Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.101658
Revised: April 10, 2025
Accepted: August 7, 2025
Published online: October 19, 2025
Processing time: 368 Days and 18.7 Hours
Schizophrenia is a complex psychiatric disorder with significant functional im
To evaluate the therapeutic efficacy and underlying mechanisms of Yueju pill combined with olanzapine in treating schizophrenia.
Ninety-seven patients with schizophrenia were randomly assigned to an inter
The intervention group demonstrated a significantly higher overall efficacy rate (93.75%) compared to the control group (73.47%, P < 0.05). Improvements in psychiatric symptoms, cognitive function, and social performance were more pronounced in the intervention group. Additionally, serum levels of BDNF, DA, and 5-HT were significantly higher in the intervention group (P < 0.05). Network pharmacology analysis revealed quercetin as a key com
Yueju pill, when combined with olanzapine, significantly improves clinical outcomes in schizophrenia patients, with safety comparable to olanzapine alone.
Core Tip: This study is the first to report the mechanism of Yueju pill combined with olanzapine in the treatment of schizophrenia. This study finds that Yueju pill combined with olanzapine does not increase adverse effects in the treatment of schizophrenia, demonstrating its safety. This study reveals the association between the upregulation of serum brain-derived neurotrophic factor, dopamine (DA), and serotonin levels and the therapeutic effects of Yueju pill combined with olanzapine in treating schizophrenia. This study preliminarily elucidates the therapeutic targets of Yueju pill through network pharmacology. This study identifies key genes related to the DA pathway, providing new theoretical foundations and molecular targets for the diagnosis and treatment of schizophrenia.
- Citation: Zhu DM, Lu Y, Xiao XD, Sun Y, Tao G, Long B, Zhao J. Enhancing schizophrenia treatment efficacy: The combined impact of Yueju pill and olanzapine through quercetin target modulation. World J Psychiatry 2025; 15(10): 101658
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/101658.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.101658
Schizophrenia is a chronic and complex mental disorder that affects millions of people worldwide[1]. The exact etiology remains unclear, but research indicates that a combination of genetic, environmental, and neurobiological changes and neurotransmitter imbalances contribute to the development of this disorder[2,3]. The primary symptoms of schizophrenia include hallucinations, delusions, disorganized thinking, reduced motivation, and social withdrawal, all of which severely impair personal life, social functioning, and occupational capacity. Currently, the mainstay of schizophrenia treatment is pharmacotherapy, particularly with antipsychotic drugs such as olanzapine[4]. However, while these medications are effective in controlling positive symptoms, their efficacy in treating negative symptoms and cognitive impairments is limited[5,6], and they are often associated with various side effects, such as weight gain and insulin resistance[7].
With the growing interest in traditional Chinese medicine (TCM) for treating mental disorders, Yueju pill, a traditional herbal formula, has garnered attention for its potential in treating schizophrenia[8]. This formula contains multiple herbal components whose combined effects are believed to play a positive role in schizophrenia treatment[9], particularly in improving negative symptoms and cognitive function[10]. However, the precise mechanisms of action of the Yueju pill remain unclear, and there is still insufficient scientific evidence to support its widespread use in clinical practice.
In recent years, network pharmacology has emerged as a novel research approach that integrates data from drug chemistry, biology, and pharmacoinformatics to explore drug mechanisms and identify potential therapeutic targets for diseases[11-13]. When combined with bioinformatics, this method effectively uncovers the complex interactions between drugs and diseases, offering a new perspective on the modern study of traditional medicines[14]. By using this approach, researchers can investigate the active components, targets, and regulatory networks of Yueju pill at the molecular level[15], providing scientific evidence for its potential mechanisms in the treatment of schizophrenia.
This study aims to evaluate the efficacy and possible mechanisms of Yueju pill combined with olanzapine in the treatment of schizophrenia. Through a randomized controlled clinical trial, the effects of the combined use of Yueju pill and olanzapine on negative symptoms, cognitive function, and social functioning in schizophrenia patients are assessed. Additionally, network pharmacology and bioinformatics methods are employed to analyze the changes in biomarkers in patients’ serum before and after treatment, revealing the molecular targets and mechanisms of action of Yueju pill and olanzapine.
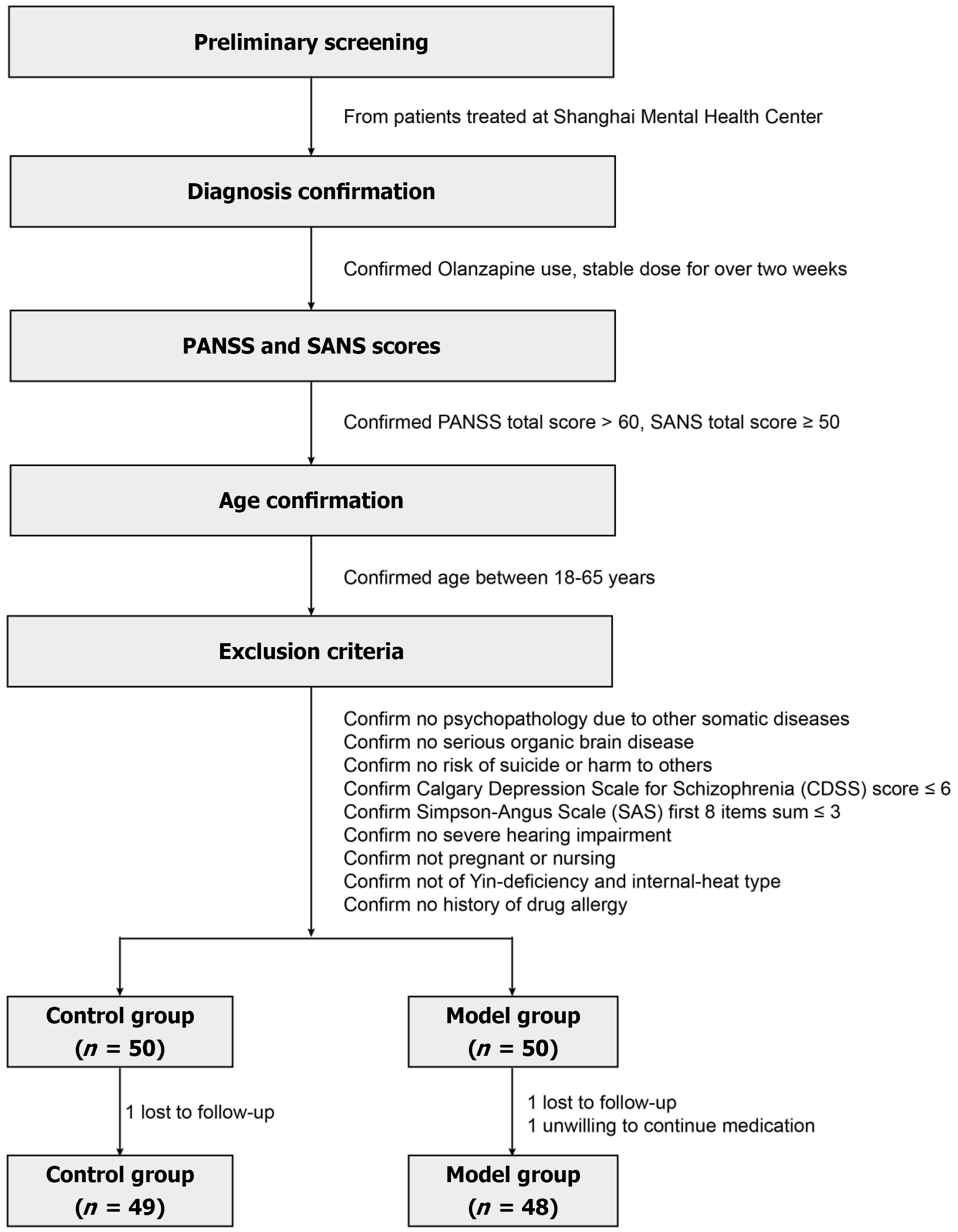
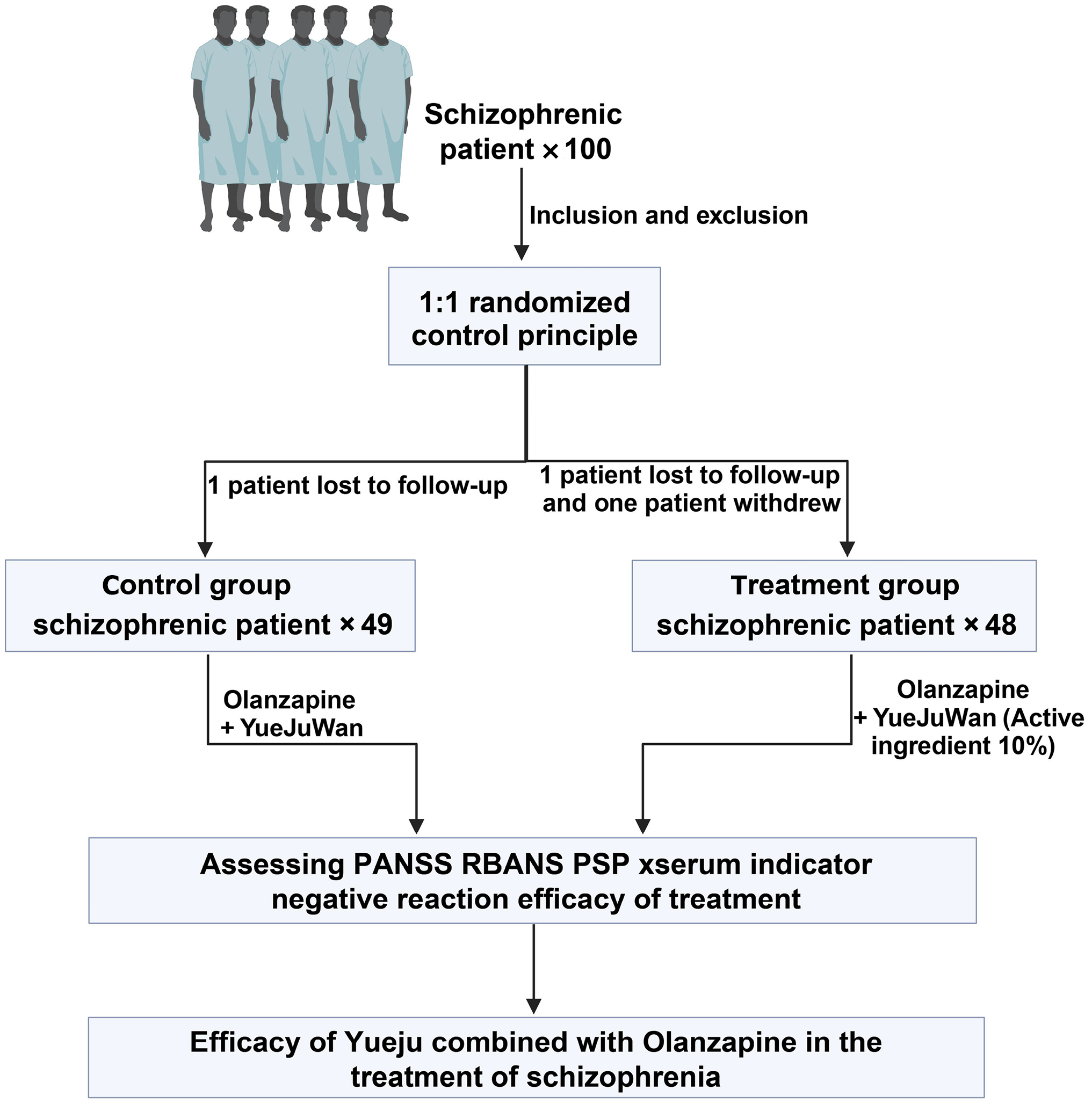
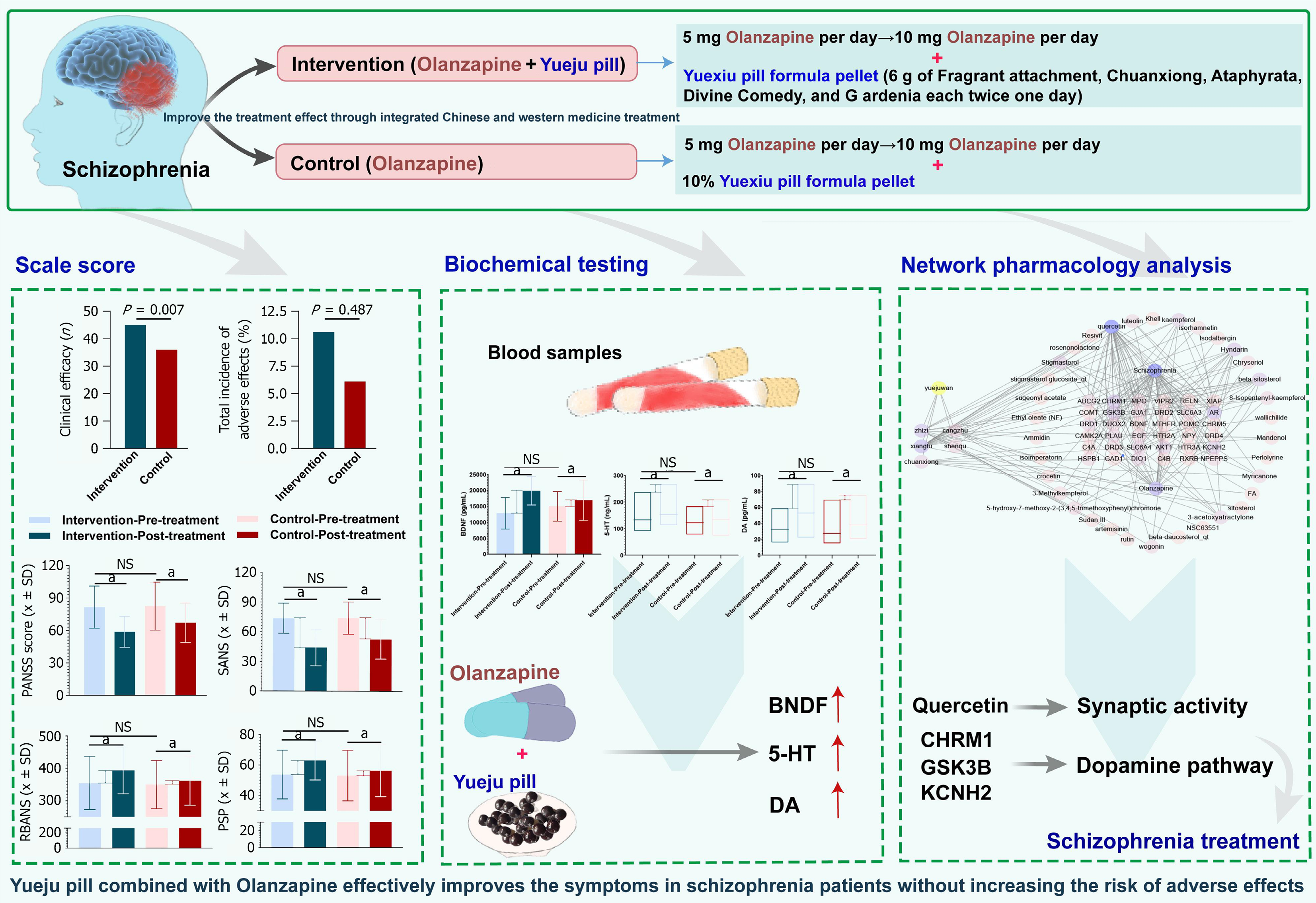
From November 2021 to January 2023, 100 patients diagnosed with schizophrenia were screened and enrolled at the Shanghai Mental Health Center for this study. Participants were randomly assigned in a 1:1 ratio to either the control group (n = 50) or the treatment group (n = 50), in accordance with the principles of a randomized controlled clinical trial (Figure 1). The study was approved by the Institutional Review Board of the Shanghai Mental Health Center (No. 2021-17). The inclusion and exclusion criteria for patient selection were as follows.
Inclusion criteria: (1) Diagnosis of schizophrenia according to the International Classification of Diseases criteria, specifically the liver-qi stagnation and phlegm-qi depression subtypes; (2) Stable use of olanzapine for more than two weeks, with the treatment regimen remaining unchanged during the study period; (3) A total positive and negative syndrome scale (PANSS) score of over 60 and a total scale for the assessment of negative symptoms (SANS) score of no less than 50; (4) Age between 18 and 65 years; and (5) Provision of signed informed consent by the patient or their legal guardian.
Exclusion criteria: (1) Psychotic symptoms are attributable to other physical illnesses; (2) Severe organic brain diseases; (3) High risk of suicide or behaviors that endanger oneself or others; (4) A Calgary depression scale score exceeding 6; (5) A total score of more than 3 on the first 8 items of the Simpson-Angus scale; (6) Severe hearing impairment; (7) Pregnant or postpartum women; (8) Patients with a yin deficiency have an internal heat pattern according to TCM pulse diagnosis; and (9) Individuals are allergic to the study medications.
Participants were allocated to the experimental or control groups based on differing treatment protocols, with an initial plan to enroll 50 participants in each group. During the course of the study, one patient in the control group was lost to follow-up, resulting in 49 patients completing the trial. In the experimental group, two patients dropped out: One due to loss to follow-up and one due to unwillingness to continue the medication, leaving 48 patients who completed the trial. Thus, the final dataset included 97 participants.
Explanation of patient attrition: In the control group, one participant was lost to follow-up. In the experimental group, one patient was lost to follow-up and another withdrew voluntarily. Consequently, data from 97 patients were included in the final analysis. Baseline comparisons between the two groups showed no statistically significant differences (P > 0.05), indicating sample homogeneity (Supplementary Table 1). All study procedures and protocols were approved by the Ethics Committee of Zhenjiang Hospital of Traditional Chinese Medicine (No. 2021-17).
All patients received a standardized treatment regimen based on olanzapine (HANSOH PHARMA, Jiangsu Province, China), starting at 5 mg/day. The dose was increased to 10 mg/day after the first week, with adjustments permitted up to a maximum of 20 mg/day to ensure safety. Participants were randomly assigned to either the intervention or control group in a 1:1 ratio using a computer-generated randomization sequence. Group allocations were concealed in sealed, opaque envelopes to maintain blinding (Figure 2). Blinding was ensured by matching the appearance, taste, and smell of Yueji pill and the control granules. Given the distinctive odor of Yueji pill, flavor-masking strategies were employed, including sealed packaging, odor-absorbing materials, and neutralizing agents. Both products were encapsulated in identical capsules containing taste-masking excipients. Due to the potential sedative effects of Yueji pill within one hour post-administration, primary outcomes were assessed 1-2 hours after dosing. Interaction with participants during this period was minimized to reduce the risk of unblinding. This study was conducted as a double-blind trial. Patients in the intervention group received Yueji pill granules, each sachet containing active ingredients equivalent to 6 g of Cyperus rotundus, Ligusticum chuanxiong, Atractylodes lancea, Massa Medicata Fermentata, and Gardenia jasminoides. The granules were administered twice daily, 30 minutes after breakfast and dinner, dissolved in hot water. Control group participants received placebo granules containing 10% of the active ingredients, along with flavoring and coloring agents to ensure indistinguishability in packaging, taste, smell, and color.
All medication preparation and distribution were managed by an independent pharmacist. Data collection and analysis were performed by blinded statisticians. The treatment period lasted 8 weeks, and adherence was monitored through returned sachet counts and patient self-reports.
All assessments were conducted by a psychiatrist blinded to group assignments to ensure objectivity and consistency. The PANSS was used to evaluate overall psychiatric status, comprising three subscales: Positive symptoms (range: 7-49), negative symptoms (range: 7-49), and general psychopathology (range: 16-112), with higher scores indicating greater symptom severity. The SANS was additionally used to assess negative symptoms in more detail, where higher scores also reflect increased severity. Evaluations were performed at baseline and after treatment to determine therapeutic effects.
Cognitive function was evaluated using the repeatable battery for the assessment of neuropsychological status (RBANS), which is designed to detect cognitive changes in patients with schizophrenia. The scale assesses five cognitive domains: Immediate memory, delayed memory, language, attention, and visuospatial/constructional abilities. Each domain is scored based on standardized subtests, with higher scores reflecting better cognitive performance. RBANS assessments were conducted at baseline and post-treatment to quantify cognitive changes and evaluate therapeutic efficacy.
Social functioning was assessed using the personal and social performance scale (PSP), which provides a global score from 0 to 100, with higher scores indicating better functioning in four domains: Socially useful activities, personal and social relationships, self-care, and disturbing and aggressive behaviors. The PSP enables quantitative evaluation of social adaptation and was used to assess treatment-related changes in functional outcomes.
Peripheral venous blood (4 mL) was collected from each participant before and after treatment to assess serological changes. Samples were centrifuged at 4 °C (3000 rpm, 13.5 cm radius) for 10 minutes to obtain serum. Serum levels of brain-derived neurotrophic factor (BDNF), dopamine (DA), and serotonin (5-HT) were measured using enzyme-linked immunosorbent assay kits (RD Systems, United States). These biomarkers were used to evaluate the neurobiological effects of the intervention.
The safety of olanzapine combined with Yueju pill granules in the treatment of schizophrenia was evaluated by monitoring adverse events (AEs) throughout the treatment period. Common AEs including excessive sleepiness, weight gain, nausea, and galactorrhea were recorded and analyzed based on their frequency, severity, and potential association with the treatment. All AEs were classified and graded according to the World Health Organization criteria. A weight gain exceeding 10% from baseline was defined as clinically significant. Nausea occurring more than five times per day was considered an adverse event. Galactorrhea was identified when prolactin receptor expression levels exceeded the normal range by more than 10% in female patients[16]. Monitoring and documentation of all AEs followed internationally accepted pharmacovigilance standards. Evaluations were performed by experienced clinicians to ensure data accuracy and reliability.
Treatment efficacy was assessed using the reduction rate of the SANS, calculated as: Reduction rate = [(SANS before treatment - SANS after treatment)/SANS before treatment] × 100%. Based on this reduction rate, patients were classified into four response categories: No improvement (reduction rate ≤ 25%), improvement (reduction rate between 25% and 50%), significant progress (reduction rate between 50% and 75%), and clinical recovery (reduction rate > 75%). The overall efficacy rate was calculated using the following formula: (Number of improved cases + significantly improved cases + recovered cases)/total number of cases × 100%.
The GSE46509 dataset, consisting of parvalbumin-expressing cell samples from the layer III of the temporal lobe hippocampus in eight healthy controls and eight patients with schizophrenia, was analyzed for gene expression differences. Differential expression analysis was performed using the “limma” package in R. Genes with an absolute log2 fold change greater than 2 and a P value less than 0.05 were considered significantly differentially expressed.
Correlation analyses were conducted with symptom improvement (e.g., the reduction in symptom scores) as the dependent variable. Independent variables included individual variability factors and changes in biomarkers (e.g., pre- to post-treatment differences in biomarker concentrations). Regression coefficients were calculated to estimate the extent of symptom improvement associated with a one-unit change in each biomarker.
When the relationship between variables was uncertain or potentially non-linear, correlation strength was evaluated using either Spearman’s rank correlation coefficient or Pearson’s correlation coefficient. Spearman’s coefficient is appropriate for non-normally distributed data and is based on ranked values, while Pearson’s coefficient is used for normally distributed data and is based on covariance and standard deviations. Correlation coefficients range from -1 to 1, with values closer to ± 1 indicating stronger associations, and 0 indicating no correlation[17-19].
Schizophrenia-related target genes were identified using the OMIM database (https://omim.org/) and the GeneCards database (https://www.genecards.org/). The search term “Cholestatic liver injury” was used, with a relevance score threshold set at > 20 to retrieve relevant genes. The differentially expressed genes (DEGs) were then merged with the schizophrenia-related target genes to obtain disease targets. The intersection of these targets with the targets of the active components of Yueju pill combined with olanzapine was identified to determine potential targets regulated by the combination therapy. A Venn analysis was conducted using the online analysis platform to visualize the results.
Using Cytoscape 3.7.2 software, we constructed a “drug-component-target-disease” network. The network topology was analyzed by calculating topological parameters using the degree centrality algorithm provided by the cytoNCA plugin.
Protein-protein interaction (PPI) analysis was performed using the STRING database (https://string-db.org), with the species limited to Homo sapiens. The confidence score threshold was set to the highest level (0.900), and disconnected nodes were removed. All other parameters were set to default values. The resulting PPI network was imported into Cytoscape version 3.7.2 for further analysis. The cytoNCA plugin was applied using the degree centrality algorithm to identify key targets. Critical genes were ranked based on degree values within the network.
Using the online analysis platform and the R package “ClusterProfiler”, we conducted Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses on the candidate targets, with a significance threshold set at P < 0.05. The GO analysis included assessments of biological processes (BP), molecular functions (MF), and cellular components (CC), ultimately identifying the primary cellular functions and signaling pathways influenced by the candidate targets (Supplementary Table 2).
Statistical analyses were performed using SPSS version 28.0. Continuous variables with a normal distribution (e.g., PANSS and SANS scores) were expressed as mean ± SD. Between-group comparisons were conducted using independent-sample t-tests, while within-group comparisons (pre- and post-treatment) were performed using paired-sample t-tests. Repeated measures analysis of variance and Pearson’s correlation analyses (based on raw values)[17] were applied where appropriate. For non-normally distributed data (e.g., DA and 5-HT levels), values were reported as median and interquartile range, and comparisons were made using non-parametric tests. Categorical variables were presented as counts (n) and percentages (%), with group differences assessed using the χ2 test or Fisher’s exact test. A P value < 0.05 was considered statistically significant.
In this study, baseline comparisons were conducted between the intervention group and the control group in terms of gender ratio, age, duration of illness, years of education, and PANSS scores. The results indicated no significant differences between the two groups in gender ratio, age, or years of education. While the difference in the duration of illness approached significance, it did not reach statistical significance. Similarly, there was no significant difference in PANSS scores between the groups. These findings suggest that the study successfully achieved a balance between the groups at baseline, providing a fair starting point for further comparison of the intervention effects.
Previous studies have demonstrated that Yueju pill alone significantly reduces Hamilton depression scale-24 (HAMD-24) scores and is effective in treating major depressive disorder[17,20]. Additionally, clinical evidence suggests that combination therapy with Yueju pill and fluoxetine results in significant symptom improvement between days 3 and 7 of treatment (P < 0.05)[21].
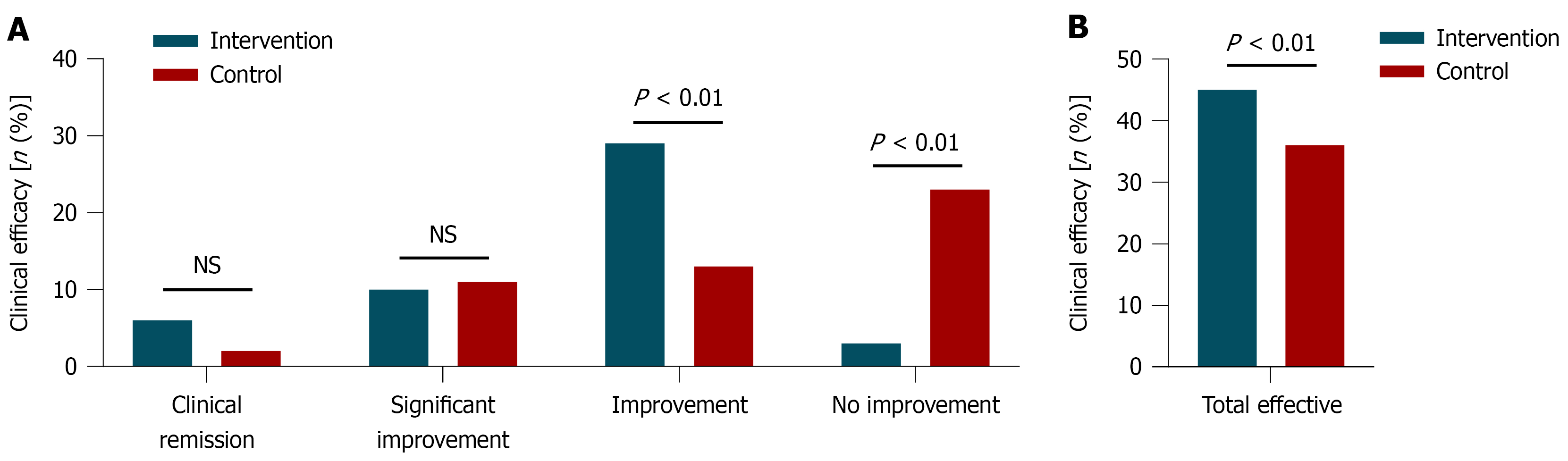
Clinical efficacy was compared between the Intervention and control groups based on SANS reduction rates (Supplementary Table 3). In the intervention group (n = 48), 6 patients achieved clinical recovery (12.50%), 10 showed significant progress (20.83%), 29 improved (60.42%), and 3 had no improvement (6.25%), resulting in an overall efficacy rate of 93.75%. In the control group (n = 49), 2 patients achieved clinical recovery (4.08%), 11 showed significant progress (22.45%), 13 improved (26.53%), and 23 had no improvement (46.94%), yielding an overall efficacy rate of 73.47%. A higher proportion of patients in the intervention group achieved clinical recovery and overall improvement compared to the control group, particularly in the clinical recovery category, where the rate was more than three times higher. The proportion of non-responders in the intervention group was also markedly lower. χ² analysis showed a statistically significant difference between groups (χ² = 7.24, P = 0.007), supporting the superior efficacy of the intervention regimen (Figure 3).
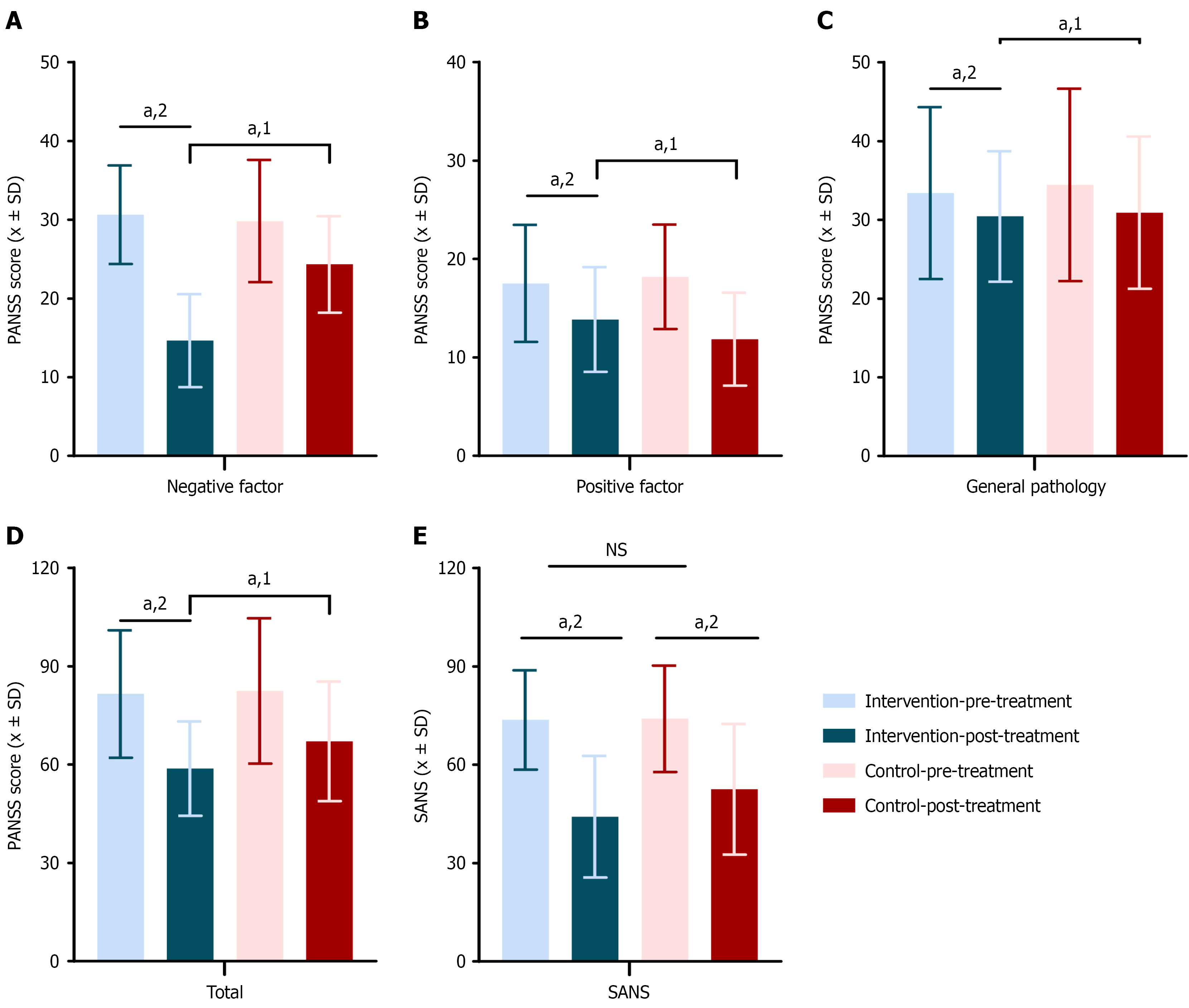
Psychiatric symptoms were systematically evaluated using the PANSS and the scale for the SANS, with results presented in Supplementary Table 4. At baseline, there were no statistically significant differences between the intervention group (n = 48) and the control group (n = 49) in PANSS and SANS scores, indicating comparability between the two groups.
Following treatment, both groups exhibited significant reductions in PANSS and SANS scores (P < 0.05), reflecting overall therapeutic effects. Notably, the Intervention group showed significantly greater improvements in PANSS negative symptom scores (P < 0.001), PANSS total scores (P = 0.015), and SANS scores (P = 0.034) compared to the control group, indicating superior efficacy in reducing negative symptoms and improving overall psychiatric status. No significant differences were observed between groups in the improvement of PANSS positive symptom scores (P = 0.054) or general psychopathology scores (P = 0.794). These findings suggest that the interventions had limited differential effects on positive symptoms and general psychopathological features (Figure 4).
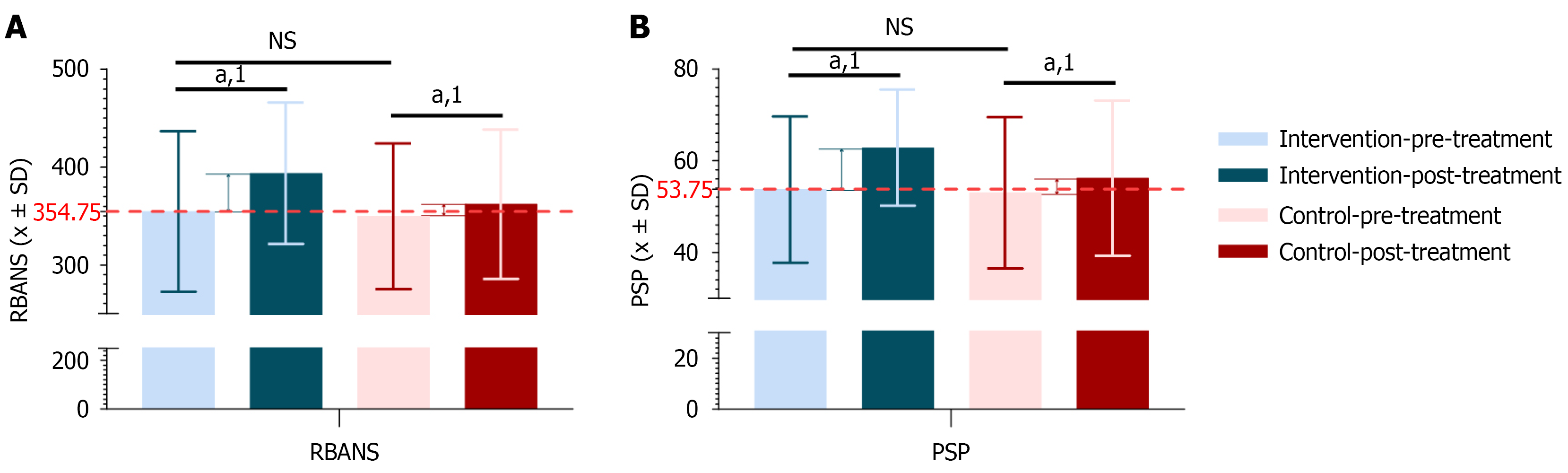
Cognitive and social functioning were evaluated using the RBANS and the PSP, respectively (Supplementary Table 5). At baseline, no significant differences were observed in RBANS and PSP scores between the Intervention group (n = 48) and the control group (n = 49), indicating comparable levels of cognitive and social functioning.
Post-treatment, both groups demonstrated significant improvements in RBANS and PSP scores (P < 0.05), indicating general benefits from the interventions. However, the magnitude of improvement was significantly greater in the Intervention group compared to the control group (RBANS: P = 0.040; PSP: P = 0.030) (Figure 5), suggesting superior effectiveness of the combined treatment in enhancing cognitive function and social adaptation.
Improvements in cognitive function are critical for enhancing daily functioning and social engagement, while better social functioning facilitates community integration and interpersonal relationships. These findings emphasize the importance of addressing not only symptom reduction but also functional recovery in the treatment of schizophrenia.
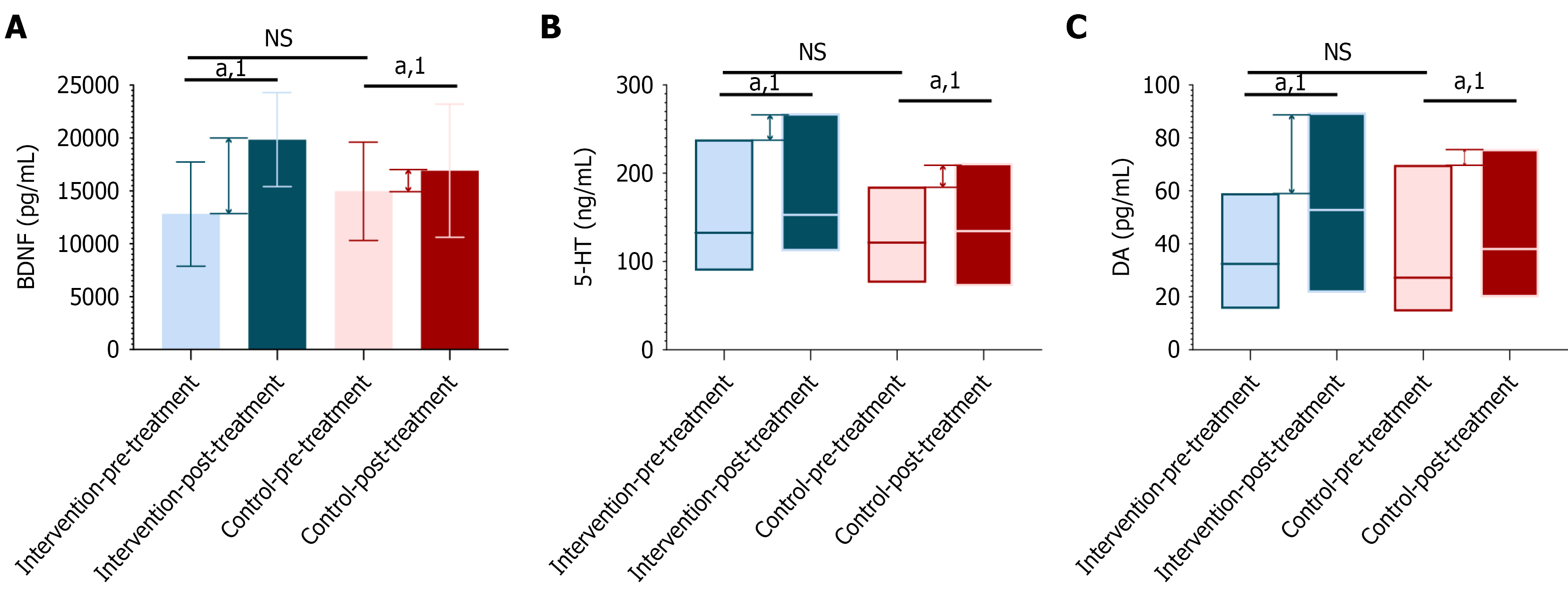
The changes in serum levels of BDNF, 5-HT, and DA in schizophrenia patients are complex and influenced by various factors, including the biological characteristics of the disease itself, the types of treatment drugs used, and their effects on these neurotransmitters. Regarding BDNF, numerous studies have shown that serum BDNF levels are generally lower in schizophrenia patients. For instance, one study found that serum BDNF levels were significantly reduced in chronic schizophrenia patients compared to healthy controls[22]. Another study supported this finding, noting that serum BDNF levels in elderly patients with depression and mild Alzheimer’s disease were lower than in normal controls[23]. Additionally, research has indicated that antipsychotic drugs and physical therapy can increase serum BDNF levels in schizophrenia patients[24]. This suggests that BDNF may play a significant role in the pathophysiology of schizophrenia, and its levels may be related to disease severity, changes in cognitive function, and treatment outcomes. Concerning 5-HT, while direct studies on serum 5-HT levels in schizophrenia patients are limited, existing research has shown that certain antidepressants can improve depressive symptoms by regulating serum 5-HT levels[25]. This implies that 5-HT may also play a crucial role in schizophrenia and other psychiatric conditions. DA is known to be a key neurotransmitter in the brain and is closely associated with many symptoms of schizophrenia[26,27]. Therefore, changes in DA levels may be an important factor in the pathophysiology of schizophrenia. As such, analyzing changes in serum BDNF, 5-HT, and DA levels can provide insight into the biological changes in schizophrenia patients before and after treatment. These changes may reveal the biological mechanisms of the disease, help evaluate treatment efficacy, guide the design of individualized treatment plans, offer new drug targets, and predict patient outcomes. In the comparison of pre-treatment levels of BDNF, 5-HT, and DA between the intervention and control groups, no statistically significant differences were found, indicating that the biochemical indicators of the two groups were comparable before treatment (Supplementary Table 6).
Following treatment, both the intervention and control groups exhibited increases in serum BDNF, 5-HT, and DA levels, indicating that the treatment methods employed were effective in activating or elevating these neurotransmitter levels. The increase in these neurotransmitters is associated with improvements in schizophrenia symptoms, particularly BDNF, which has been shown to play a crucial role in neurodevelopment, synaptic plasticity, and the maintenance of cognitive function[28-30]. The increases in 5-HT and DA are also linked to the normalization of mood regulation and reward systems[28], both of which are key targets in the treatment of schizophrenia (Supplementary Table 6).
Notably, the increases in BDNF, 5-HT, and DA levels were significantly greater in the intervention group compared to the control group, suggesting that the treatment regimen used in the Intervention group was more effective in promoting neurotransmitter production and improving psychiatric symptoms. These differences were statistically significant (BDNF: P = 0.010, 5-HT: P = 0.006, DA: P = 0.008), providing evidence of the superiority of the Intervention group’s treatment approach (Figure 6 and Supplementary Table 6).
From a biomarker perspective, these results support the positive biochemical impact of the intervention group’s treatment strategy on schizophrenia, and they provide a biological basis for further exploration of the pathophysiological mechanisms and therapeutic pathways in schizophrenia.
Monitoring AEs is essential for evaluating the safety of antipsychotic treatments. This study assessed the safety profiles of two treatment regimens for schizophrenia by recording adverse reactions during the intervention period.
A total of 97 patients were included, with 48 in the Intervention group and 49 in the control group. In the intervention group, AEs included excessive sleepiness (2.08%), weight gain (2.08%), nausea (4.16%), and galactorrhea (2.08%). In the control group, similar AEs were observed, with the exception of excessive sleepiness, which was not reported. The overall incidence of AEs was 10.42% in the intervention group and 6.12% in the control group. Statistical analysis showed no significant difference between the two groups (P = 0.487) (Supplementary Table 7).
These findings suggest that the combination of Yueju pill granules with olanzapine does not significantly increase the risk or severity of adverse reactions compared to olanzapine alone. Although excessive sleepiness was reported only in the intervention group, the overall safety profiles of both regimens were comparable. This is consistent with previous studies[31], which reported that combining TCM with conventional psychotropic agents generally yields good safety outcomes without a significant increase in AEs.
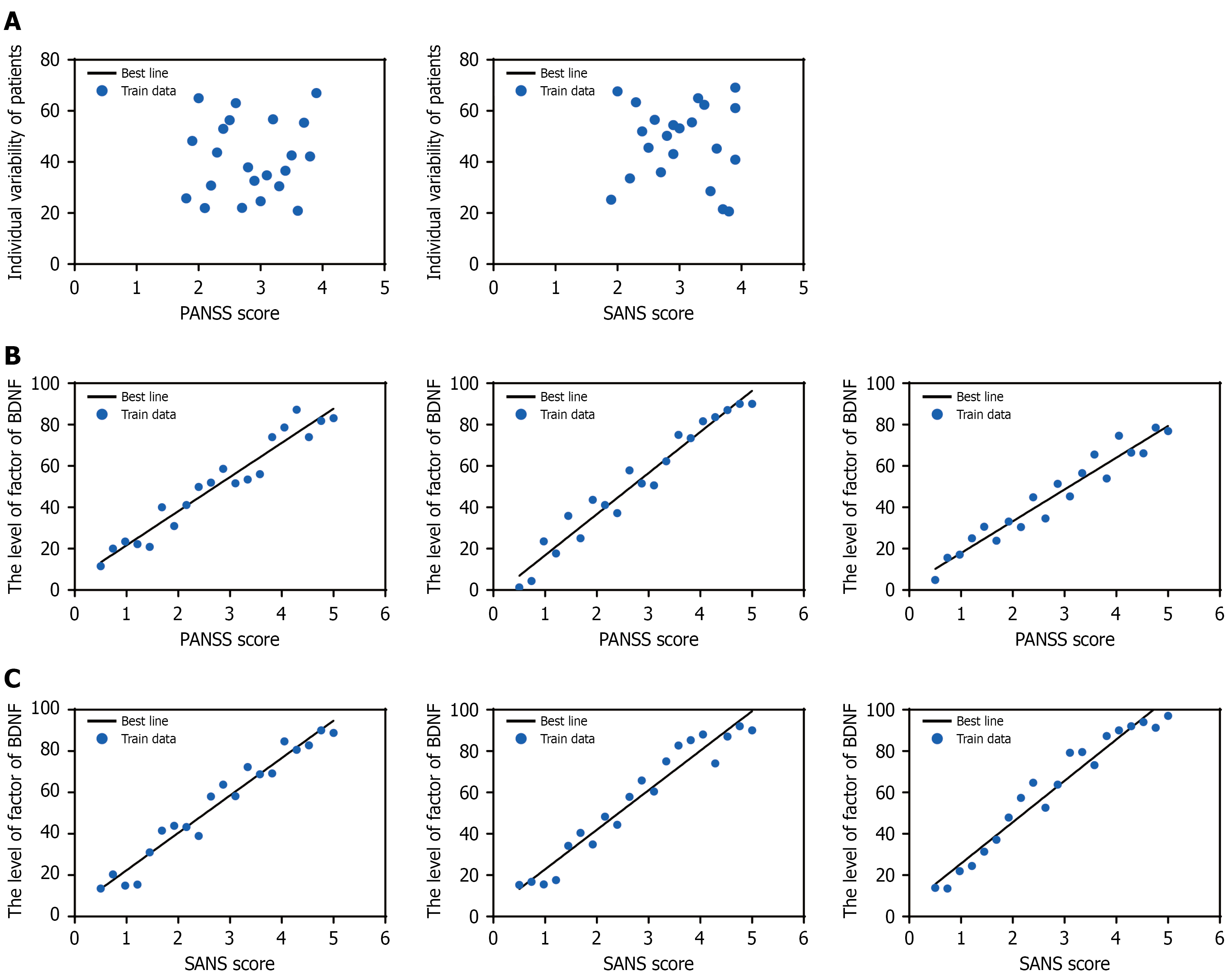
Correlation analyses were conducted to examine the relationships between individual variability, changes in serum biomarkers, and symptom improvement. No significant correlations were found between individual variability and changes in PANSS or SANS scores (Figure 7A). In contrast, changes in BDNF, DA, and 5-HT levels were positively correlated with symptom improvement (Figure 7B and C). These results suggest that alterations in these biomarkers may serve as indicators of clinical improvement and hold potential as prognostic markers. While the combination therapy demonstrated a favorable safety profile, continued monitoring for individual adverse reactions remains essential. Clinicians are advised to assess tolerability on a case-by-case basis and closely observe for AEs to ensure treatment safety and efficacy.
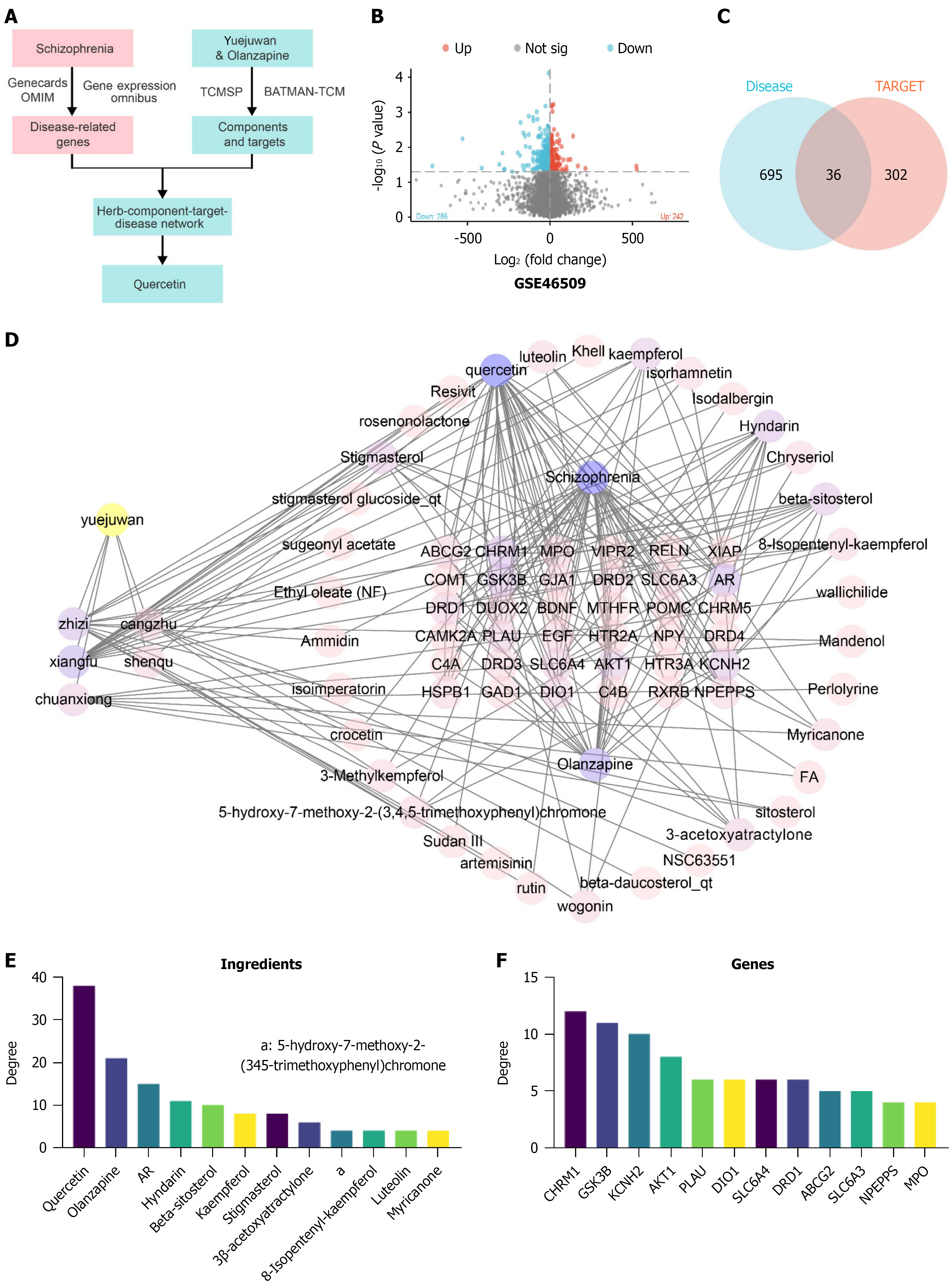
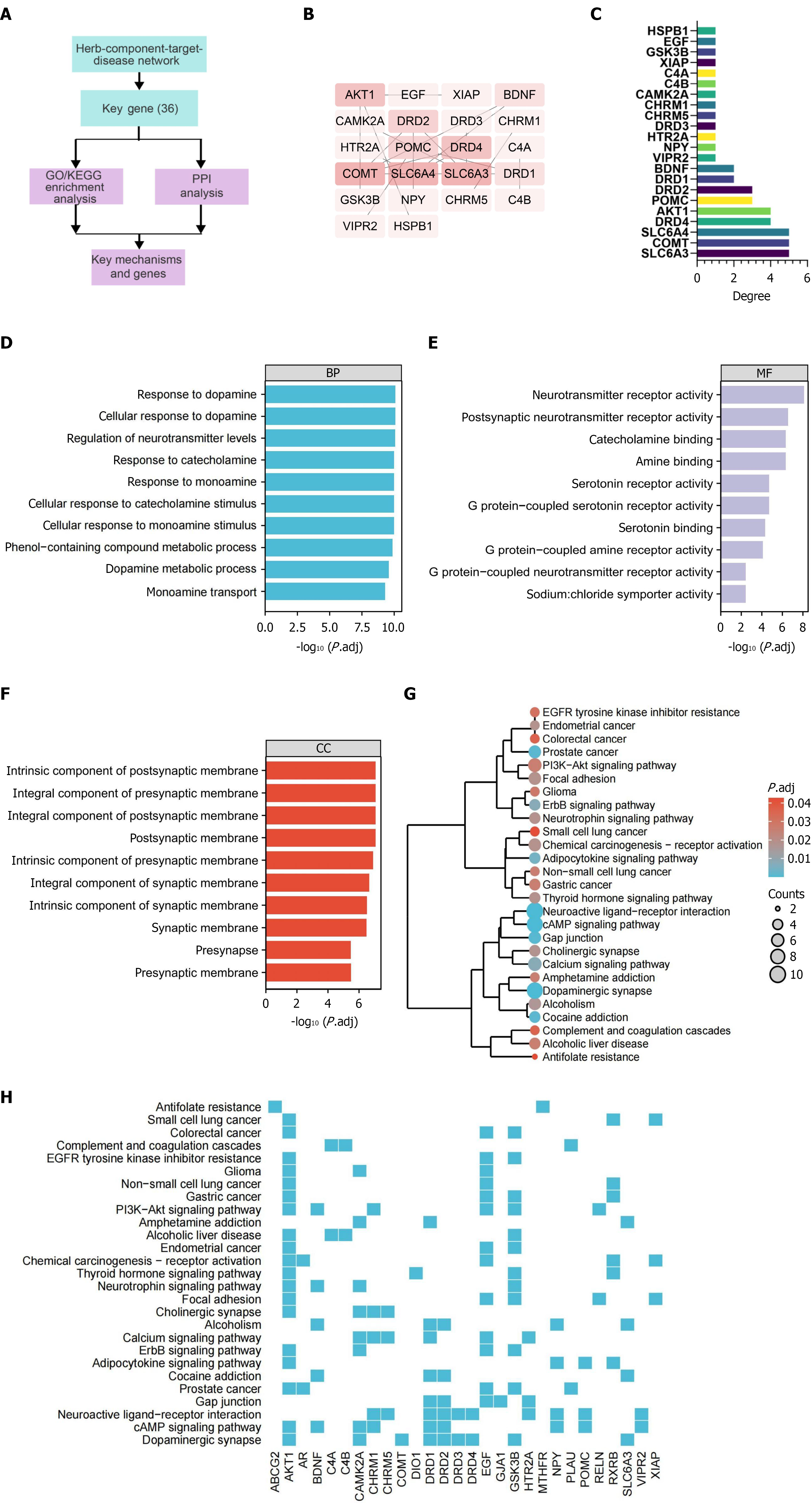
To identify candidate genes and chemical components that could alleviate schizophrenia, we conducted a network pharmacology screening (Figure 8A). Using the schizophrenia expression profiles from the GSE48072, GSE62191, and GSE46509 datasets, we applied a threshold of |log2 (fold change)| > 2 and a significance level of P < 0.05 to identify 528 differentially expressed targets (Figure 8B), noting that no DEGs were found in the GSE48072 and GSE62191 datasets. Additionally, we screened 67 schizophrenia-related genes from the GeneCards database (Supplementary Table 8) and 163 genes from the OMIM database (Supplementary Table 9). After removing duplicate targets from the three screening results, we obtained a total of 731 schizophrenia-related targets (Supplementary Table 10).
We retrieved the active chemical components of Yueju pill combined with olanzapine from the TCMSP database and performed further screening of these components. From the TCMSP database, we identified 53 active components of Yueju pill (Supplementary Table 11). The targets of these 53 chemical components were also retrieved from the TCMSP database, totaling 996 targets. Additionally, the targets for olanzapine components numbered 148. After removing duplicates, 338 unique targets of the active chemical components of Yueju pill combined with olanzapine were identified (Supplementary Table 12). By intersecting these drug targets with the 731 schizophrenia-related disease targets, we discovered 36 candidate genes (Figure 8C and Supplementary Table 13).
Next, we imported the data into Cytoscape software to construct a network that includes Yueju pill combined with olanzapine, its active components, targets, and related diseases (Figure 8D). Node analysis conducted through the software revealed that quercetin had the highest degree (the number of connections a node has with other nodes) at 38.0, followed by olanzapine with a degree of 21 (Figure 8E). This suggests that quercetin plays a significant role in the therapeutic effects of Yueju pill combined with olanzapine in alleviating schizophrenia. One study suggests that in schizophrenia, quercetin may elevate synaptic activity levels beyond a critical threshold, triggering a compensatory mechanism that restores inhibition and functional balance in the prefrontal cortex by correcting impaired GABAergic transmission[32]. Additionally, quercetin has been reported to improve behavioral disorders in schizophrenia models and can be used to enhance antipsychotic therapies[33]. Furthermore, previous studies have suggested that quercetin may serve as a potential preventive and therapeutic agent for schizophrenia by attenuating oxidative stress and cytokine toxicity, modulating neurotransmission, and preventing increased DNA fragmentation, thereby improving psychosis-like symptoms[33,34]. These findings are consistent with our results, supporting the hypothesis that quercetin may be one of the key active compounds contributing to the therapeutic effects observed in this study.
Among the key genes identified, CHRM1, GSK3B, KCNH2, AKT1, PLAU, DIO1, SLC6A4, DRD1, ABCG2 and SLC6A3 ranked in the top 10 based on degree values (Figure 8F). CHRM1, GSK3B, and KCNH2 are three genes that play crucial roles in neuropsychiatric disorders. CHRM1 is involved in cognitive function through its interaction with G-protein coupled receptors, and its abnormal expression in schizophrenia may lead to disruptions in cognitive and perceptual processes[35,36]. GSK3B plays a multifaceted role in neural cell signaling and cytoskeletal maintenance, with alterations in its function linked to mood and thought process disturbances in schizophrenia[37]. KCNH2 encodes a key potassium channel protein in the heart and nervous system, with variations associated with long QT syndrome and potential impacts on neuronal excitability and signal transmission in schizophrenia patients[38,39]. The interaction and regulation of these genes are not only critical for maintaining normal neurological function but also play key roles in the pathogenesis of neuropsychiatric disorders like schizophrenia.
In summary, through network pharmacology analysis, we have identified multiple potential therapeutic targets for the treatment of schizophrenia with Yueju pill combined with olanzapine. The analysis has also highlighted the therapeutic potential of key chemical components such as quercetin. The roles of CHRM1, GSK3B, and KCNH2 in the pathophysiology of schizophrenia underscore their importance in neural signal transmission and cardiac physiological functions.
To further elucidate the molecular mechanisms potentially involved in schizophrenia, bioinformatics analysis was performed on the 36 candidate genes identified from the network pharmacology screening (Figure 9A). PPI data were retrieved from the STRING database and visualized using Cytoscape (Figure 9B). Network topology analysis revealed that SLC6A3, COMT, SLC6A4, DRD2, and AKT1 had the highest degree values, indicating their central positions within the PPI network (Figure 9C).
GO and KEGG enrichment analyses were subsequently conducted. Under a statistical threshold of P < 0.01, GO BP terms were primarily associated with DA response, DA signaling, and regulation of neurotransmitter levels. In the CC category, enriched terms included the postsynaptic membrane and presynaptic membrane structures. In the MF category, significant enrichment was observed in neurotransmitter receptor activity, postsynaptic neurotransmitter receptor activity, and amine binding (Figure 9D-F). KEGG analysis identified 27 significantly enriched signaling pathways, including dopaminergic synapse, cyclic adenosine monophosphate signaling, and neuroactive ligand-receptor interaction pathways (Figure 9G-H). Pathway clustering using Ward’s minimum variance method indicated that the neuroactive ligand-receptor interaction pathway involved 10 of the candidate genes, while the dopaminergic synapse pathway showed the lowest P value, suggesting a predominant role for DA-related signaling in schizophrenia pathophysiology. These findings indicate that the candidate genes are enriched in pathways related to neurotransmission, with DA signaling emerging as a key functional axis.
Schizophrenia, as a chronic mental disorder, has a variety of treatment approaches, but the efficacy of these treatments varies significantly[40]. Traditionally, olanzapine, an antipsychotic drug, has been widely used, but its side effects and limited efficacy present challenges in clinical treatment[41]. This study demonstrates that the combination of the TCM Yueju pill with olanzapine provides superior efficacy compared to the use of olanzapine alone. Compared to previous studies, this approach significantly increases the overall treatment efficacy, highlighting the advantages of integrating traditional Chinese and Western medicine in the treatment of schizophrenia.
The combination of Yueju pill and olanzapine achieved a total treatment efficacy rate of 93.75%, which was significantly higher than that of olanzapine monotherapy. This improvement was reflected in greater reductions in PANSS and SANS scores, as well as enhanced social functioning outcomes. Compared with previous interventions such as cognitive behavioral therapy and social skills training, the combination therapy demonstrated superior effectiveness in functional improvement. These results highlight the potential of integrated pharmacological strategies, particularly the combination of TCM with conventional antipsychotics, in enhancing clinical outcomes in schizophrenia.
The observed clinical benefits may be attributed to the synergistic effects of multiple bioactive compounds in Yueju pill, which is a classic TCM formula composed of Cyperus rotundus, Ligusticum chuanxiong, Atractylodes lancea, Gardenia jasminoides, and Massa Medicata Fermentata. In TCM theory, Cyperus rotundus is regarded as a key herb for relieving emotional stagnation and regulating liver qi. Ligusticum chuanxiong promotes blood circulation and supports the qi-regulating action of Cyperus. Gardenia jasminoides helps clear internal heat, which is thought to arise from liver qi stagnation transforming into fire. While the pharmacological mechanisms of Yueju pill remain to be fully elucidated, our findings suggest that its efficacy may involve modulation of neurotrophic and neurotransmitter systems, offering a modern scientific basis for its application.
Following treatment, significant increases in serum levels of BDNF, DA, and 5-HT were observed. These changes offer new perspectives on the neurobiological basis of schizophrenia. BDNF plays a key role in neuronal survival, synaptic plasticity, and neurogenesis, and is abundantly expressed in the hippocampus and cortex[42]. DA, a critical neurotransmitter in motor control, reward processing, and cognition, acts predominantly in the nigrostriatal, mesolimbic, and mesocortical pathways[43]. 5-HT, primarily located in the raphe nuclei and projecting to widespread brain regions, is involved in mood regulation, sleep, appetite, and cognition[44]. The magnitude of increase in these markers following combination therapy exceeded that reported in prior studies involving monotherapies, suggesting a possible synergistic effect. This may be due to the multi-target action of Yueju pill components, which could collectively contribute to restoring neurotransmitter balance and enhancing neurobiological resilience.
Network pharmacology provides a powerful tool for understanding the complex mechanisms of drug action. In this study, quercetin was identified as a potential key component, aligning with existing literature that reports its antioxidant and neuroprotective effects[45,46]. Additionally, this study reveals possible interactions between quercetin and other chemical components, an aspect rarely reported in previous research.
Our gene analysis focused on CHRM1, GSK3B, and KCNH2, genes that are associated with the known pathological mechanisms of schizophrenia, particularly in relation to the DA pathway[47-49]. This finding is consistent with previous research, but our study provides additional insights into the roles of these genes in the context of combined traditional Chinese and Western medicine therapy. This information offers new directions for future drug discovery and optimization of treatment strategies.
Among the top five high-degree genes identified in the PPI network-SLC6A3, COMT, SLC6A4, DRD2, and AKT1-each plays a well-established role in neurotransmitter regulation relevant to schizophrenia. SLC6A3 and SLC6A4 encode transporters for DA and 5-HT, respectively, both of which are targets modulated by olanzapine, a D2 and 5-HT2A receptor antagonist[50,51]. DRD2, encoding the D2 receptor, is a direct pharmacological target of olanzapine[52]. COMT, involved in catecholamine metabolism, and AKT1, a key intracellular kinase regulating synaptic plasticity, have also been linked to antipsychotic treatment response[53]. Furthermore, several active compounds in Yueju pill have been shown to modulate GABAergic and monoaminergic systems, which may indirectly affect the expression or activity of these genes. Thus, these high-degree genes are not only central in schizophrenia-related networks but also pharmacologically relevant to both agents used in this study.
The diversity in individual genetic and biochemical profiles contributes to variability in treatment response among patients with schizophrenia. Network pharmacology provides a promising tool for identifying interactions between genes and chemical compounds, which may support the development of precision medicine strategies. In this study, we constructed a network linking the active components of Yueju pill with their potential gene targets, and identified several key genes that may be modulated by these compounds. Differences in the expression or regulation of these genes across patient subtypes may influence treatment outcomes. For instance, subtypes characterized by dysregulation of monoaminergic or GABAergic pathways may be more responsive to compounds in Yueju pill that act on these systems. Additionally, biochemical markers such as BDNF, DA, and 5-HT, which showed significant post-treatment changes, could serve as stratification tools to identify patients likely to benefit from this intervention. Therefore, network pharmacology provides a foundation for future research aimed at linking molecular signatures with therapeutic responsiveness, supporting the development of individualized treatment strategies involving Yueju pill in schizophrenia care.
In conclusion, the addition of Yueju pill to an olanzapine-based treatment regimen more effectively improves negative symptoms and cognitive function in patients with schizophrenia, promotes social recovery, and does not increase the risk of adverse effects. These therapeutic benefits may be associated with elevated serum levels of BDNF, DA, and 5-HT, which may serve as biomarkers for monitoring treatment response and prognosis. Network pharmacology and bioinformatics analyses further identified quercetin as a potential key active compound, likely exerting therapeutic effects through modulation of synaptic activity. In addition, CHRM1, GSK3B, and KCNH2 were identified as critical genes potentially involved in the dopaminergic signaling pathway underlying this treatment strategy.
This study provides molecular-level support for integrating Yueju pill with conventional antipsychotic therapy and proposes a promising treatment approach with potential clinical value, particularly for patients with comorbid depressive symptoms. Future research should aim to validate the efficacy and safety of this combination in larger and more diverse populations. Practical barriers, including herb-drug interactions, formulation standardization, and clinical acceptance, should be addressed. With multidisciplinary collaboration, policy support, and clinician education, this combined strategy may contribute to the implementation of personalized and precise treatment for schizophrenia.
This study has several limitations. First, the sample size was relatively small (n = 97), which may limit the generalizability and statistical power of the findings. Second, the study duration was limited to 8 weeks, restricting the evaluation of long-term efficacy and safety. Additionally, the trial was conducted at a single center in China, and the findings may not be generalizable to other ethnic or regional populations. The absence of a treatment arm evaluating Yueju pill alone makes it difficult to determine its standalone therapeutic contribution. Although no significant increase in AEs was observed, long-term safety remains insufficiently assessed. Moreover, key components such as quercetin and candidate genes including CHRM1, GSK3B, and KCNH2 identified through network pharmacology have not yet been validated experimentally. Lastly, the study did not adequately control for treatment adherence or baseline symptom severity, which may have influenced outcome assessments.
Future research should include larger, multicenter, and longer-term randomized controlled trials to confirm the efficacy and safety of Yueju pill combined with olanzapine. A treatment arm involving Yueju pill monotherapy is also recommended to evaluate its independent effects. Further mechanistic studies using animal or cellular models are needed to validate the roles of quercetin and the identified genes in schizophrenia pathophysiology and treatment. Stratified randomization should be adopted in future trials to ensure baseline comparability and address adherence issues. Finally, expanding the study to more diverse populations will help assess the global applicability and effectiveness of this combined treatment strategy.
This study demonstrates that the combination of Yueju pill and olanzapine significantly enhances therapeutic outcomes in patients with schizophrenia, particularly in alleviating negative symptoms, improving cognitive and social functioning, and elevating serum levels of BDNF, DA, and 5-HT. Network pharmacology and bioinformatics analyses identified quercetin and key target genes such as CHRM1, GSK3B, and KCNH2 as potential contributors to these effects, highlighting dopaminergic signaling as a central mechanism. Importantly, the combination therapy did not increase the incidence of AEs, supporting its safety and clinical feasibility. These findings provide a scientific basis for the integration of TCM and modern antipsychotic treatment, offering a novel and promising strategy for improving comprehensive outcomes in schizophrenia management (Figure 10).
We would like to express our heartfelt gratitude to Dr. Hu Q for his guidance and assistance throughout the entire process of this paper. Dr. Hu provided meticulous guidance and made numerous professional revisions from the conception to the completion of this work. His profound knowledge, rigorous academic attitude, and inspiring suggestions have significantly enhanced the quality and academic value of this paper. We have benefited immensely from his support during the research and writing process, and hereby extend our sincerest thanks.
| 1. | Zhang J, Yang H, Li W, Li Y, Qin J, He L. Automatic Schizophrenia Detection Using Multimodality Media via a Text Reading Task. Front Neurosci. 2022;16:933049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | den Braber A, van 't Ent D, Cath DC, Wagner J, Boomsma DI, de Geus EJ. Brain activation during cognitive planning in twins discordant or concordant for obsessive-compulsive symptoms. Brain. 2010;133:3123-3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Meier MA, Lemercier CE, Kulisch C, Kiss B, Lendvai B, Adham N, Gerevich Z. The novel antipsychotic cariprazine stabilizes gamma oscillations in rat hippocampal slices. Br J Pharmacol. 2020;177:1622-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Onaolapo AY, Onaolapo OJ. Schizophrenia Aetiology and Drug Therapy: A Tale of Progressive Demystification and Strides in Management. Adv Pharmacol Pharm. 2018;6:19-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Marder SR, Umbricht D. Negative symptoms in schizophrenia: Newly emerging measurements, pathways, and treatments. Schizophr Res. 2023;258:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 6. | Stępnicki P, Kondej M, Kaczor AA. Current Concepts and Treatments of Schizophrenia. Molecules. 2018;23:2087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 349] [Article Influence: 43.6] [Reference Citation Analysis (1)] |
| 7. | Luvsannyam E, Jain MS, Pormento MKL, Siddiqui H, Balagtas ARA, Emuze BO, Poprawski T. Neurobiology of Schizophrenia: A Comprehensive Review. Cureus. 2022;14:e23959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Yang Y, Chen YK, Xie MZ. Exploring the transformative impact of traditional Chinese medicine on depression: Insights from animal models. World J Psychiatry. 2024;14:607-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Wang YS, Shen CY, Jiang JG. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and prospects for drug discovery. Pharmacol Res. 2019;150:104520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Li H, Huang Y, Liang L, Li H, Li S, Feng Y, Feng S, Wu K, Wu F. The relationship between the gut microbiota and oxidative stress in the cognitive function of schizophrenia: A pilot study in China. Schizophr Res. 2024;267:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Yang SH, Zhong K, Jiang T, Zhang M, Kwan HY, Su T. Network Pharmacology-Based Strategy for the Investigation of the Anti-Obesity Effects of an Ethanolic Extract of Zanthoxylum bungeanum Maxim. Front Pharmacol. 2020;11:572387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Zhao J, Pan B, Zhou X, Wu C, Hao F, Zhang J, Liu L. Polygonum cuspidatum inhibits the growth of osteosarcoma cells via impeding Akt/ERK/EGFR signaling pathways. Bioengineered. 2022;13:2992-3006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Liu FS, Li Y, Guo XS, Liu RC, Zhang HY, Li Z. Advances in traditional Chinese medicine as adjuvant therapy for diabetic foot. World J Diabetes. 2022;13:851-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (4)] |
| 14. | Yu C, Li Y, Li Y, Li S, Zeng F, Yu J, Ji Z, Li K, Zhai H. A novel mechanism for regulating lung immune homeostasis: Zukamu granules alleviated acute lung injury in mice by inhibiting NLRP3 inflammasome activation and regulating Th17/Treg cytokine balance. J Ethnopharmacol. 2024;324:117831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 15. | Chu Y, Pang B, Yang M, Wang S, Meng Q, Gong H, Kong Y, Leng Y. Exploring the possible therapeutic mechanism of Danzhixiaoyao pills in depression and MAFLD based on "Homotherapy for heteropathy": A network pharmacology and molecular docking. Heliyon. 2024;10:e35309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Dong Y, Sun X, Li H, Han C, Zhang Y, Ding H, Xia L, Wang H, Yang S, Xu L, Xu G. Mechanisms of adverse mammary effect induced by olanzapine and therapeutic interventions in rat model. Toxicol Appl Pharmacol. 2024;485:116876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Zhang Y, Cui B, Wang T, Lu Y, Chen Z, Zou Z, Miao J, Zhao X, Yuan Y, Wang H, Chen G. Early Enhancement of Neuroplasticity Index, the Ratio of Serum Brain-Derived Neurotrophic Factor Level to HAMD-24 Score, in Predicting the Long-Term Antidepressant Efficacy. Front Behav Neurosci. 2021;15:712445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 18. | Shi Y, Hu X, Cui J, Li J, Bi Z, Li J, Fu H, Wang Y, Cui L, Xu J. Correlation Analysis of Data of Tongue and Pulse in Patients With Disease Fatigue and Sub-health Fatigue. Inquiry. 2022;59:469580211060781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Šverko Z, Vrankić M, Vlahinić S, Rogelj P. Complex Pearson Correlation Coefficient for EEG Connectivity Analysis. Sensors (Basel). 2022;22:1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Ren L, Chen G. Rapid antidepressant effects of Yueju: A new look at the function and mechanism of an old herbal medicine. J Ethnopharmacol. 2017;203:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Wu R, Zhu D, Xia Y, Wang H, Tao W, Xue W, Xia B, Ren L, Zhou X, Li G, Chen G. A role of Yueju in fast-onset antidepressant action on major depressive disorder and serum BDNF expression: a randomly double-blind, fluoxetine-adjunct, placebo-controlled, pilot clinical study. Neuropsychiatr Dis Treat. 2015;11:2013-2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Arabska J, Margulska A, Strzelecki D, Wysokiński A. Does metabolic status affect serum levels of BDNF and MMP-9 in patients with schizophrenia? Nord J Psychiatry. 2019;73:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Sun B. [Study on the Changes in Serum BDNF Levels and APOE Gene Polymorphism in Patients with Late-Life Depression and Mild Alzheimer's Disease Before and After Treatment]. M.Sc. Thesis, Soochow University 2010. Available from: https://kns.cnki.net/kcms2/article/abstract?v=VQ0ntgfwFMSC0Oe_vcTcimZENR76rBIIT8J7Sio0S7FfPEpxXpDLnhfPlDwcmg8m_P8ne3oa035XHdECysY1kQFjieSFrWjzkBhHixcx1w31LdtoxdIMvlrG8pmcZnbfg0xZrGkCntpYVQKv_-ovCD-8RidYXTr0yB0HIb5HNI-t4d0Zx7YqiA==&uniplatform=NZKPT&language=CHS. |
| 24. | Wang YX. [Changes in Serum BDNF and GDNF Levels and Cognitive Function in Patients with Schizophrenia and the Impact of Antipsychotic Medication and Physical Therapy]. Zhongguo Jiankang Xinlixue Zazhi. 2017;25:1457-1460. [DOI] [Full Text] |
| 25. | Lei Y, Butler D, Lucking MC, Zhang F, Xia T, Fujisawa K, Granzier-Nakajima T, Cruz-Silva R, Endo M, Terrones H, Terrones M, Ebrahimi A. Single-atom doping of MoS(2) with manganese enables ultrasensitive detection of dopamine: Experimental and computational approach. Sci Adv. 2020;6:eabc4250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 26. | Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 832] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 27. | Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 28. | Peng S, Li W, Lv L, Zhang Z, Zhan X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov Med. 2018;26:127-136. [PubMed] |
| 29. | Pandya CD, Kutiyanawalla A, Pillai A. BDNF-TrkB signaling and neuroprotection in schizophrenia. Asian J Psychiatr. 2013;6:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Wei YY, Lin WF, Zhang TH, Tang YX, Wang JJ, Zhong MF. Effectiveness of Traditional Chinese Medicineas as an Adjunct Therapy for Refractory Schizophrenia: A Systematic Review and Meta Analysis. Sci Rep. 2018;8:6230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Page CE, Coutellier L. Reducing inhibition: A promising new strategy for the treatment of schizophrenia. EBioMedicine. 2018;35:25-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Schwartz DL. Quercetin as an Augmentation Agent in Schizophrenia. J Clin Psychopharmacol. 2016;36:282-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Shahzad S, Batool Z, Afzal A, Haider S. Reversal of oxidative stress, cytokine toxicity and DNA fragmentation by quercetin in dizocilpine-induced animal model of Schizophrenia. Metab Brain Dis. 2022;37:2793-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Caccuri F, Bugatti A, Corbellini S, Roversi S, Zani A, Mazzuca P, Marsico S, Caruso A, Giagulli C. The Synthetic Dipeptide Pidotimod Shows a Chemokine-Like Activity through CXC Chemokine Receptor 3 (CXCR3). Int J Mol Sci. 2019;20:5287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Chen G, Hu T, Li Q, Li J, Jia Y, Wang Z. Expression of synaptosomal-associated protein-25 in the rat brain after subarachnoid hemorrhage. Neural Regen Res. 2013;8:2693-2702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Saik OV, Klimontov VV. Bioinformatic Reconstruction and Analysis of Gene Networks Related to Glucose Variability in Diabetes and Its Complications. Int J Mol Sci. 2020;21:8691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Feng L, Zhang J, Lee C, Kim G, Liu F, Petersen AJ, Lim E, Anderson CL, Orland KM, Robertson GA, Eckhardt LL, January CT, Kamp TJ. Long QT Syndrome KCNH2 Variant Induces hERG1a/1b Subunit Imbalance in Patient-Specific Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Arrhythm Electrophysiol. 2021;14:e009343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | O-Uchi J, Rice JJ, Ruwald MH, Parks XX, Ronzier E, Moss AJ, Zareba W, Lopes CM. Impaired IKs channel activation by Ca(2+)-dependent PKC shows correlation with emotion/arousal-triggered events in LQT1. J Mol Cell Cardiol. 2015;79:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Kishimoto T, Hagi K, Nitta M, Kane JM, Correll CU. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry. 2019;18:208-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | He M, Qian K, Zhang Y, Huang XF, Deng C, Zhang B, Gao G, Li J, Xie H, Sun T. Olanzapine-Induced Activation of Hypothalamic Astrocytes and Toll-Like Receptor-4 Signaling via Endoplasmic Reticulum Stress Were Related to Olanzapine-Induced Weight Gain. Front Neurosci. 2020;14:589650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Björkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 740] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 42. | Feigenbaum J, Yanai J, Blass R. Possible DA agonist properties of naloxone. Int J Neurosci. 1982;17:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Ilchibaeva T, Tsybko A, Zeug A, Müller FE, Guseva D, Bischoff S, Ponimaskin E, Naumenko V. Serotonin Receptor 5-HT(2A) Regulates TrkB Receptor Function in Heteroreceptor Complexes. Cells. 2022;11:2384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 44. | Chai GR, Liu S, Yang HW, Chen XL. Quercetin protects against diabetic retinopathy in rats by inducing heme oxygenase-1 expression. Neural Regen Res. 2021;16:1344-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 45. | Chittasupho C, Manthaisong A, Okonogi S, Tadtong S, Samee W. Effects of Quercetin and Curcumin Combination on Antibacterial, Antioxidant, In Vitro Wound Healing and Migration of Human Dermal Fibroblast Cells. Int J Mol Sci. 2021;23:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 46. | Dean B, Hopper S, Conn PJ, Scarr E. Changes in BQCA Allosteric Modulation of [(3)H]NMS Binding to Human Cortex within Schizophrenia and by Divalent Cations. Neuropsychopharmacology. 2016;41:1620-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Luo XJ, Huang L, Oord EJ, Aberg KA, Gan L, Zhao Z, Yao YG. Common variants in the MKL1 gene confer risk of schizophrenia. Schizophr Bull. 2015;41:715-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Wang X, Su Y, Yan H, Huang Z, Huang Y, Yue W. Association Study of KCNH7 Polymorphisms and Individual Responses to Risperidone Treatment in Schizophrenia. Front Psychiatry. 2019;10:633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Spaull RVV, Kurian MA, MP Adam, J Feldman, GM Mirzaa, RA Pagon, SE Wallace. SLC6A3-Related Dopamine Transporter Deficiency Syndrome. GeneReviews® [Internet]. 1993;. [PubMed] |
| 50. | Stoffel M, Rahn S, Neubauer AB, Moessner M, Aguilar-Raab C, Ditzen B. Associations of SLC6A4 methylation with salivary cortisol, salivary alpha-amylase, and subjective stress in everyday life. Psychoneuroendocrinology. 2023;153:106283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 51. | Noble EP. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics. 2000;1:309-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Scheggia D, Sannino S, Scattoni ML, Papaleo F. COMT as a drug target for cognitive functions and dysfunctions. CNS Neurol Disord Drug Targets. 2012;11:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Qian Z, Ye J, Li J, Che Y, Yu W, Xu P, Lin J, Ye F, Xu X, Su Z, Li D, Xie Z, Wu Y, Shen H. Decrotonylation of AKT1 promotes AKT1 phosphorylation and activation during myogenic differentiation. J Adv Res. 2023;50:117-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/