©The Author(s) 2025.
World J Psychiatry. Dec 19, 2025; 15(12): 112055
Published online Dec 19, 2025. doi: 10.5498/wjp.v15.i12.112055
Published online Dec 19, 2025. doi: 10.5498/wjp.v15.i12.112055
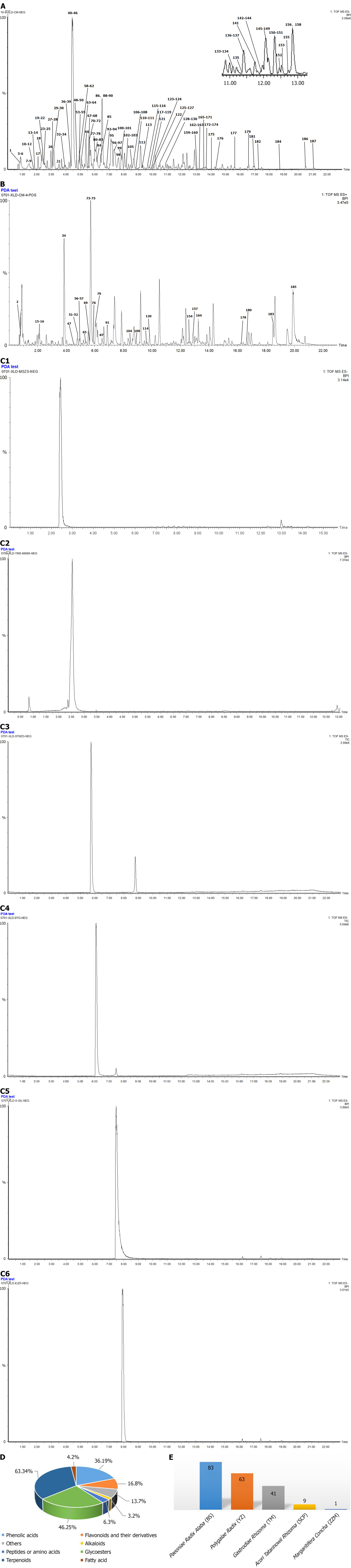
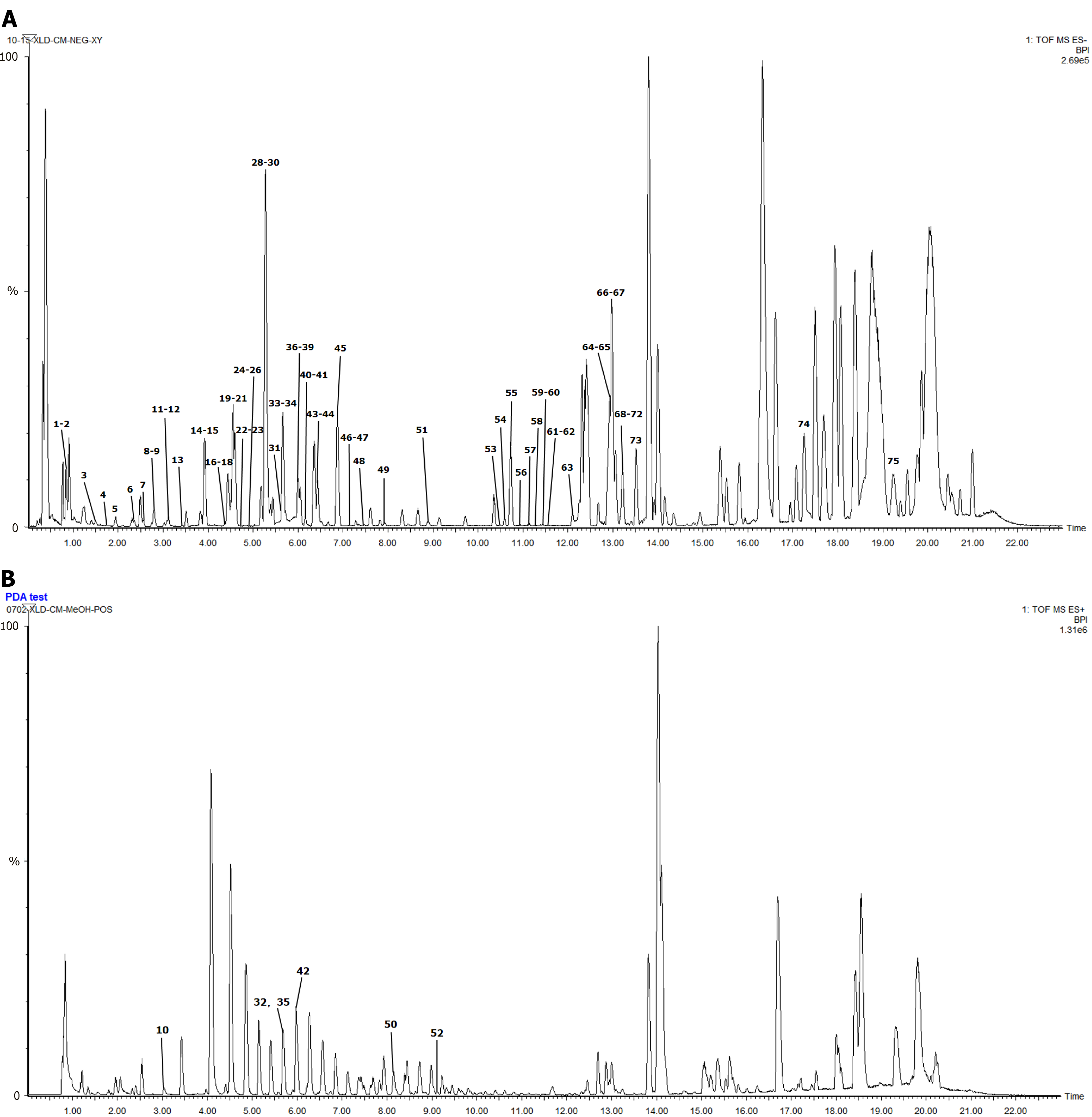
Figure 1 The results of in vitro chemical composition analysis.
A: Total ion current diagram of negative ion mode; B: Total ion current diagram of positive ion mode; C: Total ion current diagram of reference in negative ion mode; D: Class of chemical composition of Changmaxifeng granules (CG); E: Source of chemical composition of CG. C1 for gallic acid; C2 for gastrodin; C3 for albiflorin; C4 for paeoniflorin; C5 for 1,2,3,4,6-pentagalloylglucose; C6 for 3, 6′-disinapoyl sucrose.
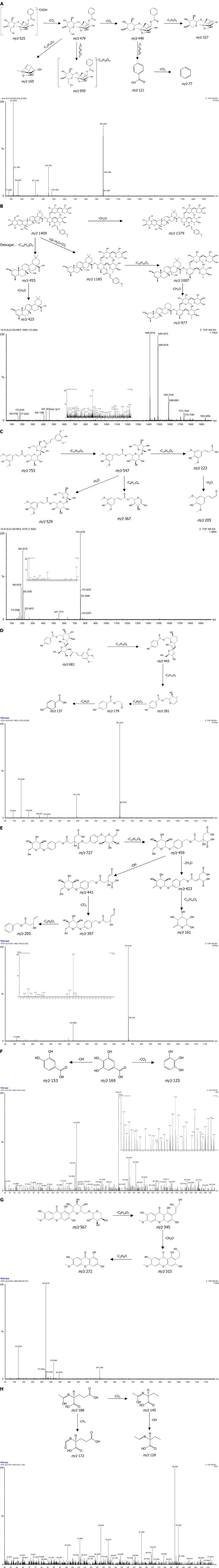
Figure 2 Possible cleavage pattern and secondary mass diagram.
A: The degradation pattern and secondary mass diagram of paeoniflorin; B: The degradation pattern and secondary mass diagram of onjisaponin Y; C: The degradation pattern and secondary mass diagram of 3,6’-disinapoyl sucrose; D: The degradation pattern and secondary mass diagram of tenuifoliside A; E: The degradation pattern and secondary mass diagram of parishin B; F: The degradation pattern and secondary mass diagram of gallic acid; G: The degradation pattern and secondary mass diagram of polygalaxanthone VIII; H: The degradation pattern and secondary mass diagram of acetyl glutamic acid.
Figure 3 Characterization results of plasma components.
A: Plasma total ion current diagram of negative ion mode; B: Plasma total ion current diagram of positive ion mode.
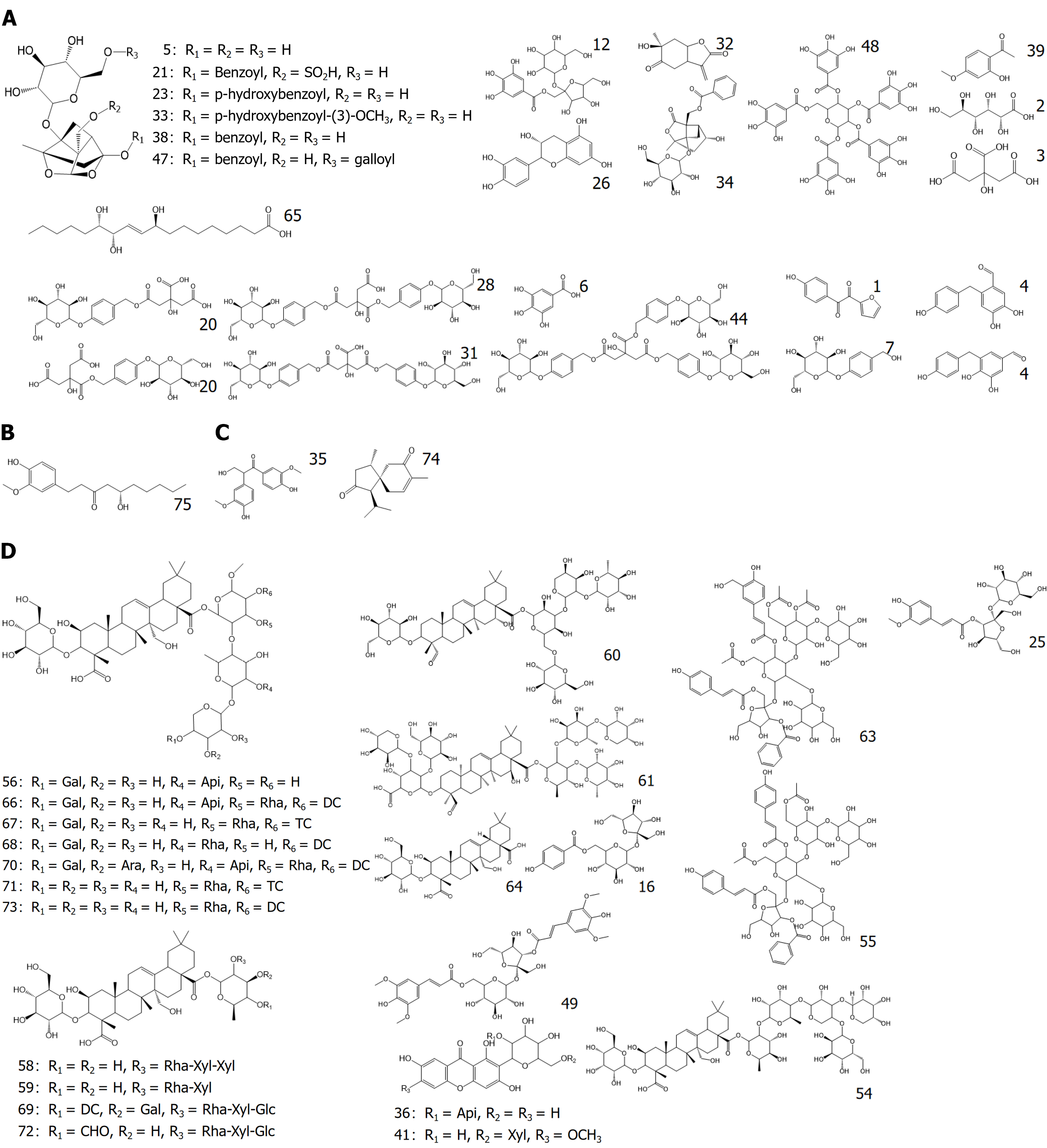
Figure 4 Structural formulas of the blood-entry prototype components.
A: Structural formulas of the blood-entry prototype components from Baishao; B: Structural formulas of the blood-entry prototype components from Tianma; C: Structural formulas of the blood-entry prototype components from Shichangpu; D: Structural formulas of the blood-entry prototype components from Yuanzhi. Gal: Galactose; Api: Apiofuranosyl; Rha: Rhamnose; Ara: Arabinose; Xyl: Xylose; TC: 3,4,5-trimethoxy cinnamoyl; DC: 3,4-dimethoxy cinnamoyl.
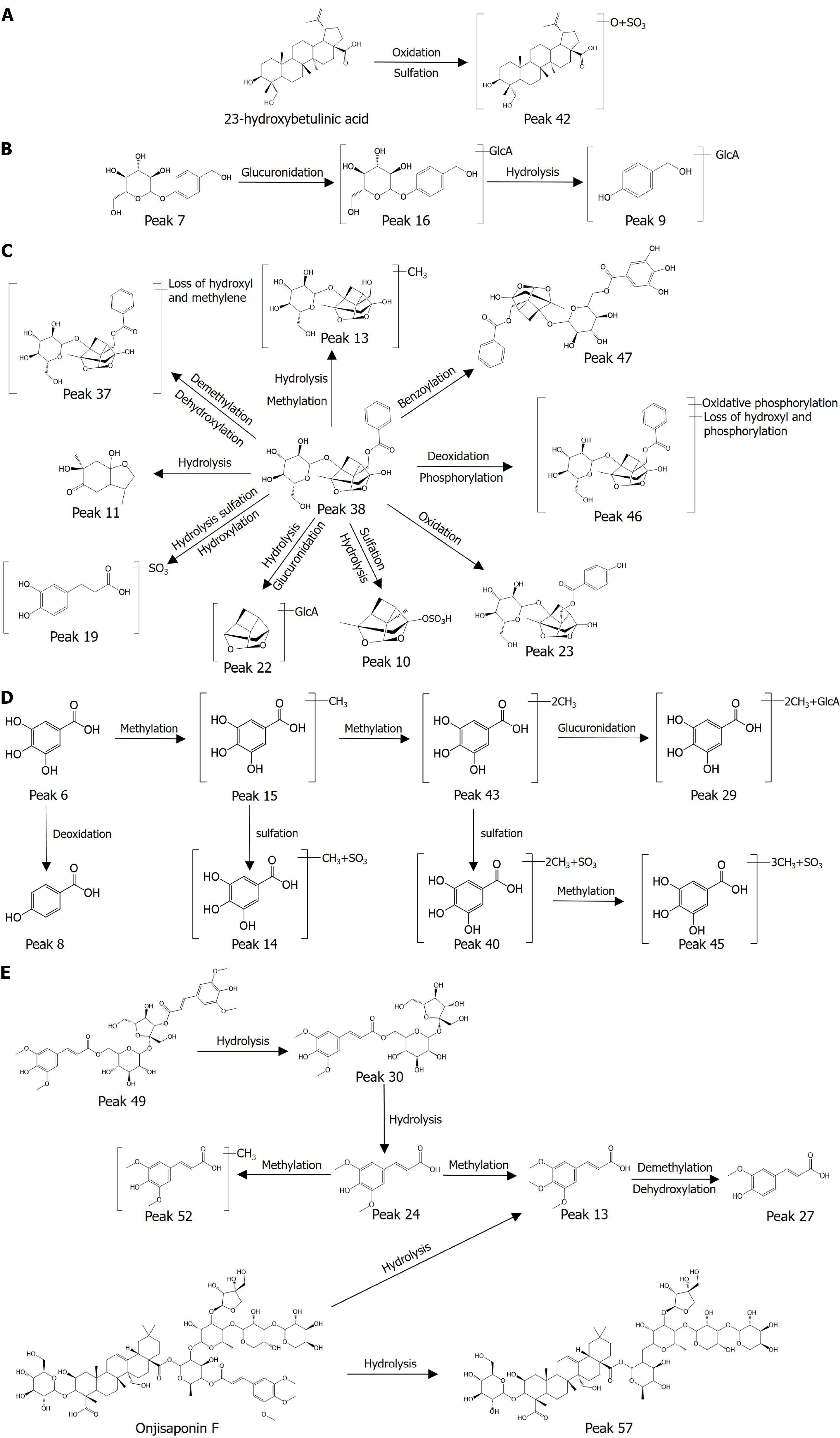
Figure 5 Possible metabolic pathways of the components that enter the bloodstream.
A: The metabolic pathways of 23-hydroxybetulinic acid; B: The metabolic pathways of gastrodin; C: The metabolic pathways of paeoniflorin; D: The metabolic pathways of gallic acid; E: The metabolic pathways of onjisaponin F, 3',6-disinapoylsucrose and sibiricose A5.
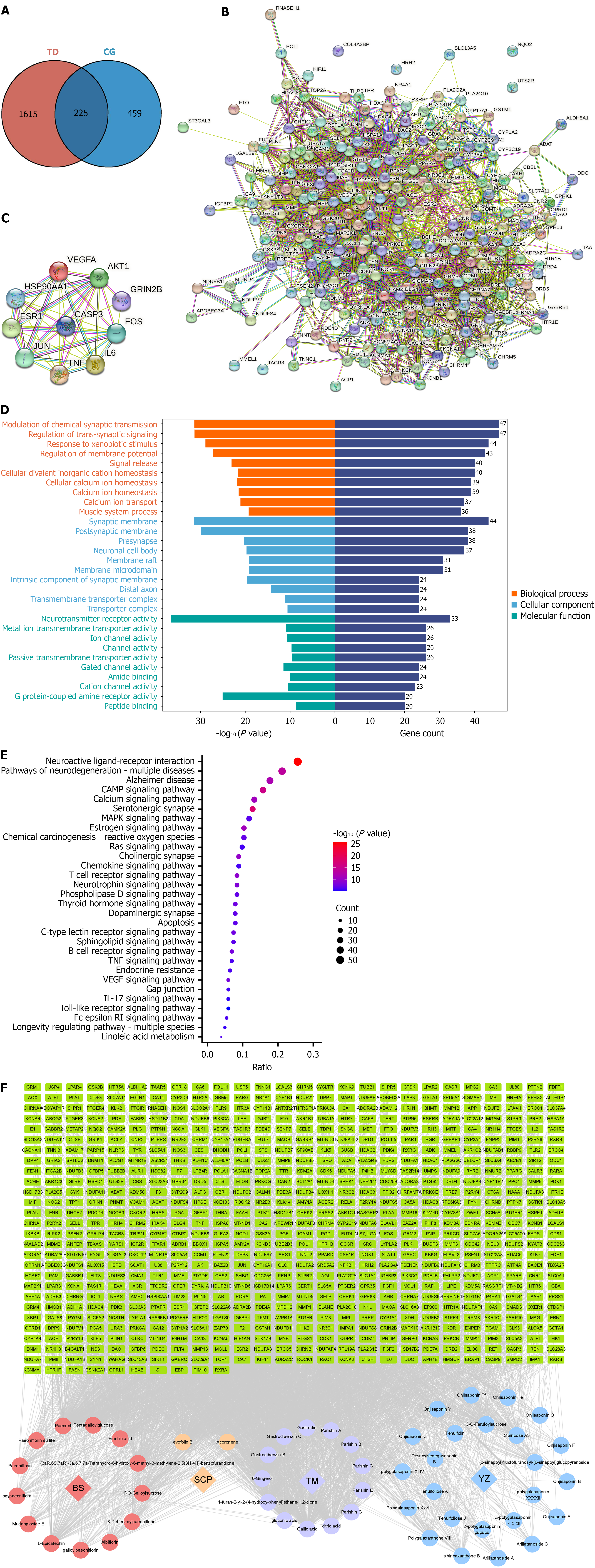
Figure 6 The results of network pharmacology.
A: The Venn diagram of the intersecting targets; B: The protein interaction network diagram; C: Top 10 of protein interaction network diagram; D: The Gene Ontology enrichment results; E: The Kyoto Encyclopedia of Genes and Genomes enrichment results; F: The component-target-pathway-disease topological network. TD: Tic disorder; CG: Changmaxifeng granules.
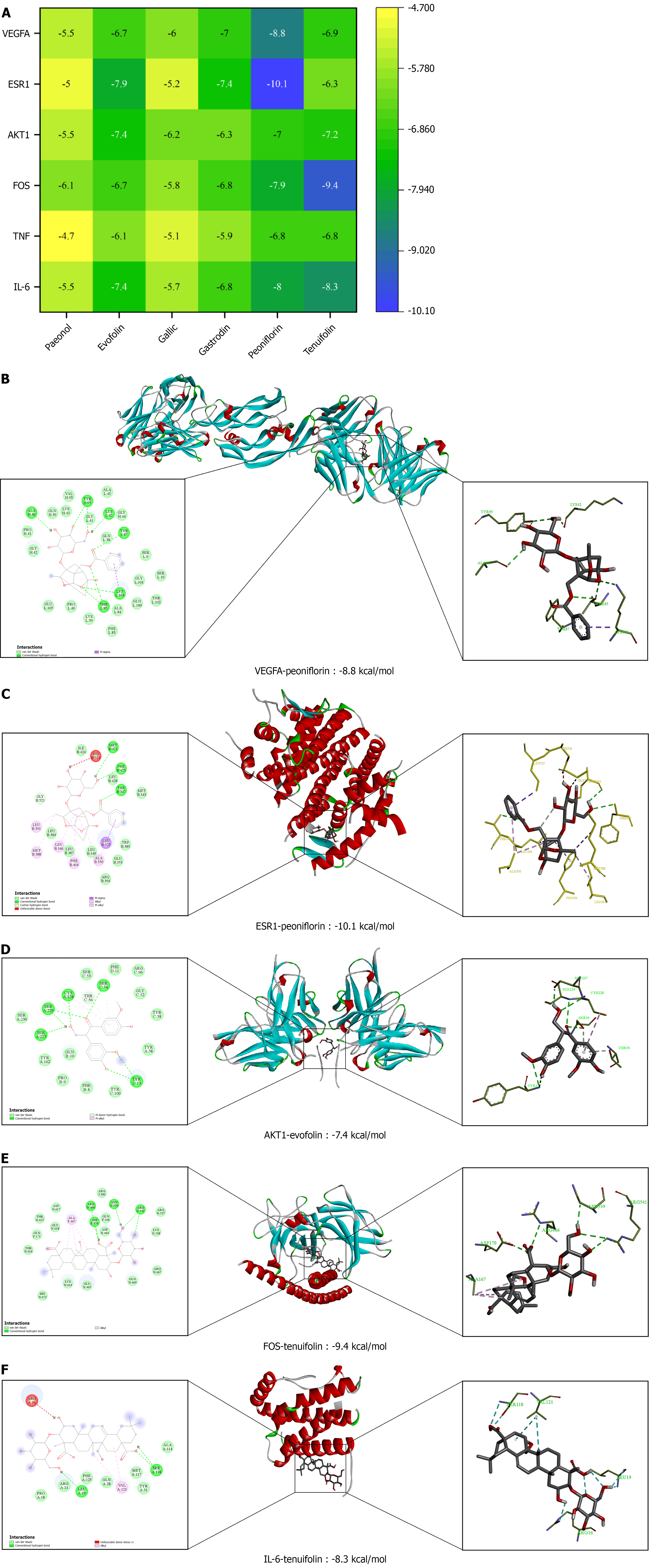
Figure 7 The results of molecular docking.
A: Results of molecular docking; B: The binding mode of VEGFA with peoniflorin; C: The binding mode of AKT1 with evofolin, and the binding mode of VEGFA with peoniflorin; D: The binding mode of ESR1 with peoniflorin; E: The binding mode of FOS with tenuifolin; F: The binding mode of IL-6 with tenuifolin.
- Citation: Xie LD, Wu JP, Liu SS, Zong Z, Hu Y, Ling N, Han B, Li WL, Yao HY. Investigating the pharmaceutical substances and action mechanisms of Changmaxifeng granules against tic disorders. World J Psychiatry 2025; 15(12): 112055
- URL: https://www.wjgnet.com/2220-3206/full/v15/i12/112055.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i12.112055