Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.109644
Revised: June 24, 2025
Accepted: August 20, 2025
Published online: September 24, 2025
Processing time: 129 Days and 15.3 Hours
Multiple primary cancers refer to the presence of two or more distinct malignant tumors in a single individual, either simultaneously or sequentially. The synch
A 74-year-old postmenopausal woman (Karnofsky performance status = 80) pre
Multidisciplinary management offers a promising strategy for treating synchronous complex malignancies with individualized treatment plans.
Core Tip: Synchronous primary cancers involving cholangiocarcinoma and cervical squamous cell carcinoma are extremely rare, posing significant diagnostic and therapeutic challenges. We report successful multidisciplinary management of such a case in a 74-year-old woman through combined laparoscopic liver resection, pelvic radiotherapy, targeted therapy, chemotherapy, and immunotherapy. At the 12-month follow-up, imaging and tumor marker data indicated no recurrence or metastasis. This case highlights the importance of personalized, multidisciplinary approaches in managing rare synchronous malignancies, providing valuable insights for clinicians encountering similar complex scenarios.
- Citation: Wu ZJ, Wang B, Zhao SC, Pan ZT. Synchronous cholangiocarcinoma and cervical squamous cell carcinoma managed via a multidisciplinary approach: A case report. World J Clin Oncol 2025; 16(9): 109644
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/109644.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.109644
Multiple primary cancers (MPCs) are defined as two or more distinct malignant tumors that arise either synchronously (diagnoses < 6 months apart) or metachronous (≥ 6 months apart), a distinction first formalized by Warren and Gates and retained by the Surveillance, Epidemiology, and End Results program and the International Agency for Research on Cancer[1,2]. The prevalence of MPC in the cancer population ranges from 4.5% to 11.7% according to some reports[3,4]. To our knowledge, a comprehensive search of PubMed, EMBASE, Scopus, and Google Scholar identified no prior peer-reviewed case reports of synchronous intrahepatic cholangiocarcinoma (CCA) and cervical squamous cell carcinoma (SCC). The nature of the diagnostic challenges and the complexities of initial treatment planning are also uniquely presented in this report on synchronous CCA and cervical SCC. Here we describe a 74-year-old woman with American Joint Committee on Cancer (AJCC) 8th T1aN0M0 CCA and International Federation of Gynecology and Obstetrics (FIGO) 2018 IB1 cervical SCC who achieved durable disease control through a multidisciplinary strategy combining laparoscopic liver resection, pelvic radiotherapy, and systemic therapy. This case study provides valuable insights into the growing body of literature advocating for personalized treatment strategies for managing rare dual malignancies.
A 74-year-old female was admitted to Dongyang People’s Hospital on March 13, 2024, with a one-day history of postmenopausal vaginal bleeding.
The patient, who had been menopausal since age 50, reported no prior episodes of abnormal vaginal bleeding. On the morning of admission, she experienced sudden, significant vaginal bleeding without associated abdominal pain, distension, urinary, or gastrointestinal symptoms. A transvaginal ultrasound revealed a solid cervical mass, raising concerns for a primary cervical malignancy.
The patient had a two years history of hypertension, which was well controlled with oral irbesartan-hydrochlorothiazide and lacidipine. She had previously undergone excision and ligation for a benign breast tumor. There was no history of diabetes, coronary artery disease, hepatitis B, tuberculosis, or other significant infections. She denied tobacco and alcohol use.
There was no family history of malignancy.
On examination, the patient was alert for stable vital signs. Cardiopulmonary findings were unremarkable. Karnofsky performance status = 80. Gynecological examination revealed a small number of blood clots in the vaginal canal. Inspection of the cervix revealed marked atrophy with a 1.5 cm × 1.5 cm erosive lesion; bimanual palpation revealed a firm mass measuring approximately 2 cm × 3 cm with involvement of the adjacent soft tissue.
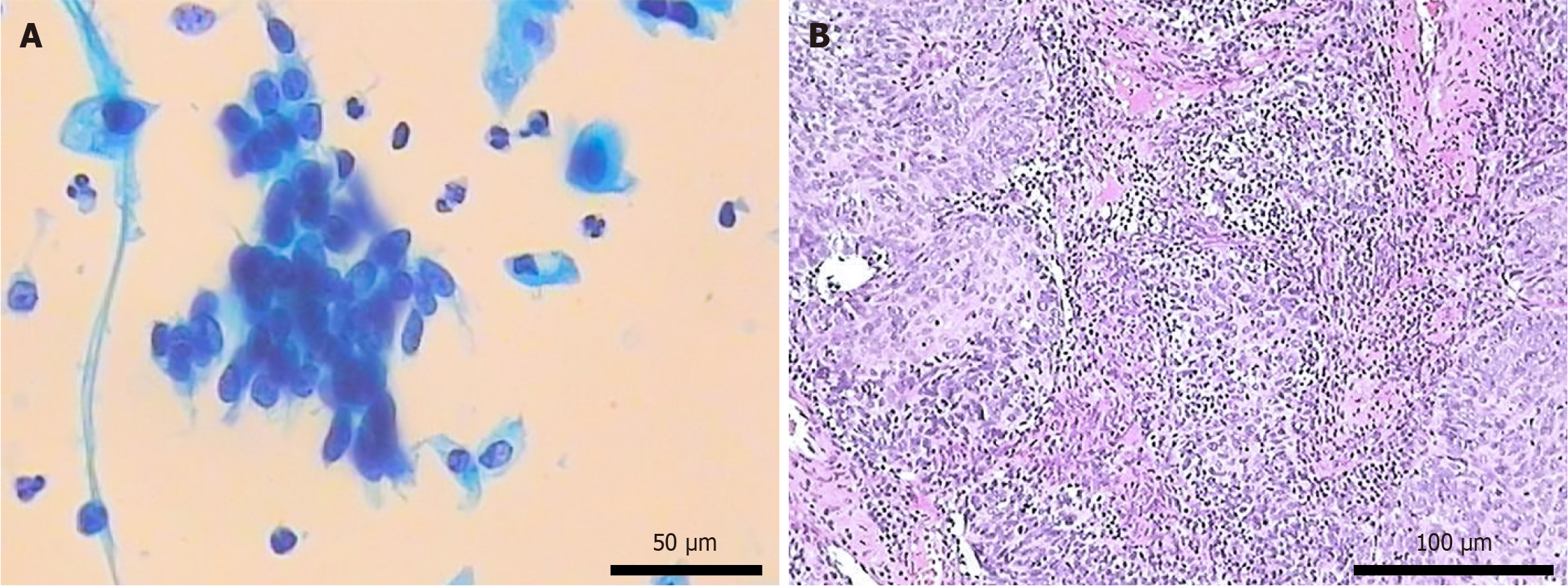
Initial laboratory tests - including complete blood count, liver and renal function panels, and coagulation studies - were within normal limits. The serum progesterone level was 4.39 nmol/L. Urinalysis was positive for occult blood and leukocytes. Hepatitis B serologies were negative. The tumor marker levels were unremarkable except for a mild increase in the level of SCC antigen (3.8 μg/L). Liquid-based cytology revealed a high-grade squamous intraepithelial lesion (Figure 1), and cervical cytology was positive for high-risk human papillomavirus (HPV). Our institution employs a “12 + 2” HPV testing protocol; therefore, a result labeled “HPV type 12” indicates the detection of one of 12 high-risk types (excluding types 16 and 18, which are reported separately). Ultrasound-guided liver biopsy confirmed CCA.
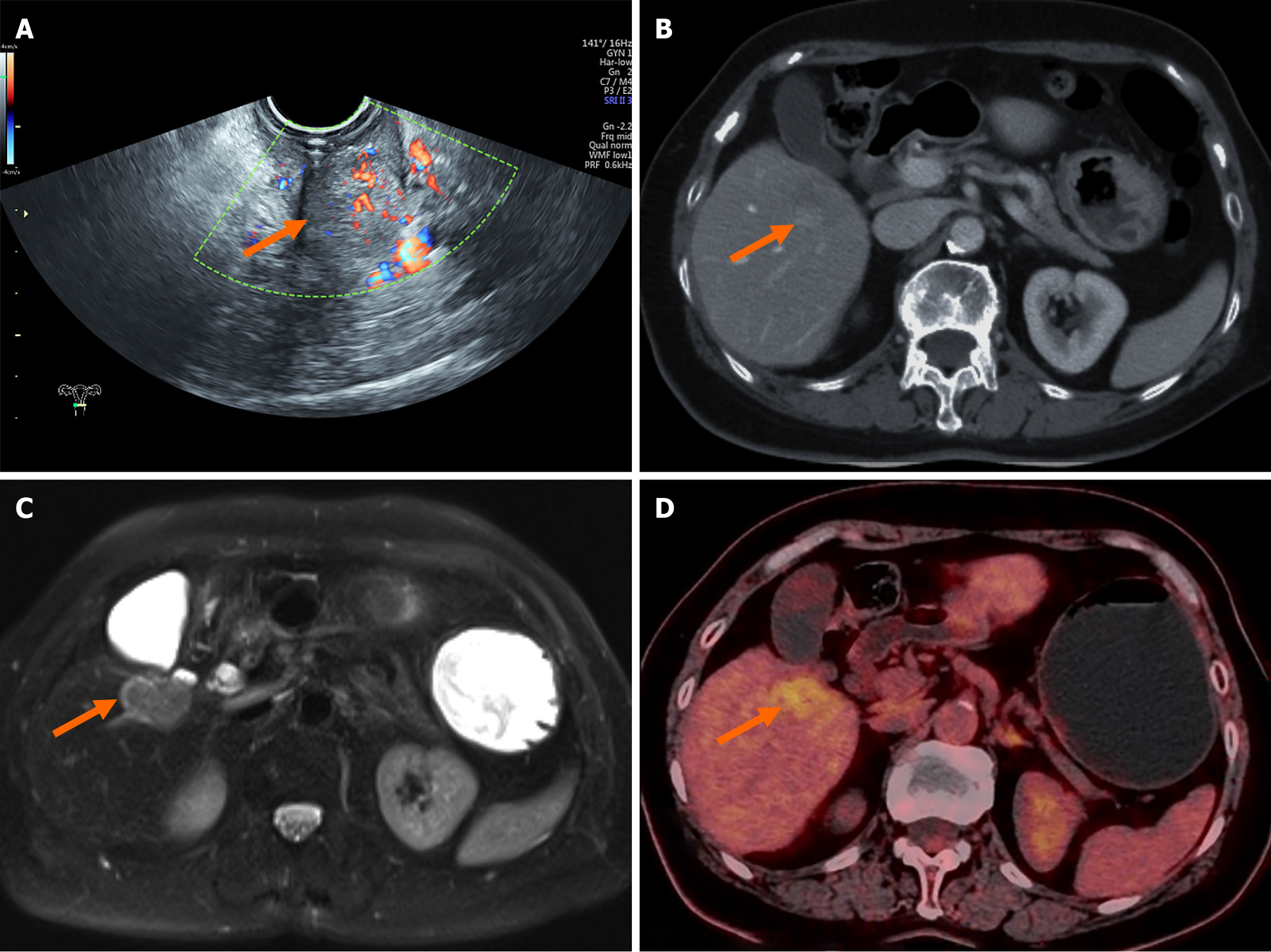
Transvaginal ultrasound confirmed the presence of a solid cervical mass (Figure 2A). Abdominal contrast-enhanced computed tomography (CT) revealed cervical thickening with enhancement and a low-density lesion in the right hepatic lobe (Figure 2B). Liver magnetic resonance imaging revealed a malignant lesion in segment V (Figure 2C), with a differential diagnosis that included primary hepatic malignancy vs metastatic disease. Contrast-enhanced ultrasound of the liver lesion revealed a rapid-in, rapid-out enhancement pattern, favoring a primary liver tumor. Positron emission tomography/CT imaging revealed increased fluorodeoxyglucose uptake in both the cervical lesion and a low-density hepatic mass in segment V (Figure 2D); however, positron emission tomography/CT was unable to definitively differentiate between benign and malignant etiologies.
AJCC 8th edition of T1aN0M0 intrahepatic CCA and FIGO 2018 IB1 cervical SCC with no radiographic evidence of distant metastasis.
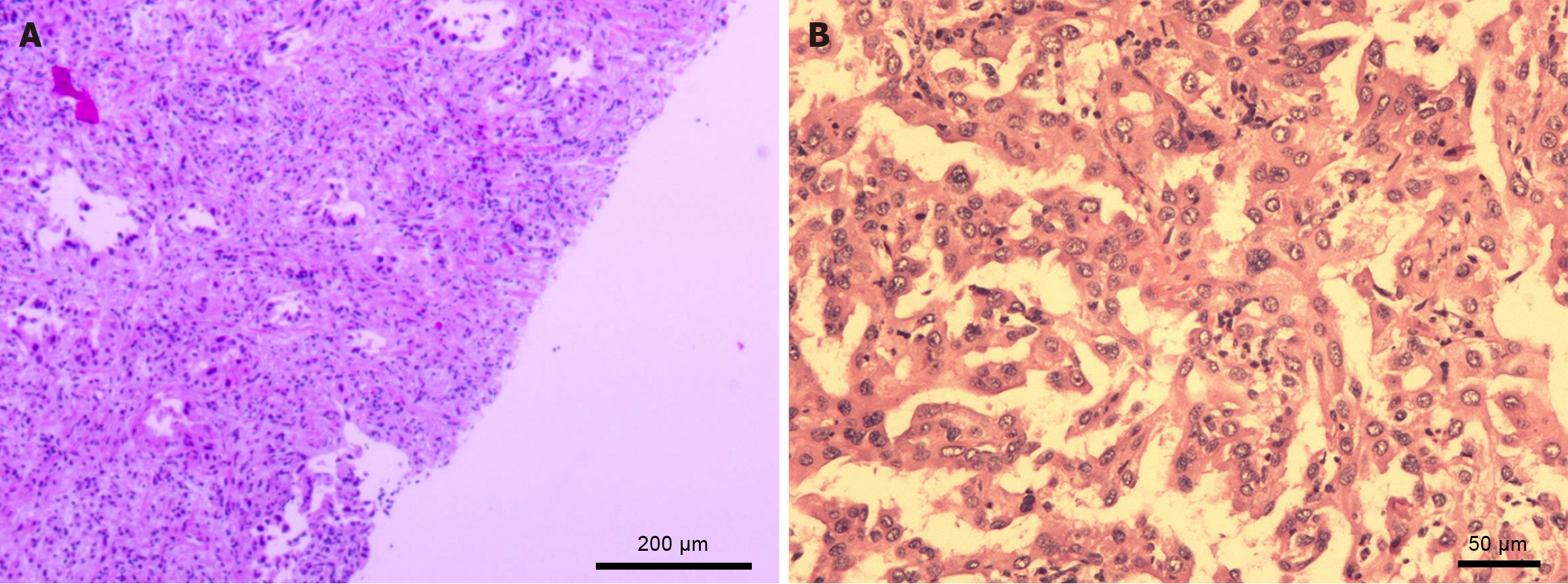
A multidisciplinary tumor board meeting was held to discuss the optimal management strategy. Owing to the aggressive nature of CCA, hepatic resection is prioritized. On March 29, 2024, the patient underwent laparoscopic liver resection. Intraoperative findings included a white, slightly elevated mass in segment V and multiple enlarged lymph nodes in the hepatoduodenal ligament. Partial resection of the liver (involving segment V and the right posterior lobe) along with lymphadenectomy was performed. Postoperative histopathology demonstrated moderately differentiated intrahepatic CCA with perineural invasion (Figure 3). Postoperatively, the patient developed fever and chills accompanied by leukocytosis and an elevated C-reactive protein level. CT imaging revealed fluid collection in the surgical bed, and blood cultures revealed Streptococcus viridans, confirming an abdominal infection. This complication was effectively managed with abdominal drainage and broad-spectrum antibiotics (vancomycin and cefoperazone-sulbactam).
After stabilization, the patient was transferred to the gynecology department. On May 2, 2024, she began pelvic radiotherapy consisting of external-beam intensity-modulated radiation therapy to 45 grays in 25 fractions (1.8 grays per fraction with 6-megavolt photons) followed by high-dose-rate 192Ir intracavitary brachytherapy delivering 35 grays to A-point in seven twice-weekly fractions of 5 grays each; no acute toxicities ≥ grade 2 (Common Terminology Criteria for Adverse Events Version 5.0) were observed and no late effects have emerged to date. Systemic therapy commenced on August 6, 2024 with lenvatinib 8 mg once daily, capecitabine 1.5 g twice daily on days 1-14 of a 21-day cycle, and camrelizumab 200 mg IV every three weeks; the eighth and final camrelizumab dose was administered on January 1, 2025, when the patient also received a two-week supply of capecitabine and a one-month supply of lenvatinib. She has not required further systemic therapy since that visit, and no clinically significant adverse events have been recorded.
The patient has maintained good functional status (Karnofsky performance status = 80) throughout therapy. At the latest follow-up on March 22, 2025 (12 months after diagnosis), contrast-enhanced abdominal CT imaging revealed no signs of recurrence or metastasis in the cervix or liver. The results of laboratory studies, including those for tumor markers, remain within normal limits. A detailed chronological overview of investigations and interventions is provided in Table 1.
| Date (2024-2025) | Event |
| March 13, 2024 | First presentation with post-menopausal bleeding |
| March 14, 2024 to March 25, 2024 | Crosssectional imaging (contrast CT/MRI) and PET/CT reveal solitary segment-V liver lesion; tissue confirmation: Cervical punch biopsy - squamouscell carcinoma; Ultrasound-guided liver biopsy - intrahepatic cholangiocarcinoma; multidisciplinary tumor board agrees treatment sequence |
| March 29, 2024 | Laparoscopic partial hepatectomy (segment V and right posterior lobe) and regional lymph-node dissection; R0 resection achieved |
| April 4, 2024 | Post-operative abdominal infection (Streptococcus viridans) managed with drainage plus IV vancomycin + cefoperazone/sulbactam |
| May 2, 2024 to June 24, 2024 | Pelvic radiotherapy for FIGO IB1 cervical SCC: External-beam IMRT 45 grays/25 fractions (May 2, 2024 to June 6, 2024); HDR 192Ir intracavitary brachytherapy 35 grays/7 fractions to point A (June 10, 2024 to June 24, 2024) |
| August 6, 2024 | Commenced systemic therapy: Lenvatinib 8 mg quaque die + capecitabine 1.5 g bis in die (day 1-14, quaque 21 die) + camrelizumab 200 mg IV quaque 3 weeks |
| January 1, 2025 | 8th (final) camrelizumab cycle administered; capecitabine and lenvatinib dispensed for last course |
| March 22, 2025 | 12-month follow-up: Contrast-enhanced CT negative for recurrence/metastasis; tumor markers normal; Karnofsky 80 |
MPCs represent a rare and complex clinical phenomenon characterized by the occurrence of two or more primary malignant tumors in a single patient[3]. The risk of developing a second primary malignancy varies significantly among different cancer sites, with reported rates ranging from 1% for primary liver cancer to 16% for primary bladder cancer[1]. There is no standardized treatment for MPCs; thus, individualized management - considering the tumor type, location, stage, and overall patient condition - is imperative, with a strong emphasis on a multidisciplinary approach[5]. In the present case, both tumors met the diagnostic criteria for MPC defined by Warren and Gates and endorsed by the Surveillance, Epidemiology, and End Results and International Agency for Research on Cancer. Both were histologically malignant, arose in distinct organs, and comprehensive imaging and immunohistochemistry excluded metastasis between them. Rigorous application of these criteria is especially important in rare scenarios like this, where distinguishing synchronous primaries from metastasis can be challenging and directly affects clinical management and prognosis.
The pathogenesis of synchronous primary malignancies is multifactorial and involves metabolic disorders, lifestyle and environmental exposures, previous cancer therapies, and genetic predispositions[6]. While HPV is widely recognized for its role in the pathogenesis of cervical SCC[7], its potential involvement in hepatobiliary malignancies remains a matter of ongoing investigation. Although some studies have suggested that HPV may interact with other oncogenic viruses, such as hepatitis B virus, to promote hepatocellular carcinoma[8,9], no clear etiological factors were identified in our patient. Distinguishing a hepatic metastasis from a second primary metastasis is pivotal, because management algorithms diverge. Currently, biliary tract oncology increasingly exploits circulating tumor DNA (ctDNA) for minimal residual disease detection and surveillance. Recent studies have demonstrated concordance between ctDNA profiles and tissue sequencing data in advanced biliary cancers and support the use of ctDNA-guided recurrence prediction after resection[10,11]. We discussed but did not perform ctDNA analysis because the assay is not yet reimbursed in our region and the patient had negative postoperative imaging results.
For single primary intrahepatic CCA (AJCC 8th edition T1aN0M0), the standard treatment is anatomic liver resection with regional lymphadenectomy, with the goal of R0 resection[5]. For FIGO 2018 stage IB1 cervical SCC, both radical hysterectomy with pelvic lymphadenectomy and definitive pelvic radiotherapy plus intracavitary brachytherapy are recommended options and have comparable survival outcomes, as demonstrated by randomized studies[12,13]. In this case, the multidisciplinary tumor board prioritized early surgical intervention for CCA and selected radiotherapy for cervical SCC, considering the patient’s advanced age, comorbidities, and recent postoperative infection. This individualized, multidisciplinary tumor board–driven approach balances oncologic control with treatment risk, and serves as a reference for managing synchronous primary malignancies in complex clinical scenarios. The incorporation of systemic therapy using a regimen of lenvatinib, capecitabine, and camrelizumab was based on the rationale of targeting multiple pathways involved in tumor progression, thereby providing a synergistic effect against both malignancies[14,15]. Although there is limited research on the combination of immunotherapy and radiotherapy in early-stage cervical cancer, studies in advanced cancers suggest that this combination can enhance treatment responses and offer survival benefits, particularly for elderly patients or those with comorbidities[16]. The collaboration among hepatologists, oncologists, gynecologists, and pathologists has enabled timely diagnosis, accurate staging, and individualized treatment planning.
Bidirectional Mendelian randomization studies indicate that both the gut microbiota and discrete immune cell phenotypes are involved in biliary tract carcinogenesis[17,18]. These data support our use of lenvatinib + capecitabine + camrelizumab and suggest that microbiome- and immunity-based surveillance may benefit patients with dual primary tumors. In parallel, a large nomogram for elderly patients with primary colorectal lymphoma showed that age-tailored therapy improved survival[19]. This evidence guided our team to moderate the radiotherapy dose and systemic therapy in this 74-year-old patient, resulting in one-year disease-free survival without unnecessary toxicity. This case, therefore, demonstrates that close multidisciplinary coordination can enable guideline-concordant, organ-specific care even when randomized data for synchronous primary tumors are unavailable. Future ctDNA-guided liquid biopsy, immunological insights and microbiome research should enable further individualization of the treatment of patients with MPCs.
This case highlights the clinical complexity and diagnostic challenges of synchronous CCA and cervical SCC, and underscores the value of a personalized, multidisciplinary approach. Successful disease control was achieved through hepatic resection, radiotherapy, and a systemic regimen incorporating lenvatinib, capecitabine, and camrelizumab, with no recurrence at one-year follow-up. This experience supports integrating surgical, radiotherapeutic, and immunotherapeutic modalities for managing rare dual malignancies, especially in elderly patients. Emerging tools such as ctDNA-based liquid biopsy may further refine post-treatment surveillance and risk assessment. Future research should investigate the underlying molecular mechanisms and long-term outcomes of multimodal therapies in larger cohorts.
| 1. | Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2:e000172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 412] [Article Influence: 45.8] [Reference Citation Analysis (1)] |
| 2. | Yang J, Zeng Y, Zhang JW. Simultaneous multiple primary malignancies diagnosed by endoscopic ultrasound-guided fine-needle aspiration: A case report. World J Clin Cases. 2022;10:5764-5769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Tan Y, Chen X, Ye M, Li X, Liu W, Liao S, Xie Z, Zuo Y. Synchronous multiple primary malignancies of clear cell renal cell carcinoma with sarcomatoid, thyroid carcinoma: a case report. Front Oncol. 2023;13:1174306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Khan RN, Kazmi Z, Vohra LM, Uddin Z. Primary synchronous malignancies of the breast and the kidney. BMJ Case Rep. 2021;14:e243563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Morawitz J, Bruckmann NM, Jannusch K, Kirchner J, Antoch G, Loosen S, Luedde T, Roderburg C, Minko P. Update on Locoregional Therapies for Cholangiocellular Carcinoma. Cancers (Basel). 2023;15:2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Osama MA, Chatterjee P, Kumar R, Saini G, Lal R, Biswas R. Synchronous Malignancies: Pathological Analysis of Three Patients, Each with Dual Malignancies. J Lab Physicians. 2023;15:608-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Baj J, Forma A, Dudek I, Chilimoniuk Z, Dobosz M, Dobrzyński M, Teresiński G, Buszewicz G, Flieger J, Portincasa P. The Involvement of Human Papilloma Virus in Gastrointestinal Cancers. Cancers (Basel). 2022;14:2607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Scinicariello F, Sato T, Lee CS, Hsu HC, Chan TS, Tyring SK. Detection of human papillomavirus in primary hepatocellular carcinoma. Anticancer Res. 1992;12:763-766. [PubMed] |
| 9. | Pang RW, Joh JW, Johnson PJ, Monden M, Pawlik TM, Poon RT. Biology of hepatocellular carcinoma. Ann Surg Oncol. 2008;15:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Wang K, Chen Y, Li Y. Evaluating concordance and clinical utility of ctDNA profiling in advanced biliary tract cancer. J Hepatol. 2025;82:e320-e321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Wang K, Song Z. Evaluating clinical utility of ctDNA monitoring for recurrence prediction in resected extrahepatic cholangiocarcinoma. J Hepatol. 2025;82:e317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, Favini G, Ferri L, Mangioni C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1173] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 13. | Cibula D, Raspollini MR, Planchamp F, Centeno C, Chargari C, Felix A, Fischerová D, Jahnn-Kuch D, Joly F, Kohler C, Lax S, Lorusso D, Mahantshetty U, Mathevet P, Naik R, Nout RA, Oaknin A, Peccatori F, Persson J, Querleu D, Bernabé SR, Schmid MP, Stepanyan A, Svintsitskyi V, Tamussino K, Zapardiel I, Lindegaard J. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer - Update 2023. Int J Gynecol Cancer. 2023;33:649-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 302] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 14. | Mi X, Tuo F, Lin T. Efficacy and safety of camrelizumab for the treatment of cervical cancer: a systematic review and meta-analysis. Front Oncol. 2024;14:1526103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Bath NM, Pawlik TM. Narrative review: current management and novel targeted therapies in intrahepatic cholangiocarcinoma. Chin Clin Oncol. 2023;12:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Yang X, Ren H, Fu J. Combinations of radiotherapy with immunotherapy in cervical cancer. J Cancer. 2022;13:1480-1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Hu Y, Wang K, Chen Y, Jin Y, Guo Q, Tang H. Causal relationship between immune cell phenotypes and risk of biliary tract cancer: evidence from Mendelian randomization analysis. Front Immunol. 2024;15:1430551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 18. | Wang K, Wang S, Qin X, Chen Y, Chen Y, Wang J, Zhang Y, Guo Q, Zhou C, Zou D. The causal relationship between gut microbiota and biliary tract cancer: comprehensive bidirectional Mendelian randomization analysis. Front Cell Infect Microbiol. 2024;14:1308742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 19. | Wang K, Zhao L, Che T, Zhou C, Qin X, Hong Y, Gao W, Zhang L, Gu Y, Zou D. Development and validation of web-based risk score predicting prognostic nomograms for elderly patients with primary colorectal lymphoma: A population-based study. J Transl Int Med. 2024;12:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/