Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.108748
Revised: May 28, 2025
Accepted: August 12, 2025
Published online: September 24, 2025
Processing time: 153 Days and 1.6 Hours
Neuroendocrine tumors are a rare cancer, with those arising in gastric tissue even less commonly. With increasing recognition through endoscopy, these tumors are diagnosed in more patients each year. As a rare and growing entity, our under
Core Tip: Gastric neuroendocrine tumors are a rare pathology, with rising incidence as endoscopic diagnoses increase. Management, including surgical decision-making, is guided by subtype as well as tumor size and grade, which have been shown to be prognostic. There still exist areas that lack consensus, namely for extent of surgery for intermediate-grade and medium-sized type III tumors, as well as in clarifying the role of systemic therapies for high-grade tumors in the perioperative settings and for nonoperative metastatic disease specifically for gastric-originating tumors. Existing practice guidelines provide surgeons the framework for treatment, however future work is needed to fill gaps in decision-making consensus.
- Citation: Agathis AZ, Lopez-May M, Brown C, Divino CM. Gastric neuroendocrine tumors: A review of pathology and updated roadmap to surgical management. World J Clin Oncol 2025; 16(9): 108748
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/108748.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.108748
Neuroendocrine neoplasms (NENs) are a rare type of cancer but are growing in incidence[1]. They arise from cell types with hormonal properties, which are often responsible for their disease manifestations. NENs range from well-differentiated neuroendocrine tumors (NETs) to poorly differentiated neuroendocrine carcinomas (NECs)[2,3]. NET malignancy potential ranges in severity, while NECs are characteristically aggressive and malignant. Many medical professionals tend to use the term carcinoid interchangeably with any neuroendocrine neoplasm, however over time it has developed a more specific definition. “Carcinoid” is a term first coined in 1907 and was used to describe these often slow-growing gastrointestinal tumors with polymorphic cytoplasm and prominent nuclei[4,5]. Now, it is used to specifically describe well-differentiated non-pancreatic gastrointestinal NETs[6,7]. These neuroendocrine tumors arise in various locations, including the stomach.
Gastric NETs (G-NETs) are a particularly rare subtype of neuroendocrine tumor but have nonetheless been rising in prevalence over the last 3 decades[1,8,9]. It is understood that most G-NETs arise from gastric enterochromaffin-like (ECL) cells. Management varies based on the location, size, histologic features, and other tumor characteristics. Gastric NETs are classified based on the World Health Organization (WHO) pathophysiological system: Type I (most common), type II (least common), and type III[10-14]. Type I and II tumors often arise in the context of hypergastrinemia, with type I related to atrophic gastritis vs type II related to syndromes such as Zollinger-Elison syndrome. They are often multifocal and less aggressive than type III. In contrast, type III tumors often arise without the context of hypergastrinemia and are often higher grade. Type III gastric NETs clinically present as an aggressive singular tumor rather than from the neuro-hormonal manifestations (Table 1).
| Type I (75%-80%) | Type II (5%) | Type III (15%-25%) | |
| Gastrin levels | High gastrin | High gastrin | Normal gastrin |
| Gastric acid | pH > 4, hypo- or achlorhydria | pH < 2, hyperchlorhydria | pH < 4, normal gastric HCL |
| Surrounding gastric mucosa | Atrophic gastritis | Corpus mucosal hypertrophy | Normal mucosa |
| Underlying cause | Autoimmune atrophic gastritis | Gastrinoma related to Zollinger-Ellison, MEN-1 syndrome | Sporadic mutations |
| Tumor number | Multifocal | Multifocal | Singular |
| Tumor size | Smaller, < 2 cm | Smaller, < 2 cm | Larger, > 2 cm |
| WHO grade | 1-2 | 1-2 | 1-3 |
| Ki-67% | Lower | Lower | Higher |
| Malignancy potential | Low | Low | High |
In this review, we seek to provide an overview of disease epidemiology and pathology to provide context before focusing on treatment clinical decision-making. We will delve deep into surgical management, providing a roadmap for treating this rising population of patients, highlighting gaps in consensus.
While uncommon and making up an estimated 0.3%-1.8% of all gastric malignancies, G-NET rates have been rising globally[1,8,15-17]. According to the American National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database, the incidence of gastric carcinoids is 1.4 per 100000 people[9]. The rate of gastric NETs has increased 15-fold from 1973 to 2012[1]. Among all neuroendocrine tumors, the percentage of which were gastric from 1950–1969 was 2.3%, which increased to 6.0% from 2000-2007[14]. Similarly, in England from 1971 to 2006 there has been a relative increase in gastric NETs, of 2325% in men and 4746% in women[15].
This rising incidence is likely in-part due to increased diagnosis facilitated by widespread endoscopy use and advances in immunohistochemistry[18-20]. Tumors are more readily diagnosed, monitored more closely, and can be endoscopically biopsied or therapeutically resected. Given the increasing rates have overlapped with the more widespread use of acid-suppressing agents like proton pump inhibitors (PPI), some studies question PPI use as a potential cause of ECL cell proliferation. It is also possible that this is a coincidental association given the routine diagnostic endoscopy-use to investigate gastric pathology in patients treated with PPIs[8]. There is presently not sufficient evidence to suggest a causal relationship with long-term proton pump inhibitor use[21].
Carcinoid tumors are most common in women, with an age-standardized incidence rate reported to be 0.5 per 100000 noted in a SEER analysis of 13279 cases from 2000-2019 in the United States. Men have a reported incidence of 0.37 per 100000. In looking at race and ethnicity, Hispanic patients were found to have the highest incidence in this SEER analysis, with Hispanic women having an incidence of 0.91 per 100000 and men having an incidence of 0.49 per 100000. They noted two-times the number of cases in patients 55 years-old and greater vs those less than 55[22]. A subsequent SEER analysis of a sample of 28199 people showed that gastric carcinoids were most common in people ages 50 and older; of people with carcinoid diagnosed 2016-2018, 81.0% were greater than or equal to 50 years of age, and 19.0% were 20-49, with similar rates of diagnoses from 2000 to 2002[23]. This is supported by other studies with similar age trends[8,22-24].
There are also epidemiologic patterns noted based on the subtype of gastric cancer. The most common subtype is type I (75%-80% of NENs), followed by type III (15%-25%), and the least common type II (5%). Aligning with the associated pathologies, type I gastric carcinoid tumors, which are associated with atrophic gastritis, are more commonly found in women, rather than men, and in people of white race[12,25,26]. For type II, there has been no specific noted association by sex[27], however given its association with gastrinomas and Zollinger-Ellison syndrome, it has been noted to affect younger patients relative to the other 2 types[28]. For type III tumors, a systematic review of 31 articles showed a median age of 58 years and slight predominance of disease in men (59.3% of the population)[29].
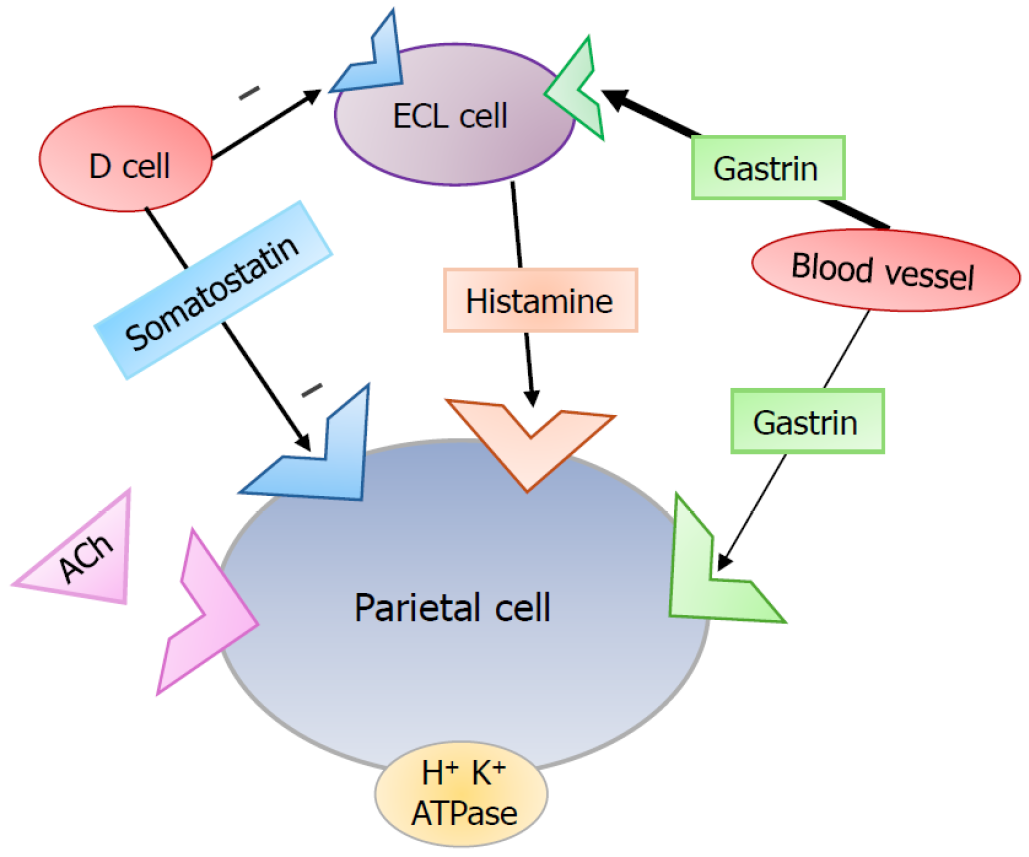
The pathogenesis of gastric NETs overall can be divided into 2 categories: Gastrin-dependent and gastrin-independent. The understanding of normal gastric physiology is vital to understanding the deviance which results in type I and II gastrin-dependent G-NETs[3,30]. Parietal cells in the stomach are responsible for secreting gastric acid upon stimulation by the vagus nerve (via acetylcholine, ACh), histamine (via ECL cell paracrine mechanism), and gastrin (endocrine mechanism) among other stimuli. ECL cells reside in the fundus of the stomach within gastric glands. ECL cells respond to the hormone gastrin which is released by G cells in the antrum of the stomach. Gastrin is normally secreted by G cells in the gastric antrum, which then bind to the gastrin receptors on ECL cells to signal the ECL-mediated release of histamine, which then serves as a potent parietal cell activator via the H2 receptor[30]. Moreover, D cells secrete somatostatin which act via paracrine mechanism to directly inhibit parietal cell activity and indirectly by inhibiting ECL cells (Figure 1). It is the dysregulation of this process via increased gastrin levels which is implicated in the pathogenesis of type I and type II NETs.
Type I gastric NETs are characterized by their association with atrophic gastritis[31]. This destruction of gastric parietal cells is an often immune-mediated process that results in hypo- or achlorhydria. This process can also be caused by Helicobacter pylori[32]. As a result of decreased gastric acid, there is a positive feedback loop that stimulates the secretion of gastrin. Thus, ECL cells are hyperstimulated by high gastrin levels and this can result in hypertrophy and neoplasia[33]. In contrast, type II G-NETs are often associated with syndromes like sporadic gastrinomas or MEN-1 related Zollinger-Ellison syndrome in which gastrin is autonomously hypersecreted by a gastrinoma in the small bowel or pancreas. Given the ectopic secretion, negative feedback loops are unable to inhibit the release of gastrin even in the presence of high gastric acid, and thus ECL cells are hyperstimulated, ultimately giving rise to type II tumors[28,34].
In comparison, type III carcinoids occur sporadically and have predominantly been noted to be gastrin-independent without associated conditions. Some have also suggested the classification of type IV gastric NETs; these tumors have been described as those that behave similarly to type III as often solitary and aggressive, but with characteristics of NECs rather than NETs. Another description of type IV tumors is with an inherent defect in acid secretion by parietal cells, effectively presenting like type I tumors[35-38]. An additional proposal of type IV carcinoid is as a non-ECL derived neu
Gastric neuroendocrine tumors can present with general signs and symptoms of gastric cancer such as weight loss, abdominal pain, nausea, vomiting, and gastrointestinal bleeding, as NETs represent a subset of gastric cancers. Specifically, type I gastric carcinoids are generally low-grade, slow-growing tumors that have a malignancy risk of less than 3%[41]. Because of their indolent nature, they are often asymptomatic and predominantly discovered incidentally on endoscopy. If the tumor is symptomatic, it can cause abdominal pain, bleeding and vomiting due to the mass effect of the lesion. They are associated with a high survival rate, comparable to the 5-year survival rate of the general population[39]. Type II tumors have a higher rate of spread than type I carcinoids with a 10%-30% chance of metastasis[42]. These tumors have a 5-year survival of roughly 70%. Type II G-NETs can often present with symptoms from Zollinger-Ellison syn
In contrast, type III gastric carcinoids are known as the most aggressive subtype with the highest malignant potential and lowest survival rate[44]. Type III carcinoids tend to only show symptoms once the disease is advanced. Patients’ symptoms associated with G-NET tumors themselves are usually nonspecific abdominal pain, nausea, vomiting, gas
The diagnosis of gastric carcinoid tumors typically begins with an upper endoscopy (esophagogastroduodenoscopy, or EGD) for direct visualization of the gastric mucosa, which also enables the biopsy of any suspicious lesions. Often times, gastric carcinoid tumors, especially type I, are noted incidentally during endoscopies for a different indication. During endoscopy, gastric carcinoids often appear as small, polypoid, or nodular lesions that are frequently found in the gastric body and fundus[47]. It is important that diagnostic endoscopies include data on location, size, appearance, and number of lesions[48]. In some cases, multiple lesions may be present, particularly in type I and type II carcinoids, which are associated with gastrin-driven hyperplasia. In some cases, endoscopic ultrasound (EUS) is used to assess the depth of invasion into the gastric wall and determine if there is lymph node involvement, which helps guide treatment decisions as will be later discussed[49].
Once a lesion is identified, diagnosis is made with histopathologic examination of the biopsy samples. Immunohistochemical staining can confirm the neuroendocrine origin of the tumor, with markers such as chromogranin A and synaptophysin[50-52]. Other tumor markers to consider include cytokeratin, CDX2, and CD56[50,53]. The Ki-67% index and mitotic rate may also help to determine the tumor's grade, further aiding in risk stratification[54]. The Ki-67% of a tumor indicates the rate at which cells are proliferating in a tumor. Higher Ki-67% values indicate a higher mitotic index with more cellular proliferation. According to WHO grading, grade 1 (G1) carcinoids have a Ki-67% of less than 3%. Grade 2 (G2) tumors have a Ki-67% between 3% and 20%, and grade 3 (G3) tumors have a Ki-67% of greater than 20%[10,11,40,50].
Laboratory tests can assess biochemical markers associated with neuroendocrine activity. Serum chromogranin A is the most utilized biomarker typically elevated in gastric carcinoids, especially in type I and type II tumors[55]. With sus
For staging and metastatic evaluation, cross-sectional imaging such as computed tomography or magnetic resonance imaging of the abdomen and pelvis is performed to assess tumor burden and organ involvement[58]. Additionally, somatostatin receptor imaging, such as a Gallium-68 DOTATATE positron emission tomography (PET) scan, is highly sensitive for detecting metastatic disease since many gastric carcinoids overexpress somatostatin receptors[59]. This advanced imaging modality can help guide treatment decisions, including the use of systemic therapies such as somato
Various classification systems exist for staging. One example is the American Joint Committee on Cancer staging criteria, which uses the tumor-node-metastasis (T-N-M) staging model. Stage I and II encompass tumors with limited local invasion, without nodal or distant metastases. Stage I denotes patients with smaller tumors (< 1.0 cm) with limited local depth invasion (into lamina propria or submucosa) (T1). Stage II includes tumors that are larger (> 1.0 cm) and more invasive (across the lamina propria and/or submucosa or invades muscularis propria) (T2). In addition, stage II also includes more invasive T3 tumors, which have grown through the muscularis propria into the subserosa. Stage III encompasses any tumors that extend deeper into the serosa or visceral peritoneum (T4) or that has spread to nearby lymph nodes (N1). The most widespread disease is stage IV, which includes metastatic tumors that spread to more distant parts of the body[61].
Given the complex pathophysiology and rarity of this disorder, as previously described in this review, managing these patients is a multi-disciplinary initiative consisting of medical oncologists, endocrinologists, gastroenterologists (including advanced endoscopists), pathologists, and surgeons. The clinical decision-making of these tumor boards is typically guided by the subtype of G-NET and more specifically on tumor grade and size[39,62]. While management strategies are highly individualized and often dependent on surgical clinical judgment, we will discuss guidelines and overarching strategy that guide treatment.
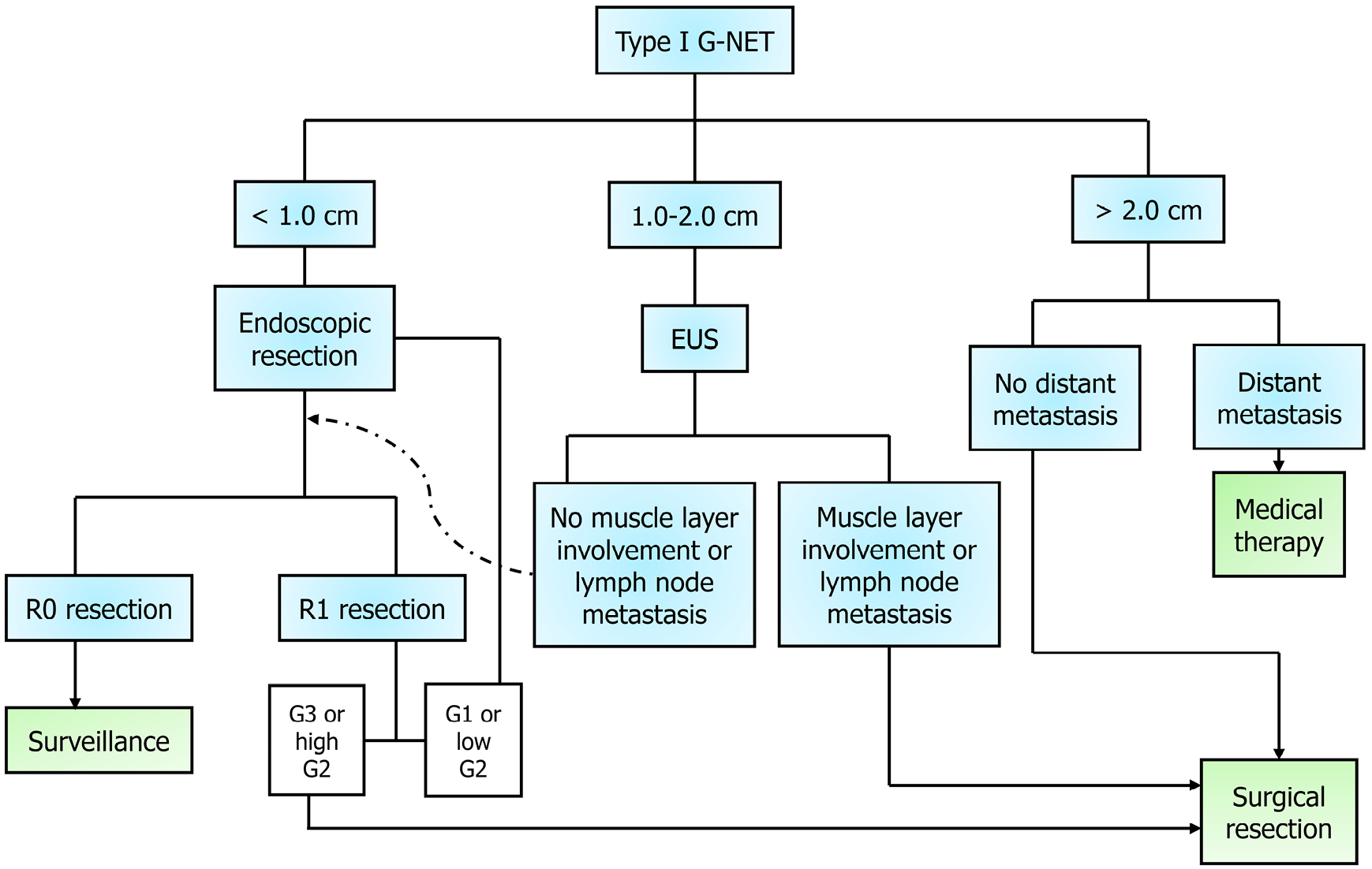
The management of type I mainly consists of endoscopic resection and surveillance for smaller (< 1.0 cm), low-grade tumors (G1 or low G2). More extensive resection or surgical consideration is reserved for larger (> 2.0 cm) tumors or those with high-risk features or grade (G3 or high G2), as per The North American Neuroendocrine Tumor Society (NANETS) among others’ recommendation[63,64]. If several small tumors are not amenable to resection or if there is recurrent disease, patients will often be initiated on somatostatin-analog therapy[64,65]. For smaller singular or few tumors, a complete resection (R0) should be performed. If there is an incomplete resection (R1), then the next steps depend on the grade; for low-grade, an endoscopic resection should be repeated, vs higher-grade tumors require surgical resection, as suggested by the European Neuroendocrine Tumor Society’s (ENETS) “step-up approach”[64].
For intermediate size (1.0-2.0 cm) tumors, ENETS suggests EUS to assess depth and local lymph node involvement[64]. If the tumor extends to the muscular layer or there is confirmed lymph node involvement on EUS, then upfront surgical resection is recommended. For these high-risk G2 type I G-NETs, it is recommended to start with a limited resection with sampling of local lymph nodes; however, if a patient has known metastases, then more aggressive upfront gastrectomy (consider subtotal vs total based on location) with D2 Lymphadenectomy may be recommended[64]. Otherwise, an intermediate size tumor is treated like a smaller G1 tumor, and the next steps depend on R0 vs R1 status (Figure 2). In terms of surgical resection for type I tumors, patients benefit from the inclusion of an antrectomy, as the elimination of gastrin-secreting cells ceases the source of gastrin[66,67].
Given that type II tumors are fed by gastrinomas, treatment of type II tumors begins with addressing the gastrinoma, as this type of G-NET has been noted to regress if its gastrin supply is impeded by somatostatin analogs or surgery[68]. The gold standard for resecting gastrinomas, most commonly found in the duodenum or head of the pancreas, is a pancreaticoduodenectomy (Whipple procedure). If a patient is not a candidate for a Whipple, then a more localized surgical resection or endoscopic excision can be attempted for cytoreduction[3,63]. Otherwise, the best option is medical therapy with a somatostatin analogue for these gastrin-dependent tumors[68].
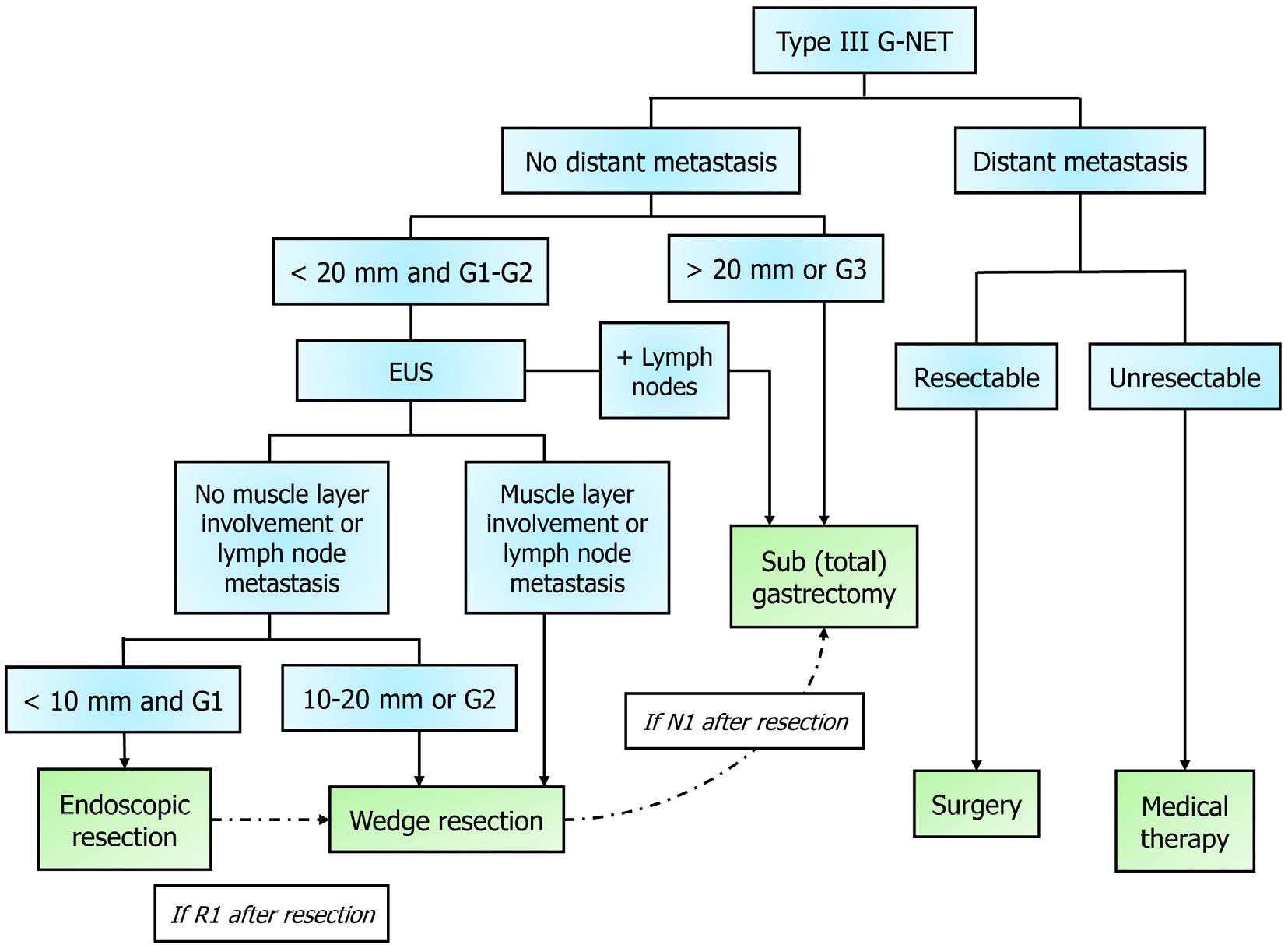
Type III tumors are the most aggressive tumors and thus require the most invasive management. Depending on the size, location, and invasiveness, there are different extents of resection offered. A surgeon will need to decide the extent of gastric resection required (wedge vs subtotal vs total gastrectomy) and the extent of lymph node sampling that is required. With improvements in endoscopic resection, now in select patients less invasive endoscopic resection or wedge resection can be performed if the tumor has favorable low-grade (G1/G2) and is small (< 1.0-1.5 cm)[64,69]. This is especially true in cases where extensive surgery would be particularly high risk. The role of wedge resection in cases of intermediate grade (G2) type III or intermediate size 1.0-2.0 cm tumors are still debated topics. While subtype is important, prior research and newer evidence confirm the impact that size and grade have on prognosis and thus these qualities guide how aggressive surgery should be[62].
For high-grade (G3) tumors, radical resection with subtotal or total gastrectomy with lymphadenectomy should be performed, as suggested by organizations like NANETS and ENETS[64,70] (Figure 3). This aggressive approach is especially important in high-grade G3 (Ki67 > 20%), metastatic disease, or large tumor (> 2.0 cm) cases[64]. Radical surgical resection may also be required subsequently after a limited wedge resection if postoperative pathology shows nodal metastasis, higher tumor grade than the original biopsy, lymphovascular invasion, or incomplete resection (R1)[69]. In terms of neoadjuvant or adjuvant therapy for these patients, there is no consensus within the community[71].
If there are isolated hepatic metastases with localization to one lobe, patients may be treated with hepatic resection, as both symptoms and survival have been shown to improve after[63,72,73]. Gastrointestinal NET metastases to the liver can also be treated with hepatic ablation or radioembolization in conjunction with or without hepatic resection, to ideally treat > 90% of bulk of disease[73-76]. However, it should be noted that much of this evidence is in the context of gas
Patients with widespread metastatic disease may also benefit from somatostatin-analog therapy, peptide receptor radionuclide therapy (PRRT), or chemotherapy. Somatostatin analogs, such as octreotide LAR and lanreotide, can help symptoms and slow the growth of well-differentiated tumors[77]. There is data that suggesting that these agents may improve disease-free survival as well[78].
PRRT is performed with beta-emitting radionuclides such as 177 Lutetium (177 LuDOTATATE). Recent prospective randomized control trials have shown great promise with mid-gut NET tumors and gastroenteropancreatic tumors in general (including stomach), in NETTER-1 and NETTER-2, respectively[79-81]. Similar to PPRT-based studies, there is lacking data for the treatment of systemic chemotherapy for specifically gastric neuroendocrine tumors; however, studies of broader gastroenteropancreatic tumors have examined the use of chemotherapy[63]. Agents including 5-fluorouracil, doxorubicin, and streptozocin have been used to treat metastatic pancreatic endocrine tumors, while other studies suggest a potential role for capecitabine, oxaliplatin, and temozolomide for patients with progressive digestive neuroendocrine carcinoma disease[70,82-85]. Everolimus, an mTOR inhibitor, is an additional agent which has been shown to have antitumor effects in advanced NETs when given with octreotide LAR in a phase II trial; however its use with car
While carcinoid syndrome is not common with gastric NET tumors, it is important that surgeons are aware of this syndrome and the potentially life-threatening complications that can occur as a result of carcinoid crisis, and how to avoid it. Providers should obtain a careful history to inquire about any carcinoid symptoms[87]. If the patient is experiencing associated diarrhea, electrolyte and vitamin levels should be assessed and corrected. If there is concern for carcinoid syndrome preoperatively, a 5-hydroxyindoleacetic acid (5-HIAA) level should be obtained, as a high 5-HIAA, chromogranin A, and tumor burden have each been correlated with increased risk of experiencing carcinoid crisis[88,89]. Minor procedures, severe emotional stress, anesthesia induction, or tumor manipulation (during physical exam or surgery) can prompt a carcinoid crisis. Patients may show signs such as labile blood pressure, alongside flushing, hyperthermia, or bronchospasm[90]. Ideally, this syndrome or crisis is avoided by preemptively giving octreotide (a somatostatin analogue), which by mimicking the action of the hormone prevents its endogenous release. However, the exact dosage and regimen of octreotide for this indication has not yet been established[90,91]. If an acute episode occurs, intravenous octreotide should be given acutely[87,91]. While initially thought to be inferior to octreotide, emerging evidence also suggests that the use of vasopressors may result in quicker resolution of crisis during intraoperative carcinoid crisis[92]. Future studies should elucidate the role for perioperative octreotide in the prevention and manage
Gastric carcinoid is a rare disease but is nonetheless increasing in incidence, largely due to the rise in endoscopic diagnosis. As the disease is more widely diagnosed, further studies have deepened our understanding of pathophy
| 1. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2665] [Article Influence: 296.1] [Reference Citation Analysis (5)] |
| 2. | Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 774] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 3. | Lamberti G, Panzuto F, Pavel M, O'Toole D, Ambrosini V, Falconi M, Garcia-Carbonero R, Riechelmann RP, Rindi G, Campana D. Gastric neuroendocrine neoplasms. Nat Rev Dis Primers. 2024;10:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Oberndorfer S. Karzinoide tumoren des dunndarms. Frankfurt Z Path 1907; 1: 426-432. |
| 5. | Tsoucalas G, Karamanou M, Androutsos G. The eminent German pathologist Siegfried Oberndorfer (1876-1944) and his landmark work on carcinoid tumors. Ann Gastroenterol. 2011;24:98-100. [PubMed] |
| 6. | Arnold R. Endocrine tumours of the gastrointestinal tract. Introduction: definition, historical aspects, classification, staging, prognosis and therapeutic options. Best Pract Res Clin Gastroenterol. 2005;19:491-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Gastrointestinal Neuroendocrine Tumors Treatment (PDQ®): Health Professional Version. 2025 May 9. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002– . [PubMed] |
| 8. | Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol. 2004;99:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Xu Z, Wang L, Dai S, Chen M, Li F, Sun J, Luo F. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw Open. 2021;4:e2124750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 10. | WHO Classification of Tumours Editorial Board. WHO Classification of Digestive Tumours. WHO, 2019. |
| 11. | WHO Classification of Tumours Editorial Board. WHO Classification of Endocrine and Neuroendocrine Tumours. WHO, 2022. |
| 12. | Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 379] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Capella C, Solcia E, Sobin LH, Arnold R. Endocrine tumours of the small intestine. In: Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumours of the Digestive System. International Agency for Research on Cancer, 2000: 67-90. |
| 14. | Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 15. | Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Das S, Dasari A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr Oncol Rep. 2021;23:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 256] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 17. | Maly M, Callebout E, Ribeiro S, Hoorens A, Carton S, Cuyle PJ, Vandamme T, Borbath I, Demetter P, Van Damme N, Van Eycken L, Verslype C, Geboes K. Neuroendocrine tumors in the stomach: An epidemiological analysis of Belgian Cancer Registry data 2010-2019. J Neuroendocrinol. 2025;37:e13473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, DiBonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-1187.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1489] [Article Influence: 106.4] [Reference Citation Analysis (1)] |
| 19. | Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gönen M, Jensen RT, Kidd M, Kulke MH, Lloyd RV, Moran C, Moss SF, Oberg K, O'Toole D, Rindi G, Robert ME, Suster S, Tang LH, Tzen CY, Washington MK, Wiedenmann B, Yao J. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34:300-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 20. | ASGE Standards of Practice Committee; Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, Pasha SF, Sharaf R, Shergill AK, Cash BD, DeWitt JM. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc. 2015;82:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 21. | Zheng Z, Lu Z, Song Y. Long-term proton pump inhibitors use and its association with premalignant gastric lesions: a systematic review and meta-analysis. Front Pharmacol. 2023;14:1244400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Aslani A, Soheili A, Mousavi SE, Ebrahimi A, Antar RM, Yekta Z, Nejadghaderi SA. Incidence trends of gastric cancer in the United States over 2000-2020: A population-based analysis. PLoS One. 2024;19:e0310040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Rustgi SD, McKinley M, McBay B, Zylberberg HM, Gomez SL, Hur C, Kastrinos F, Gupta S, Kim MK, Itzkowitz SH, Shah SC. Epidemiology of Gastric Malignancies 2000-2018 According to Histology: A Population-Based Analysis of Incidence and Temporal Trends. Clin Gastroenterol Hepatol. 2023;21:3285-3295.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Shebani KO, Souba WW, Finkelstein DM, Stark PC, Elgadi KM, Tanabe KK, Ott MJ. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999;229:815-21; discussion 822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Annibale B, Azzoni C, Corleto VD, di Giulio E, Caruana P, D'Ambra G, Bordi C, Delle Fave G. Atrophic body gastritis patients with enterochromaffin-like cell dysplasia are at increased risk for the development of type I gastric carcinoid. Eur J Gastroenterol Hepatol. 2001;13:1449-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Vannella L, Sbrozzi-Vanni A, Lahner E, Bordi C, Pilozzi E, Corleto VD, Osborn JF, Delle Fave G, Annibale B. Development of type I gastric carcinoid in patients with chronic atrophic gastritis. Aliment Pharmacol Ther. 2011;33:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 281] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Solcia E, Capella C, Fiocca R, Rindi G, Rosai J. Gastric argyrophil carcinoidosis in patients with Zollinger-Ellison syndrome due to type 1 multiple endocrine neoplasia. A newly recognized association. Am J Surg Pathol. 1990;14:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 140] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Laffi A, Lania AGA, Ragni A, Di Vito V, Liccardi A, Rubino M, Sesti F, Colao A, Faggiano A; On Behalf Of The Nike Group. Gastric Neuroendocrine Tumors (g-NETs): A Systematic Review of the Management and Outcomes of Type 3 g-NETs. Cancers (Basel). 2023;15:2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 30. | Engevik AC, Kaji I, Goldenring JR. The Physiology of the Gastric Parietal Cell. Physiol Rev. 2020;100:573-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 31. | Massironi S, Gallo C, Elvevi A, Stegagnini M, Coltro LA, Invernizzi P. Incidence and prevalence of gastric neuroendocrine tumors in patients with chronic atrophic autoimmune gastritis. World J Gastrointest Oncol. 2023;15:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology. 2021;161:1325-1332.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (3)] |
| 33. | Bordi C, D'Adda T, Azzoni C, Pilato FP, Caruana P. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol. 1995;19 Suppl 1:S8-19. [PubMed] |
| 34. | Berna MJ, Annibale B, Marignani M, Luong TV, Corleto V, Pace A, Ito T, Liewehr D, Venzon DJ, Delle Fave G, Bordi C, Jensen RT. A prospective study of gastric carcinoids and enterochromaffin-like cell changes in multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: identification of risk factors. J Clin Endocrinol Metab. 2008;93:1582-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Abraham SC, Carney JA, Ooi A, Choti MA, Argani P. Achlorhydria, parietal cell hyperplasia, and multiple gastric carcinoids: a new disorder. Am J Surg Pathol. 2005;29:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Ooi A, Ota M, Katsuda S, Nakanishi I, Sugawara H, Takahashi I. An Unusual Case of Multiple Gastric Carcinoids Associated with Diffuse Endocrine Cell Hyperplasia and Parietal Cell Hypertrophy. Endocr Pathol. 1995;6:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Nakata K, Aishima S, Ichimiya H, Yao T, Matsuura T, Seo M, Nagai E, Okido M, Kato M, Nakagaki M, Tsuneyoshi M, Tanaka M. Unusual multiple gastric carcinoids with hypergastrinemia: report of a case. Surg Today. 2010;40:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Exarchou K, Stephens NA, Moore AR, Howes NR, Pritchard DM. New Developments in Gastric Neuroendocrine Neoplasms. Curr Oncol Rep. 2022;24:77-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Borch K, Ahrén B, Ahlman H, Falkmer S, Granérus G, Grimelius L. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg. 2005;242:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 40. | Song Y, Chen E, Chiang YJ, Yao JC, Halperin DM, Chatterjee D, Badgwell BD. Classification of Gastric Neuroendocrine Tumors and Associations With Survival. J Surg Oncol. 2025;131:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Chen YY, Guo WJ, Shi YF, Su F, Yu FH, Chen RA, Wang C, Liu JX, Luo J, Tan HY. Management of type 1 gastric neuroendocrine tumors: an 11-year retrospective single-center study. BMC Gastroenterol. 2023;23:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Ogata Y, Hatta W, Kanno T, Saito M, Jin X, Asano N, Koike T, Imatani A, Yuan Y, Masamune A. Type 2 and type 3 gastric neuroendocrine tumors have high risk of lymph node metastasis: Systematic review and meta-analysis. Dig Endosc. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142:709-23; discussion, 724. [PubMed] |
| 44. | Panzuto F, Campana D, Massironi S, Faggiano A, Rinzivillo M, Lamberti G, Sciola V, Lahner E, Manuzzi L, Colao A, Annibale B. Tumour type and size are prognostic factors in gastric neuroendocrine neoplasia: A multicentre retrospective study. Dig Liver Dis. 2019;51:1456-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Davis Z, Moertel CG, McIlrath DC. The malignant carcinoid syndrome. Surg Gynecol Obstet. 1973;137:637-644. [PubMed] |
| 46. | Yadegarfar G, Friend L, Jones L, Plum LM, Ardill J, Taal B, Larsson G, Jeziorski K, Kwekkeboom D, Ramage JK; EORTC Quality of Life Group. Validation of the EORTC QLQ-GINET21 questionnaire for assessing quality of life of patients with gastrointestinal neuroendocrine tumours. Br J Cancer. 2013;108:301-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Nikou GC, Toubanakis C, Moulakakis KG, Pavlatos S, Kosmidis C, Mallas E, Safioleas P, Sakorafas GH, Safioleas MC. Carcinoid tumors of the duodenum and the ampulla of Vater: current diagnostic and therapeutic approach in a series of 8 patients. Case series. Int J Surg. 2011;9:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Borbath I, Pape UF, Deprez PH, Bartsch DK, Caplin M, Falconi M, Garcia-Carbonero R, Grozinsky-Glasberg S, Jensen RT, Arnold R, Ruszniewski P, Toumpanakis C, Valle JW, O Toole D; Members of the Advisory Board of the European Neuroendocrine Tumor Society (ENETS). ENETS standardized (synoptic) reporting for endoscopy in neuroendocrine tumors. J Neuroendocrinol. 2022;34:e13105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Yoshikane H, Tsukamoto Y, Niwa Y, Goto H, Hase S, Mizutani K, Nakamura T. Carcinoid tumors of the gastrointestinal tract: evaluation with endoscopic ultrasonography. Gastrointest Endosc. 1993;39:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC; North American Neuroendocrine Tumor Society. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 455] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 51. | Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Buffa R, Rindi G, Sessa F, Gini A, Capella C, Jahn R, Navone F, De Camilli P, Solcia E. Synaptophysin immunoreactivity and small clear vesicles in neuroendocrine cells and related tumours. Mol Cell Probes. 1987;1:367-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Georgakopoulou VE, Zygouris E, Damaskos C, Pierrakou A, Papalexis P, Garmpis N, Aravantinou-Fatorou A, Chlapoutakis S, Diamantis E, Nikokiris C, Gkoufa A, Sklapani P, Trakas N, Janinis J, Spandidos DA, Dahabreh J. Prognostic value of the immunohistochemistry markers CD56, TTF-1, synaptophysin, CEA, EMA and NSE in surgically resected lung carcinoid tumors. Mol Clin Oncol. 2022;16:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Capella C, Heitz PU, Höfler H, Solcia E, Klöppel G. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 1995;425:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 280] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 55. | Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, Krenning EP, Bouillon R, Lamberts SW. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82:2622-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Pregun I, Herszényi L, Juhász M, Miheller P, Hritz I, Patócs A, Rácz K, Tulassay Z. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion. 2011;84:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Kleĭman EI. [Complicated diverticulum of the stomach]. Vestn Rentgenol Radiol. 1986;82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Pape UF, Jann H, Müller-Nordhorn J, Bockelbrink A, Berndt U, Willich SN, Koch M, Röcken C, Rindi G, Wiedenmann B. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 59. | Shell J, Keutgen XM, Millo C, Nilubol N, Patel D, Sadowski S, Boufraqech M, Yang L, Merkel R, Atallah C, Herscovitch P, Kebebew E. 68-Gallium DOTATATE scanning in symptomatic patients with negative anatomic imaging but suspected neuroendocrine tumor. Int J Endocr Oncol. 2018;5:IJE04. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Arnold R, Trautmann ME, Creutzfeldt W, Benning R, Benning M, Neuhaus C, Jürgensen R, Stein K, Schäfer H, Bruns C, Dennler HJ. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut. 1996;38:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 227] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 61. | American Joint Committee on Cancer (AJCC). Neuroendocrine Tumors of the Stomach. In: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017: 351-359. |
| 62. | Hanna A, Kim-Kiselak C, Tang R, Metz DC, Yang Z, DeMatteo R, Fraker DL, Roses RE. Gastric Neuroendocrine Tumors: Reappraisal of Type in Predicting Outcome. Ann Surg Oncol. 2021;28:8838-8846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC, Jensen RT; North American Neuroendocrine Tumor Society (NANETS). NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 64. | Panzuto F, Ramage J, Pritchard DM, van Velthuysen MF, Schrader J, Begum N, Sundin A, Falconi M, O'Toole D. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for gastroduodenal neuroendocrine tumours (NETs) G1-G3. J Neuroendocrinol. 2023;35:e13306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 108] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 65. | Sebastian-Valles F, Bernaldo Madrid B, Sager C, Carrillo López E, Mera Carreiro S, Ávila Antón L, García NS, Sampedro-Nuñez MA, Díaz Pérez JÁ, Marazuela M. Chronic Treatment with Somatostatin Analogues in Recurrent Type 1 Gastric Neuroendocrine Tumors. Biomedicines. 2023;11:872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 66. | Eckhauser FE, Lloyd RV, Thompson NW, Raper SE, Vinik AI. Antrectomy for multicentric, argyrophil gastric carcinoids: a preliminary report. Surgery. 1988;104:1046-1053. [PubMed] |
| 67. | Hirschowitz BI, Griffith J, Pellegrin D, Cummings OW. Rapid regression of enterochromaffinlike cell gastric carcinoids in pernicious anemia after antrectomy. Gastroenterology. 1992;102:1409-1418. [PubMed] |
| 68. | Tomassetti P, Migliori M, Caletti GC, Fusaroli P, Corinaldesi R, Gullo L. Treatment of type II gastric carcinoid tumors with somatostatin analogues. N Engl J Med. 2000;343:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Exarchou K, Kamieniarz L, Tsoli M, Victor A, Oleinikov K, Khan MS, Srirajaskanthan R, Mandair D, Grozinsky-Glasberg S, Kaltsas G, Howes N, Pritchard DM, Toumpanakis C. Is local excision sufficient in selected grade 1 or 2 type III gastric neuroendocrine neoplasms? Endocrine. 2021;74:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Eads JR, Halfdanarson TR, Asmis T, Bellizzi AM, Bergsland EK, Dasari A, El-Haddad G, Frumovitz M, Meyer J, Mittra E, Myrehaug S, Nakakura E, Raj N, Soares HP, Untch B, Vijayvergia N, Chan JA. Expert Consensus Practice Recommendations of the North American Neuroendocrine Tumor Society for the management of high grade gastroenteropancreatic and gynecologic neuroendocrine neoplasms. Endocr Relat Cancer. 2023;30:e220206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 71. | Sok C, Ajay PS, Tsagkalidis V, Kooby DA, Shah MM. Management of Gastric Neuroendocrine Tumors: A Review. Ann Surg Oncol. 2024;31:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Watzka FM, Fottner C, Miederer M, Schad A, Weber MM, Otto G, Lang H, Musholt TJ. Surgical therapy of neuroendocrine neoplasm with hepatic metastasis: patient selection and prognosis. Langenbecks Arch Surg. 2015;400:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Barat M, Cottereau AS, Kedra A, Dermine S, Palmieri LJ, Coriat R, Dautry R, Tselikas L, Soyer P, Dohan A. The Role of Interventional Radiology for the Treatment of Hepatic Metastases from Neuroendocrine Tumor: An Updated Review. J Clin Med. 2020;9:2302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin N Am. 2003;12:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 75. | Musunuru S, Chen H, Rajpal S, Stephani N, McDermott JC, Holen K, Rikkers LF, Weber SM. Metastatic neuroendocrine hepatic tumors: resection improves survival. Arch Surg. 2006;141:1000-4; discussion 1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 76. | Zuckerman DA, Kennard RF, Roy A, Parikh PJ, Weiner AA. Outcomes and toxicity following Yttrium-90 radioembolization for hepatic metastases from neuroendocrine tumors-a single-institution experience. J Gastrointest Oncol. 2019;10:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, Müller HH, Arnold R; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology. 2017;104:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 277] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 78. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1352] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 79. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2439] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 80. | Hope TA, Abbott A, Colucci K, Bushnell DL, Gardner L, Graham WS, Lindsay S, Metz DC, Pryma DA, Stabin MG, Strosberg JR. NANETS/SNMMI Procedure Standard for Somatostatin Receptor-Based Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE. J Nucl Med. 2019;60:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 81. | Singh S, Halperin D, Myrehaug S, Herrmann K, Pavel M, Kunz PL, Chasen B, Tafuto S, Lastoria S, Capdevila J, García-Burillo A, Oh DY, Yoo C, Halfdanarson TR, Falk S, Folitar I, Zhang Y, Aimone P, de Herder WW, Ferone D; all the NETTER-2 Trial Investigators. [(177)Lu]Lu-DOTA-TATE plus long-acting octreotide versus highdose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): an open-label, randomised, phase 3 study. Lancet. 2024;403:2807-2817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 174] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 82. | Arnold R, Rinke A, Schmidt Ch, Hofbauer L. Endocrine tumours of the gastrointestinal tract: Chemotherapy. Best Pract Res Clin Gastroenterol. 2005;19:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Zappi A, Persano I, Galvani L, Parlagreco E, Andrini E, Campana D, Brizzi MP, Lamberti G, La Salvia A. Chemotherapy in Well Differentiated Neuroendocrine Tumors (NET) G1, G2, and G3: A Narrative Review. J Clin Med. 2023;12:717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 84. | Sorbye H, Grande E, Pavel M, Tesselaar M, Fazio N, Reed NS, Knigge U, Christ E, Ambrosini V, Couvelard A, Tiensuu Janson E. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J Neuroendocrinol. 2023;35:e13249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 85. | National Comprehensive Cancer Network. NCCN Guidelines Version 1.2025 Neuroendocrine Tumors of the Gastrointestinal Tract (Well-Differentiated Grade 1/2), Lung, and Thymus. Accessed May 23, 2025. Available from: https://www.nccn.org/guidelines/category_1. |
| 86. | Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A, Meric-Bernstam F. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311-4318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 464] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 87. | Kaltsas G, Caplin M, Davies P, Ferone D, Garcia-Carbonero R, Grozinsky-Glasberg S, Hörsch D, Tiensuu Janson E, Kianmanesh R, Kos-Kudla B, Pavel M, Rinke A, Falconi M, de Herder WW; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pre- and Perioperative Therapy in Patients with Neuroendocrine Tumors. Neuroendocrinology. 2017;105:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 88. | Kinney MA, Warner ME, Nagorney DM, Rubin J, Schroeder DR, Maxson PM, Warner MA. Perianaesthetic risks and outcomes of abdominal surgery for metastatic carcinoid tumours. Br J Anaesth. 2001;87:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 89. | Mancuso K, Kaye AD, Boudreaux JP, Fox CJ, Lang P, Kalarickal PL, Gomez S, Primeaux PJ. Carcinoid syndrome and perioperative anesthetic considerations. J Clin Anesth. 2011;23:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Massimino K, Harrskog O, Pommier S, Pommier R. Octreotide LAR and bolus octreotide are insufficient for preventing intraoperative complications in carcinoid patients. J Surg Oncol. 2013;107:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 91. | Seymour N, Sawh SC. Mega-dose intravenous octreotide for the treatment of carcinoid crisis: a systematic review. Can J Anaesth. 2013;60:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | McCully BH, Kozuma K, Pommier S, Pommier RF. Comparison of Octreotide and Vasopressors as First-Line Treatment for Intraoperative Carcinoid Crisis. Ann Surg Oncol. 2024;31:2996-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/