Published online Jan 24, 2026. doi: 10.5306/wjco.v17.i1.113612
Revised: October 16, 2025
Accepted: December 10, 2025
Published online: January 24, 2026
Processing time: 144 Days and 4.5 Hours
We have previously reported that the cancer/testis antigen Kita-Kyushu lung cancer antigen-1 (KK-LC-1) is frequently expressed in gastric cancer (GC), with a particularly high positivity rate observed in patients with multiple GCs. Thus, we conducted a preliminary study to further investigate the relationship between KK-LC-1 expression and multiple GCs. Specifically, we aimed to explore the potential clinical utility of KK-LC-1 as a biomarker for identifying patients at risk of developing multiple gastric cancers, with a focus on its expression in the initial tumor lesions.
To investigate the association between KK-LC-1 expression and the development of multiple GCs in a preliminary cohort.
Among 124 patients (177 lesions) treated for GC at Toho University Medical Center Ohashi Hospital between September 2020 and November 2023, 109 (single cancer: 82; multiple cancers: 27) with available initial tumor specimens were enrolled. KK-LC-1 expression was assessed using immunohistochemistry, and clinicopathological correlations were analyzed using Fisher’s exact test. Tumor locations were classified anatomically and analyses were performed, focusing on the initial cancer sites.
In tumors located in the upper stomach, KK-LC-1 expression did not correlate with clinicopathological features. However, in the middle and lower stomach, KK-LC-1 expression was significantly associated with older age, differentiated histological types, and multiple cancers. Notably, all patients with multiple GCs had KK-LC-1-positive tumors (100%) in their initial lesions. The KK-LC-1 positivity rate in the initial tumors of multiple cancers in the middle and lower region was significantly higher than that in solitary cancers. Conversely, the absence of KK-LC-1 expression in a solitary tumor located in the middle and lower region may suggest a reduced risk for the subsequent development of multiple primary cancers.
KK-LC-1 expression in the initial gastric tumors, particularly in the middle and lower stomach regions, may serve as a predictive biomarker for the future development of multiple GCs.
Core Tip: We have previously reported that the cancer/testis antigen Kita-Kyushu lung cancer antigen-1 (KK-LC-1) is frequently expressed in gastric cancer (GC), with high expression observed in multiple GCs. Therefore, as a preliminary study, we investigated the relationship between KK-LC-1 expression and multiple GCs, focusing on its potential clinical utility. In the middle and lower stomach regions, KK-LC-1 expression was significantly associated with older age, differentiated histological type, and the presence of multiple cancers, with all cases in the multiple cancer group exhibiting KK-LC-1 positivity (100%). KK-LC-1 expression in primary gastric tumors suggests a higher risk of subsequent development of multiple GCs.
- Citation: Futawatari N, Fukuyama T, Akimoto Y, Maehara J, Hihara D, Okamoto Y, Yokouchi Y, Takahashi K, Watanabe M, Saida Y. Kita-Kyushu lung cancer antigen-1 expression in initial gastric tumors predicts multiple cancer development: A pilot study. World J Clin Oncol 2026; 17(1): 113612
- URL: https://www.wjgnet.com/2218-4333/full/v17/i1/113612.htm
- DOI: https://dx.doi.org/10.5306/wjco.v17.i1.113612
Gastric cancer (GC) is the fourth leading cause of cancer-related death worldwide and ranks fifth in incidence among all cancers[1]. Endoscopic resection is widely used as an effective and minimally invasive local treatment for early GC with a low risk of lymph node metastasis. However, as the entire stomach is preserved following endoscopic resection, the risk of metachronous GC remains, and its incidence is higher compared to that of surgical resection[2,3]. The reported incidence of postoperative metachronous multiple GC is 2.35% following distal gastrectomy, 3.01% following pylorus-preserving gastrectomy, 6.28% following proximal gastrectomy, and 8.21% following function-preserving gastrectomy[4]. In contrast, the incidence after endoscopic treatment ranges from 9.5% to 14.0%[5].
Cancer/testis antigens are a group of tumor-associated antigens expressed in various cancer tissue and in testicular germ cells[6]. Thus, cancer/testis antigens are promising targets for cancer-specific immunotherapy. Among them, Kita-Kyushu lung cancer antigen-1 (KK-LC-1), a cancer/testis antigen with high expression levels in triple-negative breast cancer and GC, has garnered significant attention[7,8]. Currently, various therapeutic strategies targeting KK-LC-1 are under investigation, including T cell receptor-engineered T cell therapy, peptide-drug conjugate therapy, and molecular targeted therapy using small-molecule compounds[9-11].
In our previous studies, we confirmed that KK-LC-1 is a promising therapeutic target, with high expression observed in GC (81.6%) and triple-negative breast cancer (100%)[8,12]. Moreover, we reported that Helicobacter pylori (H. pylori) infection induces KK-LC-1 expression and that the antigen is highly expressed in early-stage GC (79.5%)[13]. Furthermore, KK-LC-1 expression has been detected in gastric mucosa during the precancerous stage[13], and we previously found a high rate of KK-LC-1 positivity among patients with multiple GCs[14]. Even in cases of successful H. pylori eradication, KK-LC-1 expression remained high, suggesting the involvement of pathways independent of H. pylori infection[14]. Therefore, we conducted a preliminary study to further explore the relationship between KK-LC-1 expression and multiple GCs. Specifically, we examined the clinical utility of KK-LC-1 as a biomarker for identifying patients at increased risk of developing multiple GCs, focusing on its expression in initial tumor lesions.
The Human Ethics Review Committee of the Toho University Ohashi Medical Center, Japan (Approval No. H23034_H20023) approved the study protocol, and all experiments were conducted in accordance with the relevant guidelines and regulations. All patients signed an informed consent form before the tissue samples were collected for this study.
From September 2020 to November 2023, 124 patients (177 lesions) were treated for GC at the Department of Surgery or Department of Gastroenterology at Toho University Medical Center Ohashi Hospital (Tokyo, Japan). Among them, 39 patients had multiple cancers, accounting for 77 lesions (50 treated endoscopically and 27 treated surgically). Tumor tissues from resected GC were collected and used in this study. Clinicopathological findings were classified according to the Japanese Classification of Gastric Carcinoma (14th edition)[15]. In addition, to investigate the characteristics of the first cancer lesions, 109 cases (single cancer, 82 cases; multiple cancers, 27 cases) were included in the study, wherein the initial lesion could be evaluated.
Among multiple cancers, synchronous cancers were defined as multiple GCs with a treatment interval of ≤ 1 year, whereas those with a treatment interval > 1 year were defined as metachronous. In this study, papillary and tubular adenocarcinomas were reclassified as differentiated tumors based on histological findings, whereas poorly differentiated adenocarcinoma, signet ring cell adenocarcinoma, and mucinous carcinoma were categorized as undifferentiated tumors. The tumor sites of GC were reclassified as upper, representing the upper third of the stomach, and middle and lower, representing the middle and lower thirds of the stomach. H. pylori infection was evaluated using blood anti-H. pylori antibody (DENKA Kit; DENKA Seikenn Corporation, Tokyo, Japan). A history of H. pylori eradication was confirmed through interviews. H. pylori infection was defined as uninfected when the blood anti-H. pylori antibody was negative, current infection when the blood anti-H. pylori antibody was positive, and previous infection when the blood anti-H. pylori antibody was negative with a history of eradication. Atrophic gastritis was assessed by upper gastrointestinal endoscopy to evaluate the degree of atrophy. The degree of gastric mucosal atrophy was categorized as none, closed type (C-1, C-2, C-3), and open type (O-1, O-2, O-3) according to the endoscopic atrophic-border scale described by Kimura and Takemoto[16].
Formalin-fixed, paraffin-embedded tissue sections (3 μm thick) of human GC tumors and adjacent non-tumor areas were prepared for each patient. Antigen retrieval was performed by autoclaving the sections at 97 °C for 20 minutes in a low-pH citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked using 6% hydrogen peroxide. For KK-LC-1 immunostaining, the supernatant of the previously described hybridoma clone 34B3[12], diluted 1:80, was used as the primary antibody and incubated for 1 hour. Staining was conducted using the Envision FLEX staining kit (Agilent Technologies, Santa Clara, CA, United States) on a DAKO Autostainer Link 48 platform (Agilent Technologies, Santa Clara, CA, United States), following the manufacturer’s protocol.
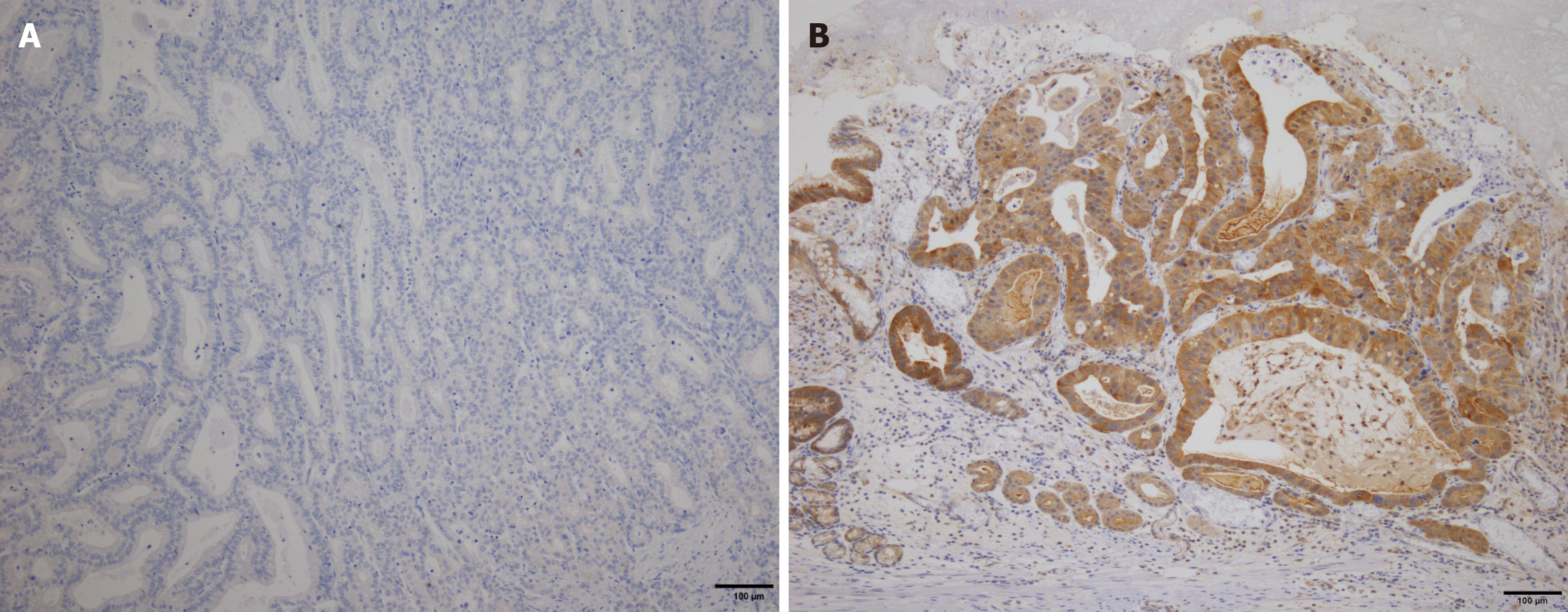
KK-LC-1 expression was evaluated in both tumor and adjacent non-tumor tissues within the same section. In tumor tissue, KK-LC-1 positivity was defined by the presence of stained tumor cells. Staining intensity was scored based on cytoplasmic expression as follows: 0 (negative), 1+ (weak), 2+ (moderate), and 3+ (strong). Specimens were considered KK-LC-1 positive if ≥ 10% of tumor or non-tumor cells exhibited 2+ or 3+ cytoplasmic staining intensity (Figure 1). The immunohistochemical staining of KK-LC-1 was independently evaluated by three gastrointestinal surgeons (Futawatari N, Akimoto Y, and Maehara J) who have extensive experience in histopathological interpretation of GC specimens. After independent scoring, all samples were reviewed with additional input from two board-certified pathologists (Yokouchi Y, and Takahashi K) to confirm the consistency and accuracy of the evaluation.
Comparisons between KK-LC-1 positive and negative groups were performed using Fisher’s exact test for categorical variables and Mann-Whitney U test for continuous variables. All P-values < 0.05 were considered significant. All statistical analyses were performed using EZR version 1.63 (Saitama Medical Center, Jichi Medical University, Saitama, Japan)[17].
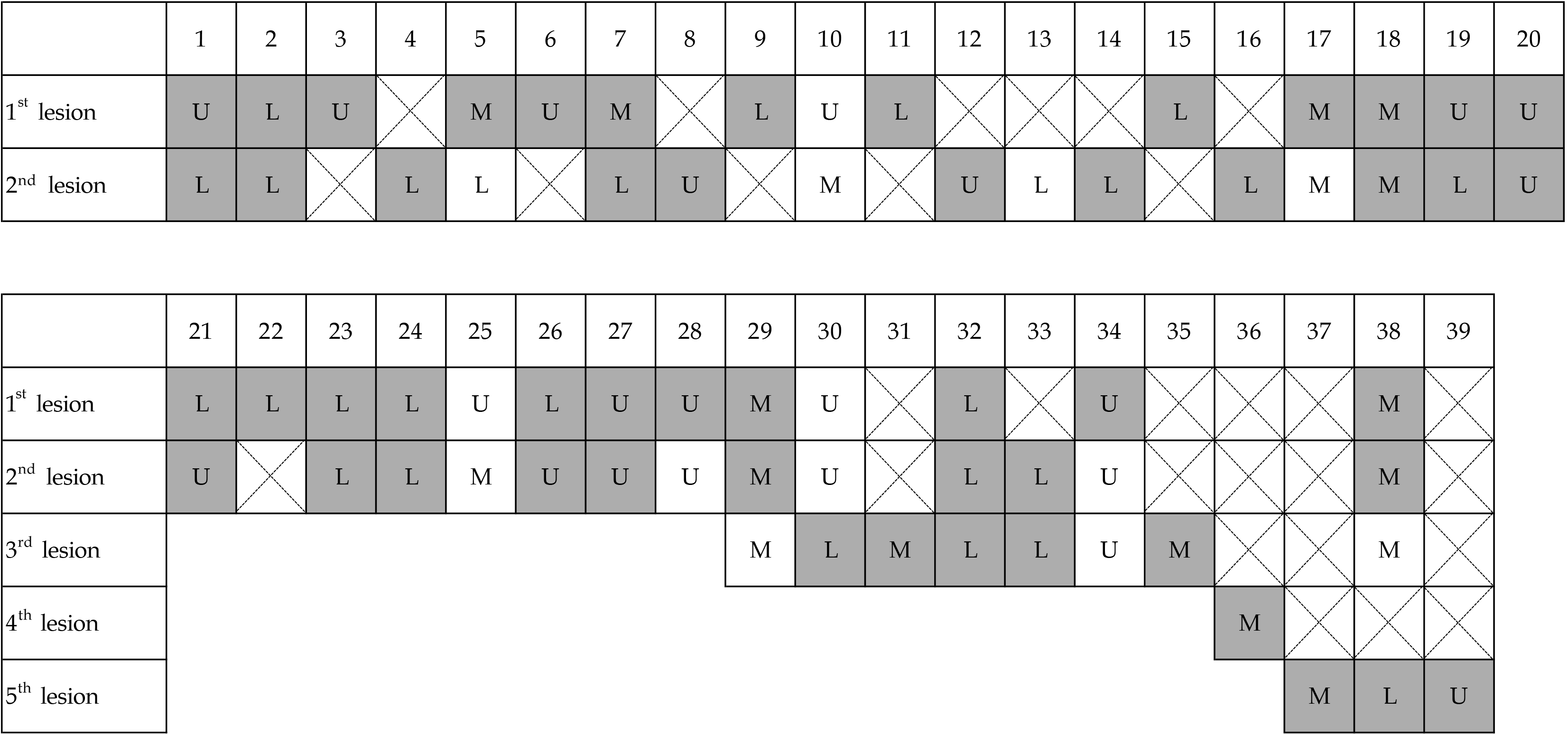
Table 1 summarizes the characteristics of 39 patients with multiple GCs. The mean age of the patients was 76.4 ± 7.50 years, and most were male (31 male vs 8 female). The numbers of cases with multiple cancers involving two, three, four, and five lesions were 28, 7, 1, and 3, respectively. At least one lesion was positive for KK-LC-1 expression in 94.9% of cases.
| Category | |
| Gender (male/female) | 31/8 |
| Age | 76.4 ± 7.503 |
| Number (2/3/4/5) | 28/7/1/3 |
| Synchronous/metachronous | 28/11 |
| Interval (month) | |
| Synchronous | 1.9 (0-10) |
| Metachronous | 30 (14-95) |
| Treatment (ESD/surgery) | 26/1 |
| Atrophic gastritis (non/closed/open), n = 37 | 1/4/32 |
| H. pylori eradication | 41.7% (15/36) |
| Blood anti-H. pylori antibody | 32.5% (12/37) |
| KK-LC-1 expression (more than 1 lesion) | 94.9% (37/39) |
Details of KK-LC-1 expression in the 39 patients are shown in Figure 2. KK-LC-1 expression was investigated in 67 lesions, of which 53 (79.1%) were KK-LC-1 positive. Only two patients (patient 10 and patient 25) did not show KK-LC-1 expression in either lesion (Table 2). Neither patient was infected with H. pylori, and the initial lesions occurred in the upper region.
| Lesion | Age | Gender | Interval | H. pylori infection | GA | Histological finding | T | Location | Size (mm) | Treatment | |
| No. 10 | 1st | 81 | Male | 0 | Negative | O-3 | por2, tub2 | 3 | Upper | 55 | Surgery |
| 2nd | tub1 | 1a | Middle | 20 | ESD | ||||||
| No. 25 | 1st | 73 | Male | 16 | Negative | None | tub2 | 1b1 | Upper | 10 | ESD |
| 2nd | tub2, tub1 | 1b1 | Middle | 11 | ESD |
Table 3 presents the correlation between KK-LC-1 expression and clinicopathological factors in initial GC. KK-LC-1 expression was significantly associated with differentiated histological type (P = 0.035), multiple cancers (P = 0.016), and a history of H. pylori eradication (P = 0.027).
| KK-LC-1 expression | |||
| Positive | Negative | P value | |
| Sex | 0.341 | ||
| Male | 54 (67.5) | 26 (43.3) | |
| Female | 23 (79.3) | 6 (20.7) | |
| Age | 77 (70.0-81.0) | 74 (59.8-79.5) | 0.078 |
| Depth of invasion | 0.110 | ||
| T1 | 61 (74.4) | 21 (25.6) | |
| T2-T4 | 16 (59.3) | 11 (40.7) | |
| Histological type | 0.035 | ||
| Differentiated | 60 (76.9) | 18 (23.1) | |
| Undifferentiated | 17 (54.8) | 14 (45.2) | |
| Tumor diameter | 1 | ||
| ≤ 20 mm | 38 (70.4) | 16 (29.6) | |
| > 20 mm | 39 (70.9) | 16 (29.1) | |
| Lymph node metastasis | 0.073 | ||
| Positive | 12 (54.5) | 10 (45.5) | |
| Negative | 65 (74.7) | 22 (25.3) | |
| Lymphatic invasion | 0.371 | ||
| Positive | 23 (63.9) | 13 (36.1) | |
| Negative | 54 (74.0) | 19 (26.0) | |
| Venous invasion | 0.254 | ||
| Positive | 20 (62.5) | 12 (37.5) | |
| Negative | 57 (74.0) | 20 (26.0) | |
| Pathological stage | 0.150 | ||
| I | 61 (74.4) | 21 (25.6) | |
| II, III, IV | 16 (59.3) | 11 (40.7) | |
| Treatment | 0.091 | ||
| Endoscopic | 46 (78.0) | 13 (22.0) | |
| Surgery | 31 (62.0) | 19 (38.0) | |
| Atrophic gastritis (n = 106) | 0.355 | ||
| Yes | 72 (72.0) | 28 (28.0) | |
| No | 3 (50.0) | 3 (50.0) | |
| Anti-H. pylori antibody titer (n = 101) | 0.279 | ||
| Positive | 27 (64.3) | 15 (35.7) | |
| Negative | 44 (74.6) | 15 (25.4) | |
| H. pylori eradication (n = 99) | 0.027 | ||
| Yes (n = 40) | 33 (82.5) | 7 (17.5) | |
| No (n = 59) | 36 (61.0) | 23 (39.0) | |
| Multiple cancers | 0.016 | ||
| Multiple | 24 (88.9) | 3 (11.1) | |
| Single | 53 (64.6) | 29 (35.4) | |
| Location | 0.800 | ||
| Upper | 17 (73.9) | 6 (26.1) | |
| Middle and lower | 60 (69.8) | 26 (30.2) | |
Table 4 summarizes the correlation between KK-LC-1 expression and clinical pathological factors in initial GC (86 cases) in the middle and lower region. KK-LC-1 expression was significantly higher in patients with older age (P = 0.020), differentiated type (P = 0.022), and multiple cancers (P = 0.002). KK-LC-1 expression was observed in all cases of multiple cancers.
| KK-LC-1 expression | |||
| Positive | Negative | P value | |
| Sex | 0.322 | ||
| Male | 39 (66.1) | 20 (33.9) | |
| Female | 21 (77.8) | 6 (22.2) | |
| Age | 77.0 (70.0-81.0) | 71.5 (58.3-77.8) | 0.020 |
| Depth of invasion | 0.192 | ||
| T1 | 46 (74.2) | 16 (25.8) | |
| T2-T4 | 14 (58.3) | 10 (41.7) | |
| Histological type | 0.022 | ||
| Differentiated | 46 (80.0) | 13(22.0) | |
| Undifferentiated | 14 (51.9) | 13 (48.1) | |
| Tumor diameter | 0.816 | ||
| ≤ 20 mm | 30 (68.2) | 14 (31.8) | |
| > 20 mm | 30 (71.4) | 12 (28.6) | |
| Lymph node metastasis | 0.158 | ||
| Positive | 10 (55.6) | 8 (44.4) | |
| Negative | 50 (73.5) | 18 (26.5) | |
| Lymphatic invasion | 0.614 | ||
| Positive | 17 (65.4) | 9 (34.6) | |
| Negative | 43 (71.7) | 17 (28.3) | |
| Venous invasion | 0.453 | ||
| Positive | 16 (64.0) | 9 (36.0) | |
| Negative | 44 (72.1) | 17 (27.9) | |
| Pathological stage | 0.417 | ||
| I | 47 (72.3) | 18 (27.7) | |
| II, III, IV | 13 (61.9) | 8 (38.1) | |
| Treatment | 0.097 | ||
| Endoscopic | 38 (77.6) | 11 (22.4) | |
| Surgery | 22 (59.5) | 15 (40.5) | |
| Atrophic gastritis (n = 84) | 0.579 | ||
| Yes | 57 (71.3) | 23 (28.8) | |
| No | 2 (50.0) | 2 (50.0) | |
| Anti-H. pylori antibody titer (n = 79) | 0.214 | ||
| Positive | 20 (60.6) | 13 (39.4) | |
| Negative | 35 (76.1) | 11 (23.9) | |
| H. pylori eradication (n = 78) | 0.142 | ||
| Yes | 26 (78.8) | 7 (21.2) | |
| No | 28 (62.2) | 17 (37.8) | |
| Multiple cancers | 0.002 | ||
| Multiple | 16 (100) | 0 (0) | |
| Single | 44 (62.9) | 26 (37.1) | |
KK-LC-1 is a cancer/testis antigen that is not expressed in normal tissues except for the testis, but has been found in cancers of multiple organs[18,19]. It has been recognized as a tumor-targeting peptide with high clinical potential for the diagnosis, imaging, and bioavailability of GC owing to its cancer/testis antigen characteristics[10]. KK-LC-1 expression may be characteristic of early-stage GC cases with a favorable prognosis, as it has been associated with longer overall survival in patients exhibiting KK-LC-1 expression[20]. In a previous study, we identified a correlation between KK-LC-1 expression and H. pylori infection in GC. However, we observed higher KK-LC-1 expression in cases with successful H. pylori eradication, suggesting that KK-LC-1 expression may be regulated by pathways independent of H. pylori infection[14]. Therefore, we hypothesize that gastric mucosa, irrespective of H. pylori infection status, expresses KK-LC-1 when it reaches a carcinogenic or precancerous state and subsequently develops into GC[13,14].
Most GCs are caused by H. pylori infection[21], which damages the gastric mucosa, leading to gastritis, chronic atrophic gastritis, intestinal metaplasia, and dysplasia, thereby increasing the risk of GC[21,22]. Some reports have suggested that H. pylori eradication reduces the incidence of metachronous carcinoma after endoscopic resection[2], whereas others report no preventive effect, especially when the gastric mucosa has progressed to the intestinal metaplasia or dysplasia stage[21,22], rendering the issue controversial[22]. This study serves as a preliminary investigation into the potential role of KK-LC-1 expression as a biomarker for the development of multiple GCs, providing a foundation for future comprehensive studies.
The expression rate of KK-LC-1 in multiple GC was 79.1%, consistent with previous reports. Among patients with multiple cancers, KK-LC-1 expression was positive in at least one lesion in 94.9% of cases. Although prior studies hypothesized that H. pylori infection induces KK-LC-1 expression[13], we observed KK-LC-1 expression in H. pylori-uninfected, currently infected, and previously infected lesions. Therefore, KK-LC-1 expression may occur during carcinogenesis independently of H. pylori infection[14]. Notably, in this study, the two cases lacking KK-LC-1 expression in two lesions were both H. pylori-uninfected, with initial lesions located in the upper region of the stomach. While there may be some relationship between H. pylori infection and KK-LC-1 expression, the small sample size limits definitive conclusions, warranting further research.
When examining KK-LC-1 expression in initial lesions alongside clinicopathological factors, differentiation type, multiple cancers, and H. pylori eradication were significantly associated with higher KK-LC-1 expression, consistent with previous findings[14]. To evaluate KK-LC-1 as a biomarker to identify the risk of multiple cancers, we analyzed characteristics of initial lesions. In cases where the initial lesion was located in the M&L stomach regions, KK-LC-1 expression was significantly higher among older patients, those with differentiated histology, and those with multiple cancers. KK-LC-1-positive tumors were detected in the initial lesions of all patients (100%) with multiple GCs in the middle and lower region, with a significantly higher incidence compared to that of patients with solitary cancers. Conversely, none of the patients with KK-LC-1-negative initial lesions in the middle and lower region developed multiple GCs, suggesting KK-LC-1 negativity may indicate a very low risk of metachronous or synchronous GC, although this finding is limited by sample size. In contrast, no similar association was observed for initial lesions located in the upper region.
This study has several limitations. It is a single-center study with a relatively small sample size and short observation period (3 years and 2 months), limiting the ability to capture all cases of multiple GCs. Future long-term follow-up studies with larger cohorts and detailed analyses of KK-LC-1 expression may clarify whether KK-LC-1 serves as a reliable risk factor for the development of multiple GCs.
Investigating KK-LC-1 expression in GC lesions may be useful in predicting multiple GCs. Specifically, if KK-LC-1 expression is positive in the middle and lower region, it may indicate the potential for the future development of multiple GCs and could function as a biomarker for predicting multiple GCs. Notably, in this study, none of the patients with KK-LC-1-negative initial lesions located in the middle and lower region developed multiple GCs. This finding suggests that the absence of KK-LC-1 expression in middle and lower lesions may be associated with an extremely low, potentially negligible, risk of developing multiple GCs.
The authors thank Yoshie Muraishi for their technical assistance.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68447] [Article Influence: 13689.4] [Reference Citation Analysis (201)] |
| 2. | Mori G, Nakajima T, Asada K, Shimazu T, Yamamichi N, Maekita T, Yokoi C, Fujishiro M, Gotoda T, Ichinose M, Ushijima T, Oda I. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: results of a large-scale, multicenter cohort study in Japan. Gastric Cancer. 2016;19:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Chen Y, He L, Zheng X. Characteristics of multiple early gastric cancer and gastric high-grade intraepithelial neoplasia. Medicine (Baltimore). 2023;102:e36439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Kinami S, Aizawa M, Yamashita H, Kumagai K, Kamiya S, Toda M, Takahata T, Fujisaki M, Miyamoto H, Kusanagi H, Kobayashi K, Washio M, Hosoda K, Kosaka T. The incidences of metachronous multiple gastric cancer after various types of gastrectomy: analysis of data from a nationwide Japanese survey. Gastric Cancer. 2021;24:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Abe S, Takizawa K, Oda I, Mizusawa J, Kadota T, Ono H, Hasuike N, Yano T, Yamamoto Y, Horiuchi Y, Nagata S, Yoshikawa T, Terashima M, Muto M. Incidence and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection of undifferentiated-type early gastric cancer: Japan Clinical Oncology Group study-post hoc analysis of JCOG1009/1010. Gastric Cancer. 2021;24:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 2385] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 7. | Paret C, Simon P, Vormbrock K, Bender C, Kölsch A, Breitkreuz A, Yildiz Ö, Omokoko T, Hubich-Rau S, Hartmann C, Häcker S, Wagner M, Roldan DB, Selmi A, Türeci Ö, Sahin U. CXorf61 is a target for T cell based immunotherapy of triple-negative breast cancer. Oncotarget. 2015;6:25356-25367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Shida A, Futawatari N, Fukuyama T, Ichiki Y, Takahashi Y, Nishi Y, Kobayashi N, Yamazaki H, Watanabe M. Frequent High Expression of Kita-Kyushu Lung Cancer Antigen-1 (KK-LC-1) in Gastric Cancer. Anticancer Res. 2015;35:3575-3579. [PubMed] |
| 9. | Stevanović S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff CA, Rosenberg SA, Hinrichs CS. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 10. | Yu X, Yan J, Chen X, Wei J, Yu L, Liu F, Li L, Liu B. Identification of a peptide binding to cancer antigen Kita-kyushu lung cancer antigen 1 from a phage-display library. Cancer Sci. 2021;112:4335-4345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Bu J, Zhang Y, Wu S, Li H, Sun L, Liu Y, Zhu X, Qiao X, Ma Q, Liu C, Niu N, Xue J, Chen G, Yang Y, Liu C. KK-LC-1 as a therapeutic target to eliminate ALDH(+) stem cells in triple negative breast cancer. Nat Commun. 2023;14:2602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Kondo Y, Fukuyama T, Yamamura R, Futawatari N, Ichiki Y, Tanaka Y, Nishi Y, Takahashi Y, Yamazaki H, Kobayashi N, Watanabe M. Detection of KK-LC-1 Protein, a Cancer/Testis Antigen, in Patients with Breast Cancer. Anticancer Res. 2018;38:5923-5928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Fukuyama T, Futawatari N, Yamamura R, Yamazaki T, Ichiki Y, Ema A, Ushiku H, Nishi Y, Takahashi Y, Otsuka T, Yamazaki H, Koizumi W, Yasumoto K, Kobayashi N. Expression of KK-LC-1, a cancer/testis antigen, at non-tumour sites of the stomach carrying a tumour. Sci Rep. 2018;8:6131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Futawatari N, Fukuyama T, Akimoto Y, Maehara J, Hihara D, Okamoto Y, Yokouchi Y, Takahashi K, Watanabe M, Saida Y. Association Between Kita-kyushu Lung Cancer Antigen-1 Expression in Gastric Cancer and Helicobacter pylori-infection Status. Anticancer Res. 2025;45:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2945] [Article Influence: 196.3] [Reference Citation Analysis (0)] |
| 16. | Kimura K, Takemoto T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 777] [Article Influence: 43.2] [Reference Citation Analysis (5)] |
| 17. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 14450] [Article Influence: 1111.5] [Reference Citation Analysis (0)] |
| 18. | Futawatari N, Fukuyama T, Yamamura R, Kobayash N. Helicobacter Pylori Infection Induces Gastric Cancer and the Cancer/Testis Antigens Expression. J Infect Dis Ther. 2017;5:345. [DOI] [Full Text] |
| 19. | Fukuyama T, Hanagiri T, Takenoyama M, Ichiki Y, Mizukami M, So T, Sugaya M, So T, Sugio K, Yasumoto K. Identification of a new cancer/germline gene, KK-LC-1, encoding an antigen recognized by autologous CTL induced on human lung adenocarcinoma. Cancer Res. 2006;66:4922-4928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Ji J, Chen J, Wang A, Zhang W, Ju H, Liu Y, Li L. KK-LC-1 may be an effective prognostic biomarker for gastric cancer. BMC Cancer. 2021;21:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer. 2016;19:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 22. | Zhu F, Zhang X, Li P, Zhu Y. Effect of Helicobacter pylori eradication on gastric precancerous lesions: A systematic review and meta-analysis. Helicobacter. 2023;28:e13013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/