Published online Feb 24, 2026. doi: 10.5306/wjco.v17.i2.114959
Revised: December 1, 2025
Accepted: January 13, 2026
Published online: February 24, 2026
Processing time: 126 Days and 20.4 Hours
Pancreatic cancer (PC) represents one of the most formidable challenges in oncology, necessitating continuous innovation in therapeutic strategies. Owing to its pivotal role in PC progression, lipid metabolism, which is characterized by dysregulated cholesterol biosynthesis, altered fatty acid profiles, and lipid-driven immunosuppression, has received increasing attention. These metabolic aber
Core Tip: This article highlights lipid metabolism reprogramming as a key driver of pancreatic cancer progression, inducing growth, metastasis, and immunosuppression. Targeting enzymes such as fatty acid synthase or cholesterol pathways shows promise in overcoming therapy resistance, offering innovative strategies to reshape treatment paradigms.
- Citation: Zhang C, Wang H, Yang YH. Lipid metabolism in pancreatic cancer treatment. World J Clin Oncol 2026; 17(2): 114959
- URL: https://www.wjgnet.com/2218-4333/full/v17/i2/114959.htm
- DOI: https://dx.doi.org/10.5306/wjco.v17.i2.114959
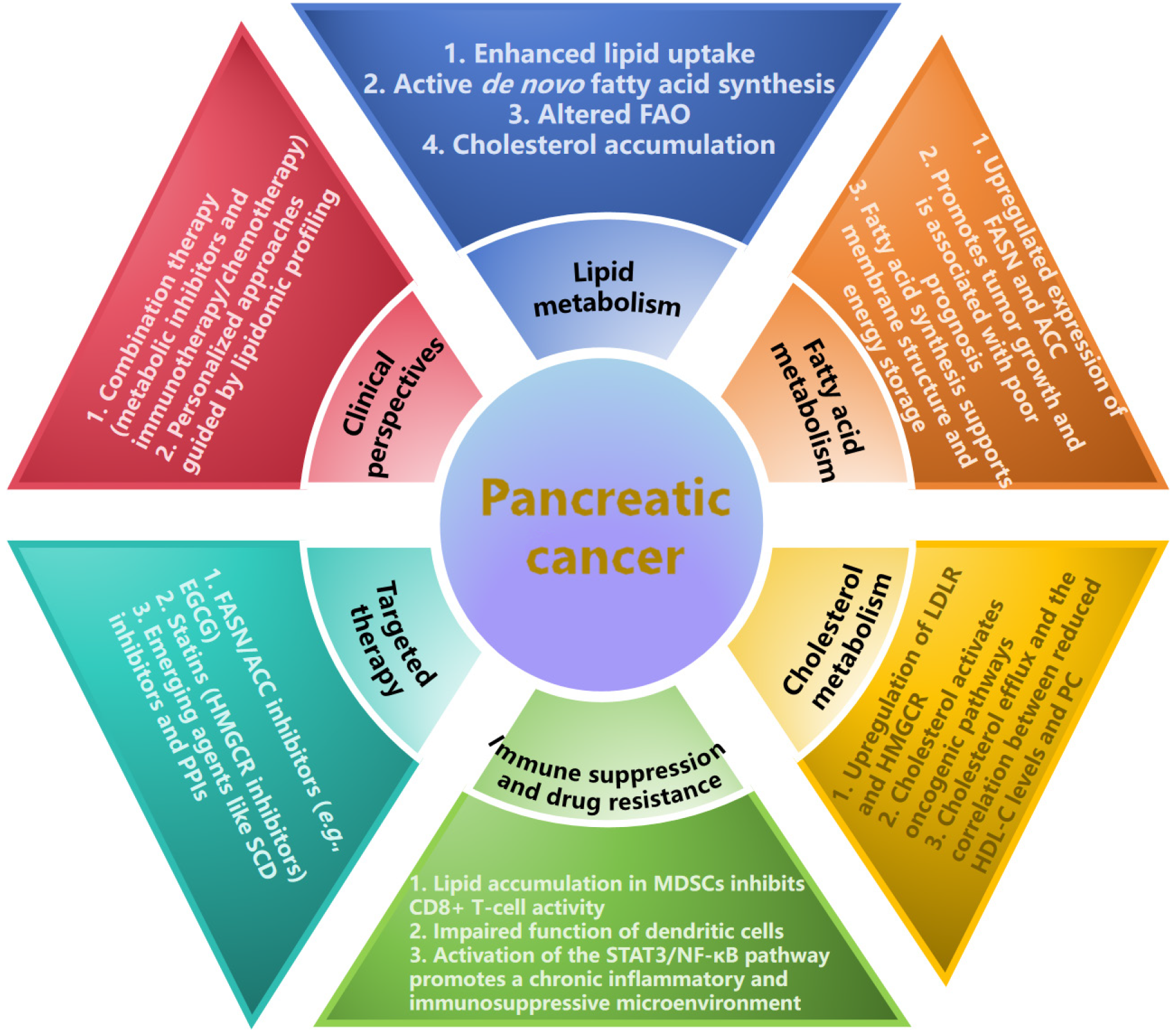
Dysregulated lipid metabolism serves as a core driver of pancreatic cancer (PC) progression and is characterized by increased fatty acid synthesis, disrupted cholesterol homeostasis, and lipid droplet accumulation. The reprogramming of lipid metabolism in cancer, notably in PC, is principally characterized by four hallmarks: Augmented uptake of ex
Lipid metabolism supports rapid tumour cell proliferation under nutrient-poor conditions, alters membrane signalling, promotes epithelial-mesenchymal transition and metastasis and mediates chemoresistance[2,3]. The dysregulation of lipid metabolism in PC not only drives tumour growth and metastasis but also provides critical entry points for the de
The contribution of lipid metabolism to cancer progression is multifaceted, encompassing the provision of substrates for membrane biogenesis, the generation of signalling molecules, and the storage of metabolic energy. Within this fra
Cholesterol is not only a critical component of cell membranes and an energy source but also acts as a signalling molecule, promoting PC progression by activating oncogenic pathways such as the Wnt/β-catenin[6] and Sonic hedgehog pathways[7]. In PC, cells overexpress the low-density lipoprotein receptor to increase cholesterol uptake and upregulate synthesis enzymes such as 3-hydroxy-3-methylglutaryl-coenzyme A reductase, which leads to excessive cholesterol ac
The efficiency of cholesterol efflux is subject to multiple determinants, including the intracellular cholesterol burden, the physicochemical properties of extracellular high-density lipoprotein (HDL) acceptors, and the expression level of specific efflux transporters. This process is governed by four principal mechanisms: (1) Passive diffusion to mature HDL; (2) Scavenger receptor class B type 1-mediated facilitated diffusion; (3) ATP-binding cassette transporter A1-dependent ATP-binding cassette transporter A1 efflux; and (4) ATP-binding cassette transporter G1-mediated efflux to mature HDL. Epidemiological studies have indicated that HDL-cholesterol levels are inversely correlated with cancer risk and that patients with PC exhibit significantly reduced blood HDL-cholesterol levels[8].
Dysregulated lipid metabolism is closely linked to key cancer biological processes in PC, including tumorigenesis, growth, metastasis, and therapeutic resistance. Through metabolic reprogramming, cancer cells gain survival advantages, such as enhanced resistance to chemotherapy and targeted therapies, while evading cytotoxic effects, thus complicating treatment outcomes. Targeting lipid metabolism pathways has the potential to reverse therapeutic resistance and im
Dysregulated lipid metabolism drives cancer initiation by altering cell membrane composition and activating oncogenic signalling pathways, which promote abnormal cell proliferation and carcinogenesis. Saturated fatty acids, such as palmitate, activate proinflammatory pathways through Toll-like receptors, increasing the secretion of inflammatory cytokines and shaping a protumourigenic microenvironment[9].
Lipid metabolism supports the energy demands and proliferation of cancer cells, which rely on lipid metabolic re
Lipid metabolism supports the energy demands and proliferation of cancer cells, which rely on lipid metabolic reprogramming for sustaining rapid growth. In the tumour microenvironment, myeloid-derived suppressor cells exhibit enhanced lipid accumulation, which fuels their immunosuppressive function by inhibiting CD8+ T-cell activity through oxidized lipids[10]. The overexpression of fatty acid transport protein 4 further promotes lipid uptake and immune suppression. Lipid overload impairs the antigen-presenting capacity of dendritic cells, reduces the expression of costimulatory molecules, and increases the secretion of tolerogenic cytokines such as interleukin-10, inducing immune tolerance instead of activation.
Moreover, dysregulated lipid metabolism activates the signal transducer and activator of transcription 3/nuclear factor kappa B pathway, promoting the secretion of proinflammatory cytokines and thereby fostering a chronic inflammatory and immunosuppressive microenvironment that accelerates tumour progression. The overexpression of FASN in PC cells activates the nuclear factor kappa B/SP1 pathway, thereby enhancing poly (adenosine diphosphate-ribose) polymerase 1-mediated DNA repair and conferring resistance to genotoxic therapies[11].
Targeting fatty acid synthesis has shown promise in the context of PC therapy. Inhibitors of key enzymes such as FASN and ACC effectively suppressed tumour growth in preclinical models. For example, EGCG blocks the β-ketoacyl-ACP synthase domain of FASN, which halts lipid production and tumour formation[12]. Similarly, proton pump inhibitors indirectly target lipid metabolism by reducing thioesterase activity and inducing cancer cell death[13].
Cholesterol metabolism is another critical therapeutic avenue. Statins, which inhibit the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase, reduce cholesterol biosynthesis and demonstrate anticancer effects even in gemcitabine-resistant PC cells[14]. Additionally, targeting cholesterol efflux pathways may reverse drug resistance by altering lipid raft-dependent signalling.
In addition to classic pathways, emerging targets such as stearoyl-CoA desaturase and lipid transporters offer new opportunities. stearoyl-CoA desaturase inhibitors reduce monounsaturated fatty acid production, thereby impairing membrane fluidity and tumour growth.
Future research must address dynamic lipid changes and tumour microenvironment interactions. Combining metabolic inhibitors with immunotherapy or chemotherapy could help overcome resistance. Personalized approaches - guided by lipidomic profiling or related protein expression levels - may optimize outcomes[15]. Despite these challenges, lipid metabolism reprogramming represents a transformative strategy for redefining PC treatment paradigms (Figure 1).
| 1. | Vassiliou E, Farias-Pereira R. Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity. Int J Mol Sci. 2023;24:12032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 2. | Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 2286] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 3. | Daya T, Breytenbach A, Gu L, Kaur M. Cholesterol metabolism in pancreatic cancer and associated therapeutic strategies. Biochim Biophys Acta Mol Cell Biol Lipids. 2025;1870:159578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond). 2018;38:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 603] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 5. | Tadros S, Shukla SK, King RJ, Gunda V, Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, Grem J, Sasson AR, Singh PK. De Novo Lipid Synthesis Facilitates Gemcitabine Resistance through Endoplasmic Reticulum Stress in Pancreatic Cancer. Cancer Res. 2017;77:5503-5517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 6. | Zheng S, Lin J, Pang Z, Zhang H, Wang Y, Ma L, Zhang H, Zhang X, Chen M, Zhang X, Zhao C, Qi J, Cao L, Wang M, He X, Sheng R. Aberrant Cholesterol Metabolism and Wnt/β-Catenin Signaling Coalesce via Frizzled5 in Supporting Cancer Growth. Adv Sci (Weinh). 2022;9:e2200750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Alexander JI, Martinez E, Vargas A, Zinshteyn D, Sodi V, Connolly DC, Hartman TR, O'Reilly AM. Cholesterol and CDON Regulate Sonic Hedgehog Release from Pancreatic Cancer Cells. J Pancreat Cancer. 2021;7:39-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Wang F, Huang L, Zhang J, Fan J, Wu H, Xu J. Dyslipidemia in Chinese Pancreatic Cancer Patients: A Two-Center Retrospective Study. J Cancer. 2021;12:5338-5344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Wang F, Baverel V, Chaumonnot K, Bourragat A, Bellenger J, Bellenger S, Zhou W, Narce M, Garrido C, Kohli E. The endoplasmic reticulum stress protein GRP94 modulates cathepsin L activity in M2 macrophages in conditions of obesity-associated inflammation and contributes to their pro-inflammatory profile. Int J Obes (Lond). 2024;48:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Zhao H, Teng D, Yang L, Xu X, Chen J, Jiang T, Feng AY, Zhang Y, Frederick DT, Gu L, Cai L, Asara JM, Pasca di Magliano M, Boland GM, Flaherty KT, Swanson KD, Liu D, Rabinowitz JD, Zheng B. Myeloid-derived itaconate suppresses cytotoxic CD8(+) T cells and promotes tumour growth. Nat Metab. 2022;4:1660-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 11. | Wu X, Dong Z, Wang CJ, Barlow LJ, Fako V, Serrano MA, Zou Y, Liu JY, Zhang JT. FASN regulates cellular response to genotoxic treatments by increasing PARP-1 expression and DNA repair activity via NF-κB and SP1. Proc Natl Acad Sci U S A. 2016;113:E6965-E6973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Calle RA, Amin NB, Carvajal-Gonzalez S, Ross TT, Bergman A, Aggarwal S, Crowley C, Rinaldi A, Mancuso J, Aggarwal N, Somayaji V, Inglot M, Tuthill TA, Kou K, Boucher M, Tesz G, Dullea R, Bence KK, Kim AM, Pfefferkorn JA, Esler WP. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: two parallel, placebo-controlled, randomized phase 2a trials. Nat Med. 2021;27:1836-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 13. | Alkhushaym N, Almutairi AR, Althagafi A, Fallatah SB, Oh M, Martin JR, Babiker HM, McBride A, Abraham I. Exposure to proton pump inhibitors and risk of pancreatic cancer: a meta-analysis. Expert Opin Drug Saf. 2020;19:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Kawashiri T, Tokunaga A, Kobayashi D, Shimazoe T. Anti-tumor Activities of 3-Hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) Reductase Inhibitors and Bisphosphonates in Pancreatic Cell Lines Which Show Poor Responses to Gemcitabine. Biol Pharm Bull. 2020;43:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Yang Z, Liu Y, Pei J, Li R, Yang Y. Targeting lipid metabolism: novel insights and therapeutic advances in pancreatic cancer treatment. Lipids Health Dis. 2025;24:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/