressive emergence of negative symptoms, often precipitates deteriorating cognitive impairment, which invariably results in an inevitable decline in social functionality[23]. A small-scale systematic review indicated that lurasidone exerts a more pronounced effect on improving cognitive ability compared with placebo, quetiapine, ziprasidone, or conventional treatment[24]. A study on a rat model pointed out that the neuroprotective effect of lurasidone can be attributed, to some extent, to its increase in the expression of the language-related protein forkhead box P2 in hippocampal cells, and the increase in the levels of the neuroprotective SxIP motif and microtubule-plus-end-binding proteins[25]. The SQLS data also showed that the treatment of patients with schizophrenia with lurasidone plus sulpiride can more effectively improve the quality of life in terms of psychosocial scores, symptom, and side effect scores, and motivation and energy scores. Dembek et al[26] reported that lurasidone significantly enhances the quality of life of patients with bipolar depression in various aspects, including overall life satisfaction, social and family relationships, medication satisfaction, and the ability to perform activities of daily living, which corroborates our findings. As regards serum biomarkers, with the administration of lurasidone plus sulpiride, the levels of IL-6, IL-17, and PRL in patients with schizophrenia have more substantially improved. Accumulated evidence reveals that the IL-6 Level is intricately linked to the chronic manifestations of patients with schizophrenia; moreover, a pronounced positive correlation has been established between IL-17 and the severity of mental disorders. Concurrently, low PRL levels have been significantly associated with more severe symptomatology in schizophrenia[27-29]. The immunopathogenesis of schizophrenia may be partly driven by IL-6 through its involvement in the IL-6/retinoic acid receptor-related orphan receptor C/IL-22 axis, whereas disturbances in IL-17 signaling might induce neuropsychiatric symptoms in affected individuals[30,31]. Numerous investigators have put forward novel treatment strategies for individuals with schizophrenia. For instance, Bredin et al[32] elucidated that the application of aerobic, resistance, and combined exercise training modalities in patients with schizophrenia can notably mitigate psychiatric symptoms. Additionally, Achtyes et al[33] demonstrated that agonists of the trace amine-associated receptor 1, when administered in the treatment of patients with schizophrenia, not only effect a remarkable improvement in clinical symptoms but also exhibit a certain degree of tolerability.
The findings of this study are constrained by several factors. The small sample size calls for larger, multicenter studies to enhance reliability. Second, the uneven group sizes might weaken the statistical power, indicating a need for prospective analyses with balanced sampling. Lastly, incorporating mechanistic investigations, such as basic experimental studies, could provide deeper insights into treatment-related mechanisms.
Compared with sulpiride monotherapy, lurasidone plus sulpiride offers a significantly greater improvement in therapeutic efficacy while maintaining a satisfactory safety profile in patients with schizophrenia. This combination also effectively alleviates psychiatric symptoms, enhances cognitive and social functioning, and improves quality of life. Moreover, it favorably modulates abnormal serum levels of IL-6, IL-17, and PRL. These findings provide meaningful clinical insights into the potential of this combination therapy and offer promising guidance for more effective treatment strategies in schizophrenia.
| 1. | Rantala MJ, Luoto S, Borráz-León JI, Krams I. Schizophrenia: The new etiological synthesis. Neurosci Biobehav Rev. 2022;142:104894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 2. | Fišar Z. Biological hypotheses, risk factors, and biomarkers of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2023;120:110626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 3. | Li X, Zhou W, Yi Z. A glimpse of gender differences in schizophrenia. Gen Psychiatr. 2022;35:e100823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 4. | Zhu J, Lang X, Shangguan F, Zhang XY. Prevalence, demographics, and clinical characteristics of suicide attempts in first episode drug-naïve schizophrenia patients with comorbid severe depression. Int J Psychiatry Clin Pract. 2024;28:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Sippel LM, Myers AL, Brooks JM, Storm M, Mois G, Fortuna KL. Risk and protective factors in relation to early mortality among people with serious mental illness: Perspectives of peer support specialists and service users. Psychiatr Rehabil J. 2022;45:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Mucci A, Galderisi S, Gibertoni D, Rossi A, Rocca P, Bertolino A, Aguglia E, Amore M, Bellomo A, Biondi M, Blasi G, Brasso C, Bucci P, Carpiniello B, Cuomo A, Dell'Osso L, Giordano GM, Marchesi C, Monteleone P, Niolu C, Oldani L, Pettorruso M, Pompili M, Roncone R, Rossi R, Tenconi E, Vita A, Zeppegno P, Maj M; Italian Network for Research on Psychoses. Factors Associated With Real-Life Functioning in Persons With Schizophrenia in a 4-Year Follow-up Study of the Italian Network for Research on Psychoses. JAMA Psychiatry. 2021;78:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 7. | Tsapakis EM, Treiber M, Mitkani C, Drakaki Z, Cholevas A, Spanaki C, Fountoulakis KN. Pharmacological Treatments of Negative Symptoms in Schizophrenia-An Update. J Clin Med. 2024;13:5637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Strube W, Wagner E, Luykx JJ, Hasan A. A review on side effect management of second-generation antipsychotics to treat schizophrenia: a drug safety perspective. Expert Opin Drug Saf. 2024;23:715-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Lü W, Liu F, Zhang Y, He X, Hu Y, Xu H, Yang X, Li J, Kuang W. Efficacy, acceptability and tolerability of second-generation antipsychotics for behavioural and psychological symptoms of dementia: a systematic review and network meta-analysis. BMJ Ment Health. 2024;27:e301019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Shahien MM, Alshammari A, Ibrahim S, Ahmed EH, Atia HA, Elariny HA, Abdallah MH. Development of Glycerosomal pH Triggered In Situ Gelling System to Ameliorate the Nasal Delivery of Sulpiride for Pediatric Psychosis. Gels. 2024;10:608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Liang Y, Yang Y, Huang R, Ning J, Bao X, Yan Z, Chen H, Ding L, Shu C. Conjugation of sulpiride with a cell penetrating peptide to augment the antidepressant efficacy and reduce serum prolactin levels. Biomed Pharmacother. 2024;174:116610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Wang J, Omori IM, Fenton M, Soares B. Sulpiride augmentation for schizophrenia. Cochrane Database Syst Rev. 2010;CD008125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Guarro Carreras MT, Jiménez Suárez L, Lago García L, Montes Reula L, Neyra Del Rosario A, Rodríguez Batista FA, Velasco Santos M, Prados-Ojeda JL, Diaz-Marsà M, Martín-Carrasco M, Cardenas A. Towards full recovery with lurasidone: effective doses in the treatment of agitation, affective, positive, and cognitive symptoms in schizophrenia and of dual psychosis. Drugs Context. 2024;13:2024-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Miura I, Horikoshi S, Ichinose M, Suzuki Y, Watanabe K. Lurasidone for the Treatment of Schizophrenia: Design, Development, and Place in Therapy. Drug Des Devel Ther. 2023;17:3023-3031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 15. | Maruyama H, Sano F, Sakaguchi R, Okamoto K, Miura I. Effect of Lurasidone on Life Engagement in Schizophrenia: Post-Hoc Analysis of the JEWEL Study. Neuropsychiatr Dis Treat. 2024;20:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Japanese Society of Neuropsychopharmacology. Japanese Society of Neuropsychopharmacology: "Guideline for Pharmacological Therapy of Schizophrenia". Neuropsychopharmacol Rep. 2021;41:266-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Correll CU, Cucchiaro J, Silva R, Hsu J, Pikalov A, Loebel A. Long-term safety and effectiveness of lurasidone in schizophrenia: a 22-month, open-label extension study. CNS Spectr. 2016;21:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Patel PJ, Weidenfeller C, Jones AP, Nilsson J, Hsu J. Long-Term Assessment of Lurasidone in Schizophrenia: Post Hoc Analysis of a 12-Month, Double Blind, Active-Controlled Trial and 6-Month Open-Label Extension Study. Neurol Ther. 2021;10:121-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Citrome L, Cucchiaro J, Sarma K, Phillips D, Silva R, Tsuchiya S, Loebel A. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Loebel A, Cucchiaro J, Silva R, Mao Y, Xu J, Pikalov A, Marder SR. Efficacy of lurasidone across five symptom dimensions of schizophrenia: pooled analysis of short-term, placebo-controlled studies. Eur Psychiatry. 2015;30:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Meyer JM, Loebel AD, Schweizer E. Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs. 2009;18:1715-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Riva MA, Albert U, de Filippis S, Vita A, De Berardis D. Identification of clinical phenotypes in schizophrenia: the role of lurasidone. Ther Adv Psychopharmacol. 2021;11:20451253211012250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | García-López M, Alonso-Sánchez M, Leal I, Martín-Hernández D, Caso JR, Díaz-Caneja CM, Andreu-Bernabeu Á, Arango C, Rodriguez-Jimenez R, Sánchez-Pastor L, Díaz-Marsá M, Mellor-Marsá B, Ibáñez Á, Malpica N, Bravo-Ortiz MF, Baca-Garcia E, Ayuso-Mateos JL, Izquierdo A; Grupo AGES-CM. The relationship between negative symptoms, social cognition, and social functioning in patients with first episode psychosis. J Psychiatr Res. 2022;155:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Olivola M, Bassetti N, Parente S, Arienti V, Civardi SC, Topa PA, Brondino N. Cognitive Effects of Lurasidone and Cariprazine: A Mini Systematic Review. Curr Neuropharmacol. 2023;21:2431-2446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | He B, Yu L, Li S, Xu F, Yang L, Ma S, Guo Y. Neuroprotective effect of lurasidone via antagonist activities on histamine in a rat model of cranial nerve involvement. Mol Med Rep. 2018;17:6002-6008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Dembek C, Fan Q, Niu X, Mao Y, Anupindi VR, Laubmeier K, Tocco M. Impact of lurasidone on health-related quality of life in adults with bipolar depression: a post-hoc analysis. Curr Med Res Opin. 2022;38:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Facal F, Arrojo M, Páramo M, Costas J. Association between psychiatric admissions in patients with schizophrenia and IL-6 plasma levels polygenic score. Eur Arch Psychiatry Clin Neurosci. 2024;274:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Ghasemi Noghabi P, Shahini N, Salimi Z, Ghorbani S, Bagheri Y, Derakhshanpour F. Elevated serum IL-17 A and CCL20 levels as potential biomarkers in major psychotic disorders: a case-control study. BMC Psychiatry. 2024;24:677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Gragnoli C, Reeves GM, Reazer J, Postolache TT. Dopamine-prolactin pathway potentially contributes to the schizophrenia and type 2 diabetes comorbidity. Transl Psychiatry. 2016;6:e785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Subbanna M, Shivakumar V, Talukdar PM, Narayanaswamy JC, Venugopal D, Berk M, Varambally S, Venkatasubramanian G, Debnath M. Role of IL-6/RORC/IL-22 axis in driving Th17 pathway mediated immunopathogenesis of schizophrenia. Cytokine. 2018;111:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Lv H, Guo M, Guo C, He K. The Interrelationships between Cytokines and Schizophrenia: A Systematic Review. Int J Mol Sci. 2024;25:8477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Bredin SSD, Kaufman KL, Chow MI, Lang DJ, Wu N, Kim DD, Warburton DER. Effects of Aerobic, Resistance, and Combined Exercise Training on Psychiatric Symptom Severity and Related Health Measures in Adults Living With Schizophrenia: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2021;8:753117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Achtyes ED, Hopkins SC, Dedic N, Dworak H, Zeni C, Koblan K. Ulotaront: review of preliminary evidence for the efficacy and safety of a TAAR1 agonist in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023;273:1543-1556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/